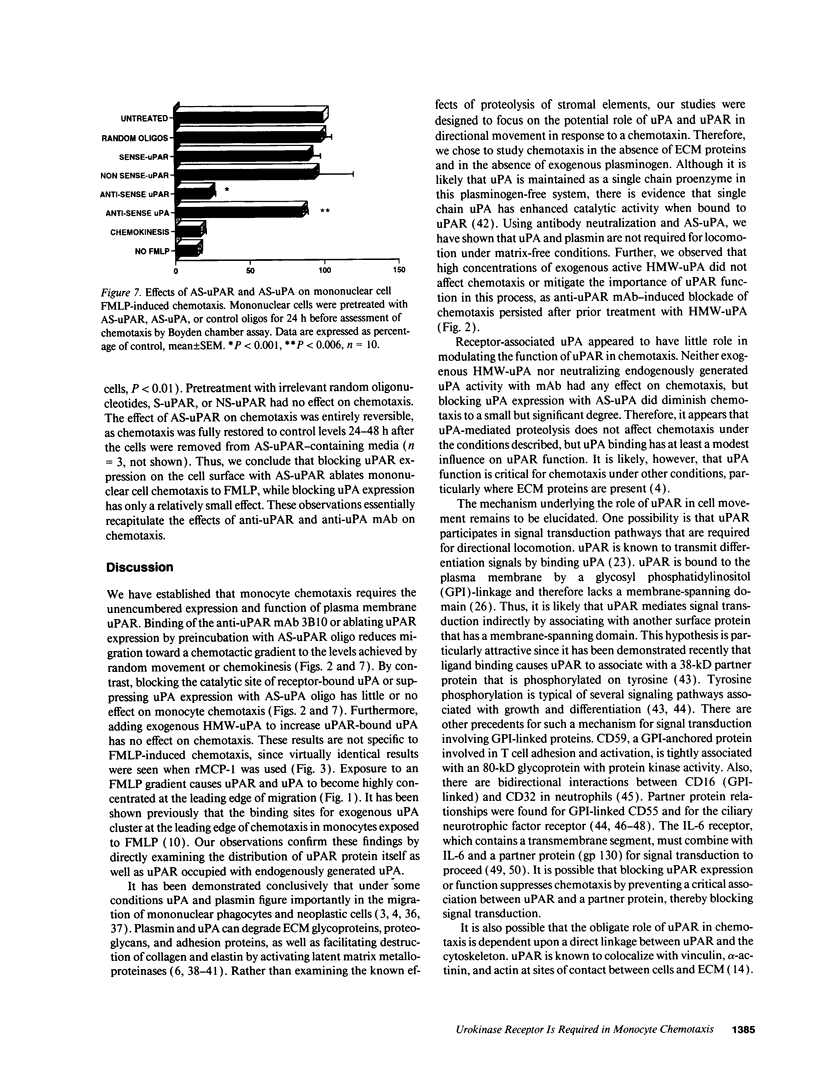
Abstract
Mononuclear phagocytes (Mphi) produce urokinase-type plasminogen activator (uPA) and also express a specific cell-surface receptor for urokinase, uPAR. The concomitant expression of these proteins provides a mechanism by which Mphi can degrade extracellular matrix proteins during directed cell migration. In this study, we sought to determine if uPAR plays a role in Mphi chemotaxis that is distinct from its role in matrix proteolysis. Exposing adherent monocytes to a chemotactic gradient causes plasma membrane uPAR to localize strongly to the leading edge of cell migration. Adherence alone or exposure to FMLP had no effect on uPAR expression. Using Boyden chamber chemotaxis assays, we demonstrate that treating mononuclear cells with an anti-uPAR mAb (either as an intact mAb or F[ab']2) ablates chemotaxis induced by FMLP and monocyte chemotactic peptide-1 (P < 0.001). Inactivating the catalytic activity of uPAR-bound uPA had no effect on chemotaxis. Similarly, blocking uPAR expression with an antisense oligonucleotide to uPAR completely ablates chemotaxis, but blocking uPA expression with an antisense oligonucleotide to uPA has a minimal effect. We therefore demonstrate that expression and unimpeded function of uPAR plays an obligate role in M phi chemotaxis by mechanisms that are largely independent of its ligand, uPA. Combined with its known role in mediating pericellular proteolysis, these observations demonstrate that uPAR is essential for both locomotion and traversing tissue barriers during M phi migration.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle M. D., Chiodo V. A., Lawman M. J., Gee A. P., Young M. Urokinase: a chemotactic factor for polymorphonuclear leukocytes in vivo. J Immunol. 1987 Jul 1;139(1):169–174. [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L., Vavrin Z. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J Clin Invest. 1984 Mar;73(3):806–815. doi: 10.1172/JCI111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Macrophage fibrinolytic activity: identification of two pathways of plasmin formation by intact cells and of a plasminogen activator inhibitor. Cell. 1982 Mar;28(3):653–662. doi: 10.1016/0092-8674(82)90220-3. [DOI] [PubMed] [Google Scholar]
- Ciambrone G. J., McKeown-Longo P. J. Plasminogen activator inhibitor type I stabilizes vitronectin-dependent adhesions in HT-1080 cells. J Cell Biol. 1990 Nov;111(5 Pt 1):2183–2195. doi: 10.1083/jcb.111.5.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana N., Styrt B., Griffin J. D., Todd R. F., 3rd, Klempner M. S., Arnaout M. A. Two functional domains in the phagocyte membrane glycoprotein Mo1 identified with monoclonal antibodies. J Immunol. 1986 Nov 15;137(10):3259–3263. [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- Davies P. F. Vascular cell interactions with special reference to the pathogenesis of atherosclerosis. Lab Invest. 1986 Jul;55(1):5–24. [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Valenzuela D. M., Wong V. V., Furth M. E., Squinto S. P., Yancopoulos G. D. The receptor for ciliary neurotrophic factor. Science. 1991 Jul 5;253(5015):59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- Dumler I., Petri T., Schleuning W. D. Interaction of urokinase-type plasminogenactivator (u-PA) with its cellular receptor (u-PAR) induces phosphorylation on tyrosine of a 38 kDa protein. FEBS Lett. 1993 May 3;322(1):37–40. doi: 10.1016/0014-5793(93)81106-a. [DOI] [PubMed] [Google Scholar]
- Estreicher A., Mühlhauser J., Carpentier J. L., Orci L., Vassalli J. D. The receptor for urokinase type plasminogen activator polarizes expression of the protease to the leading edge of migrating monocytes and promotes degradation of enzyme inhibitor complexes. J Cell Biol. 1990 Aug;111(2):783–792. doi: 10.1083/jcb.111.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfaro S., Berman J. S. The relation between cell migration and activation in inflammation: beyond adherence. Am J Respir Cell Mol Biol. 1992 Sep;7(3):248–250. doi: 10.1165/ajrcmb/7.3.248. [DOI] [PubMed] [Google Scholar]
- Gold L. I., Rostagno A., Frangione B., Passalaris T. Localization of the cleavage sites on fibronectin following digestion by urokinase. J Cell Biochem. 1992 Dec;50(4):441–452. doi: 10.1002/jcb.240500412. [DOI] [PubMed] [Google Scholar]
- Goldstein S. C., Todd R. F., 3rd Structural and biosynthetic features of the Mo5 human myeloid differentiation antigen. Tissue Antigens. 1993 Apr;41(4):214–218. doi: 10.1111/j.1399-0039.1993.tb02007.x. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Seifert P. S., Stemme S. Immune mechanisms in atherosclerosis. Arteriosclerosis. 1989 Sep-Oct;9(5):567–578. doi: 10.1161/01.atv.9.5.567. [DOI] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Huber A. R., Weiss S. J. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989 Apr;83(4):1122–1136. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Lohi J., Tuuttila A., Tryggvason K., Vartio T. Proteolytic processing of the 72,000-Da type IV collagenase by urokinase plasminogen activator. Exp Cell Res. 1992 Oct;202(2):471–476. doi: 10.1016/0014-4827(92)90101-d. [DOI] [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G. Endogenous receptor-bound urokinase mediates tissue invasion of human monocytes. J Immunol. 1989 Oct 15;143(8):2634–2639. [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G. Functional characteristics of receptor-bound urokinase on human monocytes: catalytic efficiency and susceptibility to inactivation by plasminogen activator inhibitors. Blood. 1989 Sep;74(4):1396–1402. [PubMed] [Google Scholar]
- Kirchheimer J. C., Wojta J., Christ G., Binder B. R. Proliferation of a human epidermal tumor cell line stimulated by urokinase. FASEB J. 1987 Aug;1(2):125–128. doi: 10.1096/fasebj.1.2.3038646. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D. A. Models for receptor-mediated cell phenomena: adhesion and migration. Annu Rev Biophys Biophys Chem. 1991;20:387–414. doi: 10.1146/annurev.bb.20.060191.002131. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Goldfarb R. H., Brundage R., Siegal G. P., Terranova V., Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981 Nov;41(11 Pt 1):4629–4636. [PubMed] [Google Scholar]
- Liu D. Y., Todd R. F., 3rd A monoclonal antibody specific for a monocyte-macrophage membrane component blocks the human monocyte response to migration inhibitory factor. J Immunol. 1986 Jul 15;137(2):448–455. [PubMed] [Google Scholar]
- Manchanda N., Schwartz B. S. Single chain urokinase. Augmentation of enzymatic activity upon binding to monocytes. J Biol Chem. 1991 Aug 5;266(22):14580–14584. [PubMed] [Google Scholar]
- Miller M. D., Krangel M. S. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12(1-2):17–46. [PubMed] [Google Scholar]
- Min H. Y., Semnani R., Mizukami I. F., Watt K., Todd R. F., 3rd, Liu D. Y. cDNA for Mo3, a monocyte activation antigen, encodes the human receptor for urokinase plasminogen activator. J Immunol. 1992 Jun 1;148(11):3636–3642. [PubMed] [Google Scholar]
- Mizukami I. F., Vinjamuri S. D., Trochelman R. D., Todd R. F., 3rd A structural characterization of the Mo3 activation antigen expressed on the plasma membrane of human mononuclear phagocytes. J Immunol. 1990 Mar 1;144(5):1841–1848. [PubMed] [Google Scholar]
- Mochan E., Keler T. Plasmin degradation of cartilage proteoglycan. Biochim Biophys Acta. 1984 Aug 21;800(3):312–315. doi: 10.1016/0304-4165(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Rifkin D. B. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim Biophys Acta. 1988 Aug 3;948(1):67–85. doi: 10.1016/0304-419x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Naldini L., Tamagnone L., Vigna E., Sachs M., Hartmann G., Birchmeier W., Daikuhara Y., Tsubouchi H., Blasi F., Comoglio P. M. Extracellular proteolytic cleavage by urokinase is required for activation of hepatocyte growth factor/scatter factor. EMBO J. 1992 Dec;11(13):4825–4833. doi: 10.1002/j.1460-2075.1992.tb05588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A. R., Chapman H. A., Jr An autocrine role for urokinase in phorbol ester-mediated differentiation of myeloid cell lines. J Clin Invest. 1991 Mar;87(3):1091–1097. doi: 10.1172/JCI115070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowski L., Russo-Payne H., Wilson E. L. Inhibition of urokinase-type plasminogen activator by antibodies: the effect on dissemination of a human tumor in the nude mouse. Cancer Res. 1991 Jan 1;51(1):274–281. [PubMed] [Google Scholar]
- Pepper M. S., Sappino A. P., Stöcklin R., Montesano R., Orci L., Vassalli J. D. Upregulation of urokinase receptor expression on migrating endothelial cells. J Cell Biol. 1993 Aug;122(3):673–684. doi: 10.1083/jcb.122.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty H. R., Todd R. F., 3rd Receptor-receptor interactions of complement receptor type 3 in neutrophil membranes. J Leukoc Biol. 1993 Nov;54(5):492–494. doi: 10.1002/jlb.54.5.492. [DOI] [PubMed] [Google Scholar]
- Pöllänen J., Hedman K., Nielsen L. S., Danø K., Vaheri A. Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol. 1988 Jan;106(1):87–95. doi: 10.1083/jcb.106.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani S. A., Mazar A. P., Bernier S. M., Haq M., Bolivar I., Henkin J., Goltzman D. Structural requirements for the growth factor activity of the amino-terminal domain of urokinase. J Biol Chem. 1992 Jul 15;267(20):14151–14156. [PubMed] [Google Scholar]
- Remold-O'Donnell E., Savage B. Purification, composition, and structure of macrophage adhesion molecule. Biochemistry. 1988 Jan 12;27(1):42–45. doi: 10.1021/bi00401a008. [DOI] [PubMed] [Google Scholar]
- Riccio A., Grimaldi G., Verde P., Sebastio G., Boast S., Blasi F. The human urokinase-plasminogen activator gene and its promoter. Nucleic Acids Res. 1985 Apr 25;13(8):2759–2771. doi: 10.1093/nar/13.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksela O., Hovi T., Vaheri A. Urokinase-type plasminogen activator and its inhibitor secreted by cultured human monocyte-macrophages. J Cell Physiol. 1985 Jan;122(1):125–132. doi: 10.1002/jcp.1041220119. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Release of basic fibroblast growth factor-heparan sulfate complexes from endothelial cells by plasminogen activator-mediated proteolytic activity. J Cell Biol. 1990 Mar;110(3):767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Shapiro S. D. Introduction: the matrix metalloproteinase family. Am J Respir Cell Mol Biol. 1992 Aug;7(2):119–119. doi: 10.1165/ajrcmb/7.2.119. [DOI] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V. Association of the CD59 and CD55 cell surface glycoproteins with other membrane molecules. J Immunol. 1991 Sep 1;147(5):1587–1592. [PubMed] [Google Scholar]
- Stossel T. P. On the crawling of animal cells. Science. 1993 May 21;260(5111):1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Taga T., Hibi M., Hirata Y., Yamasaki K., Yasukawa K., Matsuda T., Hirano T., Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989 Aug 11;58(3):573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Ikeo K., Gojobori T., Tanifuji M. Local function of urokinase receptor at the adhesion contact sites of a metastatic tumor cell. Thromb Res Suppl. 1990;10:55–61. doi: 10.1016/0049-3848(90)90378-p. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Alvarez P. A., Brott D. A., Liu D. Y. Bacterial lipopolysaccharide, phorbol myristate acetate, and muramyl dipeptide stimulate the expression of a human monocyte surface antigen, Mo3e. J Immunol. 1985 Dec;135(6):3869–3877. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Meuer S. C., Romain P. L., Schlossman S. F. A monoclonal antibody that blocks class II histocompatibility-related immune interactions. Hum Immunol. 1984 May;10(1):23–40. doi: 10.1016/0198-8859(84)90083-1. [DOI] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Sappino A. P., Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991 Oct;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg M. A., Israel E., Margolese R. G. Further studies on the mitogenic and immune-modulating effects of plasminogen activator. Immunology. 1982 Apr;45(4):715–720. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. C., Haston W. S. Chemotaxis: an overview. Methods Enzymol. 1988;162:3–16. doi: 10.1016/0076-6879(88)62059-3. [DOI] [PubMed] [Google Scholar]
- Zetter B. R., Brightman S. E. Cell motility and the extracellular matrix. Curr Opin Cell Biol. 1990 Oct;2(5):850–856. doi: 10.1016/0955-0674(90)90083-q. [DOI] [PubMed] [Google Scholar]