Abstract
Objective
To evaluate thyroid structure and function in patients with enlargement of the vestibular aqueduct (EVA) and sensorineural hearing loss.
Design
Prospective cohort survey.
Setting
National Institutes of Health Clinical Center, a federal biomedical research facility.
Patients
The study population comprised 80 individuals, aged 1.5 to 59 years, ascertained on the basis of EVA and sensorineural hearing loss.
Main Outcome Measures
Associations among the number of mutant alleles of SLC26A4; volume and texture of the thyroid; percentage of iodine 123 (123I) discharged at 120 minutes after administration of perchlorate in the perchlorate discharge test; and peripheral venous blood levels of thyrotropin, thyroxine, free thyroxine, triiodothyronine, thyroglobulin, antithyroid peroxidase and antithyroglobulin antibodies, and thyroid-binding globulin.
Results
Thyroid volume is primarily genotype dependent in pediatric patients but age dependent in older patients. Individuals with 2 mutant SLC26A4 alleles discharged a significantly (P ≤ .001) greater percentage of 123I compared with those with no mutant alleles or 1 mutant allele. Thyroid function, as measured by serologic testing, is not associated with the number of mutant alleles.
Conclusions
Ultrasonography with measurement of gland volume is recommended for initial assessment and follow-up surveillance of the thyroid in patients with EVA. Perchlorate discharge testing is recommended for the diagnostic evaluation of patients with EVA along with goiter, nondiagnostic SLC26A4 genotypes (zero or 1 mutant allele), or both.
ENLARGEMENT OF THE VESTIBU-lar aqueduct (EVA) (OMIM 600791) is the most common radiologic malformation of the inner ear associated with sensorineural hearing loss (SNHL).1 It is a fully penetrant feature of Pendred syndrome (PDS)(OMIM274600).2 Pendred syndrome is an autosomal recessive disorder that comprises SNHL, EVA with or without cochlear and vestibularmal formations,3 and an iodine organification defect that may lead to goiter.4,5
 CME available online at www.jamaarchivescme.com and questions on page 633
CME available online at www.jamaarchivescme.com and questions on page 633
Pendred syndrome is caused by biallelic mutations in the SLC26A4 gene (OMIM 605646)6 encoding pendrin, a transmembrane exchanger of iodide, chloride, and bicarbonate ions that is expressed in the inner earandthyroid. SLC26A4 mutations have also been detected in patients with nonsyndromic SNHL and EVA (NSEVA).7,8 Two mutant alleles of SLC26A4 can be identified in approximately one-third of patients with EVA, 1 mutant allele of SLC26A4 can be found in another third, and no mutant alleles are detectable in the other third.9-11 Biallelic SLC26A4 mutations typically cause bilateral EVA,although there may be rare cases of uni-lateral EVA associated with biallelic variants encoding pendrin with residual anion exchange function.12 The genotypic and phenotypic overlap of NSEVA and PDS has led to uncertainty in their nosologic relationship.
The original sisters described by Pendred13 both had goiter, which is still frequently used to distinguish between NSEVA and PDS. However, the majority of patients with SNHL and EVA are ascertained prior to the potential onset of goiter in late childhood to early adulthood.14 In addition, goiter due to PDS is incompletely penetrant, even in adulthood.14 Goiter is common in the general population15; therefore, PDS phenocopies are common among patients with EVA. Goiter is typically screened via palpation, which is not as sensitive as ultrasonography for detecting nodules, nonnodular structural abnormalities, and enlargement of the thyroid.16 When ultrasonography has been used, sex- and age-specific normative data are rarely compared to objectively confirm enlargement.
The perchlorate discharge test (PDT) is thought to be a more accurate way of detecting the iodine organification defect associated with PDS. SLC26A4 mutations are believed to reduce pendrin-mediated transport of iodide from the thyroid folliculocyte across its apical membrane into the follicular lumen for organification. This results in an increase of iodide discharged from the thyroid gland in response to potassium perchlorate, a competitive inhibitor of the sodium-iodide symporter located in the basal membrane of the folliculocyte. However, the conditions and criteria for the PDT are often not presented. The reported criterion for a positive PDT result has ranged from 6% to 15% discharge at time points ranging from 30 to 120 minutes after the administration of perchlorate. We previously observed a strong correlation of 2 detectable SLC26A4 mutations and a positive PDT result when consistent test interpretation, radioisotope, route of administration, and pre-test counseling were used.11
We sought to clarify the nosologic status of PDS and NSEVA by rigorous thyroid evaluations of 80 patients with EVA and hearing loss. We searched for correlations of thyroid ultrasonography, PDT, and serologic test results with the number of pathogenic SLC26A4 mutations. We confirmed the strong association of 2 mutations with a positive PDT result and found that thyroid gland volume is primarily genotype-dependent in pediatric patients with EVA but age-dependent in older patients.
METHODS
SUBJECTS
This study was approved by the Combined Neuroscience Institutional Review Board (National Institutes of Health [NIH], Bethesda, Maryland). The eligibility criterion was EVA in at least 1 ear imaged by computed tomography (CT) or magnetic resonance imaging (MRI). Enlargement of the vestibular aqueduct was defined as a diameter exceeding 1.5 mm at the midpoint between the posterior cranial fossa and the vestibule of the inner ear or grossly malformed overall morphologic features of the VA.1 Written informed consent was obtained from all subjects or their legal guardians. Subjects comprised 80 previously reported individuals with EVA and SNHL (43 female and 37 male [mean age, 11.5 years; range, 1.5-59 years]).11,12 Subjects self-identified race and ethnicity in accordance with our institutional guidelines. Of the subjects, 89% were “white,” 5% were “more than 1 race,” 4% were “black or African American,” and 2% were “unknown or not reported.” Ninety-six percent of subjects reported their ethnicity as “not Hispanic or Latino” and 4% as “Hispanic or Latino.” All subjects were evaluated at the NIH Clinical Center. Evaluations included a detailed medical history and physical examination (S.P.P. or A.J.G.). Results of previous endocrinology evaluations were obtained, when available, for review.
ULTRASONOGRAPHY
Ultrasonograms were reviewed by 1 radiologist (T.H.S.) to determine thyroid size and structure. Three of the original examinations were not available for review. The patients were scanned with a 12-MHz linear array transducer. The volume of each lobe was measured using an ellipsoid model: width × length × thickness × 0.479.17,18 Lobe volumes were summed to determine total thyroid volume (Tvol). Because Tvol is a function of age and sex, it was compared with sex- and age-specific data to identify subjects with thyroid gland enlargement and to permit comparison of Tvol across subjects. Thyroid volume was compared with the 97th percentile of sex- and age-specific normative data for ages 6 to 12 years in the study by Zimmerman et al18 and for ages 15 years and older in the study by Maravall et al.17
Normative Tvol data was not available for individuals younger than 6 years or aged 13 to 14 years. To classify goiter status in these subjects, 4 criteria were used. Each subject's Tvol measurement was compared with a 97th percentile calculated from a sex- and age-specific regression equation modeled on the data of Maravall et al17 and Zimmerman et al.18 The second method was clinical judgment by a single radiologist (T.H.S.). In the third and fourth methods, Tvol was compared with the adjacent age- and sex-specific normative data reported by Zimmerman et al18 and Vitti et al.19 Thyroid volume was classified as goitrous if the findings from 3 or more of the methods indicated that it was goitrous. Goiter status was classified as equivocal when only the findings from 2 of the methods were concordant and within normal limits when the findings from 3 or 4 of the methods indicated that Tvol was not enlarged. Thyroid structural abnormalities were coded on the basis of presence or absence of nodules and their number and size, presence or absence of calcifications or other inhomogeneities, and the laterality of these features.
SEROLOGIC TESTING
Thyroid function was evaluated by measuring the blood concentration of thyrotropin (TSH); thyroxine (T4) (Elecsys [Roche Diagnostics, Olathe, Kansas] and Immulite 2000/2500 [Siemens USA, Deerfield, Illinois] analyzers); free thyroxine (FT4) (Elecsys, Nichols [Nichols Institute Diagnostics, San Clemente, California] and Immulite 2000/2500 analyzers); triiodothyronine (T3) (Mayo Medical Laboratories, Rochester, Minnesota), thyroglobulin (Tg) (Mayo Medical Laboratories, Immulite 2000, Nichols, and Immulite 2000/2500 analyzers), antithyroid peroxidase (TPO) and antithyroglobulin (Anti-Tg) (Labotech Automated EIA [Adaltis, Montreal, Quebec, Canada] and DSX Automated EIA [Magellan, Chelmsford, Massachusetts] analyzers); and thyroid-binding globulin (TBG) (Immulite 1000 and Immulite 2000/2500 analyzers).
Results of TSH and FT4 tests were analyzed in parallel to score each subject as frankly or subclinically hypothyroid, euthyroid, or frankly or subclinically hyperthyroid. Frank hypothyroidism was defined as an elevated TSH level in combination with a decreased FT4 level. Subclinical hypothyroidism was defined as an elevated TSH level in combination with an FT4 level within normal limits. Frank hyperthyroidism was defined as a decreased TSH level in combination with an increased FT4 level. Subclinical hyperthyroidism was defined as a decreased TSH level in combination with an FT4 level within normal limits.
Antithyroid antibody status was classified on the basis of TPO and anti-Tg results: status was positive if both results were positive, equivocal if 1 result was negative and the other positive, and negative if both results were negative.
The methods and normative ranges for all of the serologic tests ordered through the NIH Department of Laboratory Medicine changed in the years that testing was completed. Serologic test results were therefore converted to categorical variables (eg, low, within normal limits, high, positive, or negative) to permit direct comparison of data for all subjects. All T3 results were discarded owing to a problem in sample processing after the use of serum separator tubes for sample collection.20 The TSH, FT4, and T4 results of individuals (subjects 1530, 1556, and 1616) who did not discontinue levothyroxine therapy prior to evaluations at the NIH were discarded from further analysis.
PERCHLORATE DISCHARGE TEST
A PDT was performed as previously described11 on subjects with bilateral EVA, goiter, or both. The PDT results were reviewed by a single nuclear medicine physician (J.C.R.). The result was scored as positive if the subject had greater than 15% discharge of iodine at 120 minutes after the administration of perchlorate. The test was considered uninterpretable if the discharge values fluctuated above and below 15%. If a measurement was not available at 120 minutes, the nearest (within 30 minutes) time point was used.
SLC26A4 GENOTYPES
SLC26A4 genotypes have been previously reported.11,12 Six variants (c.-60A>G, IVS1-2A>G, p.F335L, p.C565Y, p.L597S, and p.M775T) of indeterminate pathogenicity12 were considered nonpathogenic for analyses in this study.
STATISTICAL ANALYSIS
One-way analysis of variance (ANOVA) was used to assess differences in mean ages among independent groups categorized on the basis of number of SLC26A4 mutations. The Tukey honestly significant difference test was used to perform post-ANOVA comparisons between groups. Logistic regression was used to model a binary dependent variable (goiter) vs independent factors (ie, number of mutant alleles, age, and sex). Linear regression was used to model a continuous dependent variable (ie, percentage of iodine 123 [123I] discharge in PDT) with respect to the same independent factors. Some regression analyses were stratified to investigate age-specific relationships when appropriate.
The Fisher exact test was performed to assess association among categorical variables. Nonparametric binomial tests were used to compare the prevalence of goiter in 2 populations. Statistical significance was defined according to the criterion P<.05. All statistical analyses were computed with SPSS software for Windows version 15 (SPSS Inc, Chicago, Illinois).
RESULTS
SUBJECTS
There were 47 subjects with no detectable mutant alleles, 12 subjects with 1 mutant allele, and 21 subjects with 2 mutant alleles of SLC26A4. One-way ANOVA with Tukey honestly significant difference post hoc testing revealed no statistically significant differences in the mean ages of individuals with 1 mutant allele compared with those with 2 or zero mutant alleles. There was a statistically significant difference (P=.008) in the mean ages of individuals with 2 mutant alleles compared with individuals with no mutant alleles. This difference likely reflects an ascertainment bias due to increased use of temporal bone imaging in the diagnosis of childhood hearing loss within the past 20 years. To account for this between-group variance, we stratified the subjects into subcohorts 10 years or younger and older than 10 years for some of the analyses.
Seven subjects (subjects 1529, 1530, 1556, 1564, 1616, 1618, and 1377) were being treated with levothyroxine prior to their evaluations at the NIH. One subject (subject 1556) had a right partial thyroidectomy at approximately age 17 years. At age 35 years, a 24-hour 131I uptake scan revealed significant thyroid hyperplasia with a possible cold nodule. At age 57 years, she developed chest pain and a deviated trachea due to a large substernal left-sided goiter. She underwent a left completion thyroidectomy at age 58 years. Pathological examination revealed multifocal microscopic papillary carcinoma. The subject was treated with postoperative 131I and levothyroxine.
ULTRASONOGRAPHIC RESULTS
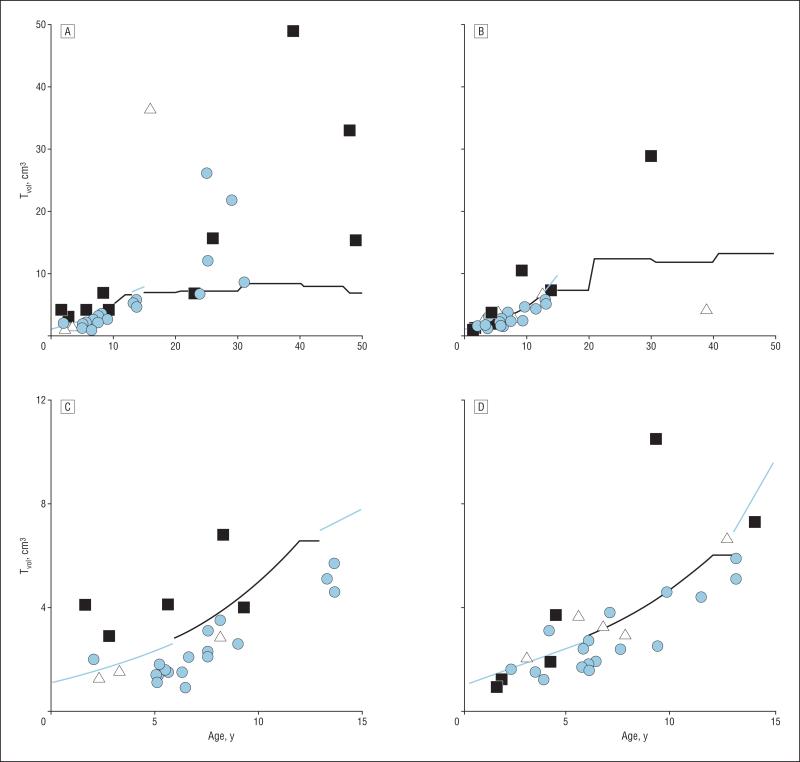
Seventy-three subjects received thyroid ultrasonography (Figure and Table 1). One individual (subject 1556) was classified as goitrous on the basis of her surgical history and findings. Another (subject 1627) was classified as equivocal and dropped from further analysis. Of 70 subjects whose results were available for review, 23 (33%) had a Tvol that exceeded the 97th percentile for age- and sex-specific normative data. Of 23 subjects with goiter, 13 (57%) had 2 SLC26A4 mutant alleles, and 6 of 47 subjects (13%) without goiter had 2 mutant alleles. Among this cohort of 70 subjects, the sensitivity and specificity of thyroid ultrasonography (Tvol >97th percentile) for 2 SLC26A4 mutant alleles was 68% (13 of 19) and 80% (41 of 51), respectively (Figure and Table 1). The sensitivity and specificity were 60% (6 of 10) and 89% (33 of 37), respectively, for subjects 10 years or younger and 78% (7 of 9) and 57% (8 of 14), respectively, for subjects older than 10 years.
Figure.
Measured thyroid volume (Tvol) of patients with enlargement of the vestibular aqueduct and 2 (black squares), 1 (white triangles), or no (light blue circles) mutant alleles of SLC26A4. A, All female subjects; B, all male subjects; C, female subjects 15 years or younger; and D, male subjects 15 years or younger. Sex-specific 97th percentiles are indicated by dark (Zimmerman et al18 and Maravall et al17) or light blue lines (regression equation derived from Zimmerman et al18 and Maravall et al17 97th percentile). The Tvol (151 cm3) for subject 1564 (44-year-old man with 2 mutant alleles) is not shown because it is a distant outlier.
Table 1.
Subjects With EVA and Thyroid Abnormalities
| Subject No.a | Age, yb/Sex/No. of Mutant Alleles | Goiter | Structural Abnormality | Functional Abnormality | Antithyroid Antibodies | Abnormal Perchlorate Discharge |
|---|---|---|---|---|---|---|
| 1159 | 4/M/2 | + | – | – | – | ND |
| 1163 | 5/F/2 | + | – | – | – | + |
| 1167 | 7/M/0 | + | – | – | – | – |
| 1171 | 8/F/2 | + | Inhomogeneity | – | – | + |
| 1290 | 3/M/0 | – c | – c | Hypothyroid (subclinical) | – | ND |
| 1303 | 12/M/1 | + | – | ND | – | – |
| 1343 | 6/M/0 | – d | – d | – | ± | ND |
| 1377 | 27/F/2 | + | Nodules (bilateral) | – | – | + |
| 1412 | 9/M/2 | + | Nodules (bilateral) | – | – | + |
| 1447 | 3/F/1 | – | – | – | – | + |
| 1459 | 7/M/0 | + | – | – | ND | – |
| 1460 | 13/F/0 | – | Nodules (bilateral) | – | – | – |
| 1470 | 4/M/0 | + | Nodule (unilateral) | – | – | – |
| 1473 | 31/F/0 | + | Inhomogeneity | – | – | – |
| 1529 | 49/F/2 | + | Nodules (bilateral) | Levothyroxinee | – | ND |
| 1530 | 48/F/2 | + | Nodules (bilateral) | – | – | + |
| 1556 | 59/F/2 | + d,f | Nodulesd,g | Levothyroxinee | – | ND |
| 1564 | 44/M/2 | + | Nodules (bilateral); calcifications | – | ND | + |
| 1571 | 16/12/F/2 | + | – | – | – | ND |
| 1598 | 13/F/0 | – | Nodules (bilateral) | – | – | – |
| 1604 | 4/M/2 | – | Nodule (unilateral) | – | – | ND |
| 1616 | 16/F/1 | + | Nodules (bilateral) | Levothyroxinee | – | +g |
| 1618 | 24/F/0 | + | Nodules (bilateral) | – | – | – |
| 1619 | 28/F/0 | + | Nodules (bilateral) | – | ± | – |
| 1623 | 24/F/0 | – | Nodules (bilateral) | – | – | ND |
| 1625 | 30/M/2 | + | Nodules (bilateral) | – | – | + |
| 1627 | 5/M/1 | ± | – | – | – | ND |
| 1645 | 6/M/1 | + | – | – | – | – |
| 1663 | 210/12/F/2 | + | – | ND | – | Uninterpretable |
| 1691 | 3/M/0 | – | – | Hypothyroid (subclinical) | – | – |
| 1693 | 25/F/0 | + | Nodules (bilateral) | – | ± | – |
| 1712 | 9/F/0 | – | Nodule (unilateral) | – | – | – |
| 1770 | 6/M/0 | – | Nodule (unilateral) | – | – | ND |
| 1823 | 7/M/0 | – | – | – | + | – |
| 1847 | 39/F/2 | + | Nodules (unilateral); inhomogeneity | Hyperthyroid (subclinical) | – | – |
Abbreviations: EVA, enlargement of the vestibular equeduct; ND, not done; +, positive result; –, negative result; ±, equivocal result.
Subjects 1159 to 1618 were reported in Pryor et al,11 and subjects 1619 to 1847 were reported in Choi et al.12
Age at time of initial National Institutes of Health evaluation. Superscript numbers refer to number of months of 12 months (only done for ages <3 years).
Evaluated only by manual palpation.
Based on consultation report.
Levothyroxine therapy for goiter suppression, not functional replacement.
Based on previous records of physical examination, chest radiography, and surgical pathologic findings.
Not performed at the National Institutes of Health Clinical Center.
We modeled the relationship between goiter and age, sex, and number of SLC26A4 mutant alleles (Table 2). A statistically significant increase (P=.004) in the odds ratio (OR) of developing goiter (OR, 8.3; 95% confidence interval [CI], 2.0 to 34.7) was identified in the total cohort when comparing individuals with 2 SLC26A4 mutant alleles with those with no mutant alleles. Adjusting for sex and mutation status, the odds of developing goiter increased by a factor of 1.1 for each 1-year increase in age. Thus, a 10-year difference in age translates to a 2.6-fold increase in odds of developing goiter. In subjects 10 years or younger, only 2 mutant alleles had a statistically significant effect (OR, 21.9; 95% CI, 2.9 to 162.2) on the likelihood of goiter. In the subjects older than 10 years, the only independent variable that approached statistical significance (P=.08) was age (OR, 1.1; 95% CI, 1.0 to 1.3).
Table 2.
Logistic Regression Relationships of Goiter With Age, Sex, and Number of Mutant Alleles of SLC26A4 in Subjects With EVA
| Subjects With EVA | Independent Factor | No. of Subjects | Odds Ratio (95% CI) | P Valuea |
|---|---|---|---|---|
| All ages | Age, y | 70 | 1.1 (1.0-1.2) | .008 |
| Male sex | 31 | 1.0 (0.3-3.7) | .97 | |
| 1 Mutant allele | 9 | 2.5 (0.4-14.5) | .32 | |
| 2 Mutant alleles | 19 | 8.3 (2.0-34.7) | .004 | |
| Age ≤ 10 y | Age, y | 47 | 1.2 (0.9-1.8) | .24 |
| Male sex | 23 | 0.4 (0.1-2.4) | .33 | |
| 1 Mutant allele | 6 | 2.1 (0.2-26.8) | .55 | |
| 2 Mutant alleles | 10 | 21.9 (2.9-162.2) | .003 | |
| Age > 10 y | Age, y | 23 | 1.1 (1.0-1.3) | .08 |
| Male sex | 8 | 4.6 (0.3-73.5) | .29 | |
| 1 Mutant allele | 3 | 7.4 (0.2-256.0) | .27 | |
| 2 Mutant alleles | 9 | 2.4 (0.1-46.0) | .55 |
Abbreviations: CI, confidence interval; EVA, enlargement of the vestibular aqueduct.
Statistically significant differences are indicated in bold.
The prevalence of goiter was compared in subjects with EVA with no SLC26A4 mutant alleles vs the general population (Table 3). Adult (age, 17-59 years) subjects had a statistically significantly (P<.001) higher prevalence of goiter compared with the reference population. There was no significant increase for subjects aged 10 to 16 years (P=.78) and 0 to 5 years (P=.06) but there was a significantly (P=.045) higher prevalence of goiter among subjects aged 6 to 9 years compared with the reference population.
Table 3.
Comparison of Goiter Prevalence Among Subjects With EVA With No Mutant Alleles of SLC26A4 vs the General Population Reported by Trowbridge et al15
| Prevalence of Goiter |
|||
|---|---|---|---|
| Age Range,y | Present Study, No./Total No. (%) | Trowbridge et al,15 % | P Valuea |
| 0-5 | 1/13 (7.7) | 0.05 | .06 |
| 6-9 | 2/18 (11.1) | 1.9 | .045 |
| 10-16 | 0/6 (0) | 3.9 | .78 |
| 17-59 | 4/5 (80) | 4 1 | <.001 |
Abbreviation: EVA, enlargement of the vestibular aqueduct.
Statistically significant differences are indicated in bold.
Of 70 ultrasonograms, thyroid structural abnormalities were identified in 20 (29%) (Table 1). Of 20 glands, 18 (90%) were uninodular or multinodular, 3 (15%) were inhomogeneous, and 1 (5%) had calcifications. Logistic regression models of the relationship between thyroid structural abnormalities and the factors age, sex, number of SLC26A4 mutations, and goiter were not interpretable owing to significant correlations among these factors.
PDT RESULTS
A PDT was completed at the NIH for 39 subjects. Three tests were uninterpretable and discarded from further analysis One subject (subject 1616) received a PDT at an outside facility. These results were not included in our analysis. Of 8 subjects with a positive PDT result, 7 (88%) had 2 SLC26A4 mutant alleles (Table 1). Conversely, 7 of 8 subjects (88%) with 2 mutant alleles had a positive PDT result. The sensitivity and specificity of the PDT for 2 SLC26A4 mutant alleles were 88% and 96%, respectively.
We used linear regression to model the relationship between the percentage of 123I discharged at 120 minutes after administration of perchlorate and 3 factors (age, sex, and number of SLC26A4 mutant alleles) (Table 4). There were statistically significant (P≤.001) increases in the percentage of 123I discharged in subjects with 2 mutant alleles compared with either those with no mutant alleles (27.3% [95% CI, 15.5% to 39.0%]) (Table 4) or those with 1 mutant allele (27.0% [95% CI, 12.3% to 41.7%]) (data not shown). There was not a statistically significant (P>.99) difference in percentage of 123I discharged (0.3%, 95% CI, −11.8% to 12.3%) in subjects with 1 mutant allele compared with those with no mutant alleles. These relationships persisted in the age-stratified subcohorts (data not shown).
Table 4.
Linear Regression Relationships of PDT Percentage of 123I Discharge With Age, Sex, and Number of Mutant Alleles of SLC26A4
| Independent Factor | Adjusted Percentage Change in 123I Discharge (95% CI) | P Valuea |
|---|---|---|
| Age | 0.0 (–0.4 to 0.4) | .88 |
| Male sex | –4.3 (–13.2 to 4.5) | .33 |
| 1 Mutant allele | 0.3 (–11.8 to 12.3) | .97 |
| 2 Mutant alleles | 27.3 (15.5 to 39.0) | <.001 |
Abbreviations: CI, confidence interval; 123I, iodine 123; PDT, perchlorate discharge test.
Statistically significant differences are indicated in bold.
SEROLOGIC TEST RESULTS
Levels of FT4 and TBG were within normal limits for all patients and were discarded from further analyses as individual variables. There were no significant differences in the serologic test results when analyzed on the basis of number of SLC26A4 mutant alleles, goiter status, or structural abnormalities of the thyroid. Of 71 subjects, 2 (3%) were subclinically hypothyroid and 1 (1%) was subclinically hyperthyroid. Of 70 subjects, 3 (4%) had equivocal and 1 (1%) had positive antithyroid antibody results (Table 1).
COMMENT
Although there was a statistically significantly higher prevalence of goiter in the 6- to 9-year age group with zero SLC26A4 mutant alleles compared with the general population, this difference is likely due to the detection method (palpation) used to identify goiter in the control group.15 Manual palpation and routine clinical ultrasonographic examination of the 2 goitrous subjects with EVA in this age group did not detect thyroid enlargement. Thyroid enlargement was revealed only with quantitative volume measurements and comparison with age- and sex-specific normative data. Our algorithm for classifying goiter status in subjects younger than 6 years or 13 to 14 years old, for whom normative Tvol data are not available, is probably too complex for routine clinical use. A more limited but practical approach would be comparison with adjacent age- and sex-specific normative data.18,19
The thyroid associated with PDS has been described as initially smooth and diffuse and later becoming multinodular.14 Those conclusions may not fully reflect the natural history as revealed by ultrasonographic investigation of a primarily pediatric population. Although our cross-sectional study design did not permit the definition of “pregoitrous” structural or volume changes in subjects with EVA, the possibility of presymptomatic detection and intervention warrants a longitudinal study. Follow-up of structural abnormalities should include periodic thyroid ultrasonography and may include other diagnostic tests or endocrinology consultation for atypical nodules, gland enlargement, or thyroid function abnormalities or symptoms.
Our data do not support the hypothesis that thyroid serologic test results can be used to distinguish among individuals with zero, 1, or 2 mutant SLC26A4 alleles. This may reflect the cross-sectional design of our study and the comparatively young age distribution (mean age, 11.5 years) of our cohort. Some studies have reported a significant prevalence of subclinical or frank hypothyroidism among individuals presumed to have 2 SLC26A4 mutant alleles, but they were largely based on adult populations.14,21 In contrast, none of our subjects had clinical evidence of frank hypothyroidism, even those who received levothyroxine therapy. For some subjects, the goal of levothyroxine therapy may have been to stop or retard goiter progression for cosmetic or compressive indications. Although our results indicate that thyroid serologic testing is not a useful initial diagnostic screen for PDS, it remains important in the evaluation and management of symptoms or signs of hypothyroidism, hyperthyroidism, goiter, or thyroid structural abnormalities.
There was 1 case of thyroid cancer in our series of 80 individuals. There are published case reports, but no estimates of the incidence, of thyroid cancer in individuals with PDS. It has been suggested that thyroid cancer in individuals with PDS and untreated goiter may be a result of chronic exposure to excessive thyrotropin.22,23 However, subject number 1556 had no history of elevated thyrotropin levels. Thyroid cancer is a comparatively common malignant neoplasm and may be a co-incidental comorbid condition in some individuals with PDS. Our cohort with 2 mutant alleles of SLC26A4 is too small and too young to test the hypothesis that PDS is associated with an increased risk of thyroid cancer.
Our current results confirm our previous observation of a strong correlation of 2 mutant alleles of SLC26A4 with a positive PDT result.11 The 2 exceptions to this association are subjects 1447 (p. E384G and p.L597S alleles of SLC26A4) and 1847 (p.G209V and p.Q514R alleles of SLC26A4). We classified subject 1447 as having 1 mutant allele, since p.E384G is a functional null mutation but p.L597S is a hypofunctional variant with indeterminate pathogenic potential.12 Her positive PDT result could thus reflect a pathogenic role for p.L597S in trans configuration with a functional null allele.12 Alternatively, we cannot exclude the possibilities of a subtle but significant motion artifact for the 123I measurements or that subject 1447 represents a genuine exception to the geno-type-phenotype correlation. In contrast, subject 1847 has 2 clearly pathogenic SLC26A4 alleles. However, some atypical aspects of her thyroid phenotype may confound the interpretation of the PDT result. She had decreased TSH levels, FT4 results within normal limits, negative results for antithyroid antibodies, and multiple solid nodules. A fine-needle aspiration revealed an adenomatoid nodule with cystic changes. The patient had no signs or symptoms of hyperthyroidism. She was counseled to have a thyroidectomy and has not returned to the NIH.
A baseline thyroid ultrasonogram at initial diagnosis, followed by regular ultrasonographic studies to monitor for enlargement, may be appropriate clinical surveillance for individuals with EVA and hearing loss. Perchlorate discharge testing is recommended for the diagnostic evaluation of goiter, nondiagnostic SLC26A4 genotypes (zero or 1 mutant alleles), or both in patients with EVA. Further study will be necessary to evaluate the natural history and prognostic significance of a slightly but significantly enlarged thyroid gland or a normally sized gland with evidence of structural abnormalities. The clinician must weigh the potential benefit of early identification of an enlarged gland and presymptomatic initiation of levothyroxine therapy24 against the cost of ultrasonography and the identification of thyroid nodules of indeterminate clinical significance. Levothyroxine therapy is more clearly indicated in an individual with goiter accompanied by evidence of functional hypothyroidism. Because SLC26A4 mutations may lower the threshold for goiter when there is a slight decrease in dietary iodine intake,25 iodine supplementation should be considered as a prophylactic treatment for goiter. Longitudinal ultrasonographic studies of the thyroid gland in patients with EVA would provide a foundation for the design and interpretation of those studies.
Funding/Support
This study was supported by NIH intramural research funds Z01-DC000060 and Z01-DC000064 and the Intramural Research Program of the National Human Genome Research Institute.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Valvassori GE, Clemis JD. The large vestibular aqueduct syndrome. Laryngoscope. 1978;88(5):723–728. doi: 10.1002/lary.1978.88.5.723. [DOI] [PubMed] [Google Scholar]
- 2.Phelps PD, Coffey RA, Trembath RC, et al. Radiological malformations of the ear in Pendred syndrome. Clin Radiol. 1998;53(4):268–273. doi: 10.1016/s0009-9260(98)80125-6. [DOI] [PubMed] [Google Scholar]
- 3.Mondini C. Anatomica surdi nati sectio: bononiensi scientarium et artium instituto atque academia commentarii. Bonaniae VII. 1791;7:419–428. [Google Scholar]
- 4.Madeo AC, Pryor SP, Brewer C, et al. Pendred syndrome. Semin Hear. 2006;27(3):160–170. [Google Scholar]
- 5.Pryor SP, Park HJ, Madeo AC, Griffith AJ, Butman JA. Pendred syndrome. In: Willems PJ, editor. Genetic Hearing Loss. Vol. 1. Marcel Dekker, Inc; New York, NY: 2003. pp. 75–96. [Google Scholar]
- 6.Everett LA, Glaser B, Beck JC, et al. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet. 1997;17(4):411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- 7.Li XC, Everett LA, Lalwani AK, et al. A mutation in PDS causes non-syndromic recessive deafness. Nat Genet. 1998;18(3):215–217. doi: 10.1038/ng0398-215. [DOI] [PubMed] [Google Scholar]
- 8.Usami S, Abe S, Weston MD, Shinkawa H, Van Camp G, Kimberling WJ. Nonsyndromic hearing loss associated with enlarged vestibular aqueduct is caused by PDS mutations. Hum Genet. 1999;104(2):188–192. doi: 10.1007/s004390050933. [DOI] [PubMed] [Google Scholar]
- 9.Albert S, Blons H, Jonard L, et al. SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in Caucasian populations. Eur J Hum Genet. 2006;14(6):773–779. doi: 10.1038/sj.ejhg.5201611. [DOI] [PubMed] [Google Scholar]
- 10.Coyle B, Reardon W, Herbrick JA, et al. Molecular analysis of the PDS gene in Pendred syndrome. Hum Mol Genet. 1998;7(7):1105–1112. doi: 10.1093/hmg/7.7.1105. [DOI] [PubMed] [Google Scholar]
- 11.Pryor SP, Madeo AC, Reynolds JC, et al. SLC26A4/PDS genotype-phenotype correlation in hearing loss with enlargement of the vestibular aqueduct (EVA): evidence that Pendred syndrome and non-syndromic EVA are distinct clinical and genetic entities. J Med Genet. 2005;42(2):159–165. doi: 10.1136/jmg.2004.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi BY, Stewart AK, Madeo AC, et al. Hypo-functional SLC26A4 variants associated with nonsyndromic hearing loss and enlargement of the vestibular aqueduct: genotype-phenotype correlation or coincidental polymorphism? Hum Mutat. 2009;30(4):599–608. doi: 10.1002/humu.20884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pendred V. Deaf-mutism and goitre. Lancet. 1896;2:532. [Google Scholar]
- 14.Reardon W, Coffey R, Chowdhury T, et al. Prevalence, age of onset, and natural history of thyroid disease in Pendred syndrome. J Med Genet. 1999;36(8):595–598. [PMC free article] [PubMed] [Google Scholar]
- 15.Trowbridge FL, Hand KE, Nichaman MZ. Findings relating to goiter and iodine in the Ten-State Nutrition Survey. Am J Clin Nutr. 1975;28(7):712–716. doi: 10.1093/ajcn/28.7.712. [DOI] [PubMed] [Google Scholar]
- 16.Christensen SB, Tibblin S. The reliability of the clinical examination of the thyroid-gland: a prospective-study of 100 consecutive patients surgically treated for hyperparathyroidism. Ann Chir Gynaecol. 1985;74(4):151–154. [PubMed] [Google Scholar]
- 17.Maravall FJ, Gomez-Arnaiz N, Guma A, Abos R, Soler J, Gomez JM. Reference values of thyroid volume in a healthy, non-iodine-deficient Spanish population. Horm Metab Res. 2004;36(9):645–649. doi: 10.1055/s-2004-825901. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann MB, Hess SY, Molinari L, et al. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for Health and Development Iodine Deficiency Study Group Report. Am J Clin Nutr. 2004;79(2):231–237. doi: 10.1093/ajcn/79.2.231. [DOI] [PubMed] [Google Scholar]
- 19.Vitti P, Martino E, Aghini-Lombardi F, et al. Thyroid volume measurement by ultrasound in children as a tool for the assessment of mild iodine deficiency. J Clin Endocrinol Metab. 1994;79(2):600–603. doi: 10.1210/jcem.79.2.8045982. [DOI] [PubMed] [Google Scholar]
- 20.Bowen RA, Chan Y, Cohen J, et al. Effect of blood collection tubes on total triiodothyronine and other laboratory assays. Clin Chem. 2005;51(2):424–433. doi: 10.1373/clinchem.2004.043349. [DOI] [PubMed] [Google Scholar]
- 21.Berrettini S, Neri E, Forli F, et al. Large vestibular aqueduct in distal renal tubular acidosis. High-resolution MR in three cases. Acta Radiol. 2001;42(3):320–322. doi: 10.1080/028418501127346710. [DOI] [PubMed] [Google Scholar]
- 22.Cooper DS, Axelrod L, DeGroot LJ, Vickery ALJ, Jr, Maloof F. Congenital goiter and the development of metastatic follicular carcinoma with evidence for a leak of nonhormonal iodide: clinical, pathological, kinetic, and biochemical studies and a review of the literature. J Clin Endocrinol Metab. 1981;52(2):294–306. doi: 10.1210/jcem-52-2-294. [DOI] [PubMed] [Google Scholar]
- 23.Camargo R, Limbert E, Gillam M, et al. Aggressive metastatic follicular thyroid carcinoma with anaplastic transformation arising from a long-standing goiter in a patient with Pendred's syndrome. Thyroid. 2001;11(10):981–988. doi: 10.1089/105072501753211073. [DOI] [PubMed] [Google Scholar]
- 24.Illum P, Kiaer HW, Hvidberg-Hansen J, Sondergaard G. Fifteen cases of Pendred's syndrome. Congenital deafness and sporadic goiter. Arch Otolaryngol. 1972;96(4):297–304. doi: 10.1001/archotol.1972.00770090473001. [DOI] [PubMed] [Google Scholar]
- 25.Kopp P, Arseven OK, Sabacan L, et al. Phenocopies for deafness and goiter development in a large inbred Brazilian kindred with Pendred's syndrome associated with a novel mutation in the PDS gene. J Clin Endocrinol Metab. 1999;84(1):336–341. doi: 10.1210/jcem.84.1.5398. [DOI] [PubMed] [Google Scholar]