Abstract
The concept of immunosurveillance against cancer has been an extensively debated question over the last decades. Multiple indirect arguments have supported the view that the immune system may control, at least in certain cases, malignant cell growth while direct demonstration is still lacking in the human. In an attempt to address this issue, we have selected a study model, namely spontaneously regressive melanoma. In previous series of experiments, the variability of T cell receptors (TCRs) in the lymphocytes infiltrating a regressive tumor lesion was investigated. Results demonstrated that clonal T cell populations, precisely defined through their V-D-J junctional sequences, were amplified in situ. One clone was predominant, expressing the V beta 16 variable gene segment. A specific anti-V beta 16 TCR mAb was generated here to purify and functionally characterize the corresponding cells. A tumor-infiltrating lymphocyte-derived V beta 16+ T cell line was developed using this reagent. These in vitro cultured cells were found to express the in vivo predominant TCR sequence exclusively and to display an HLA-B14-restricted cytotoxic activity against the autologous tumor cells. Immunohistochemical experiments, performed with the anti-V beta 16 mAb, showed that the corresponding CTLs are present in the tumor area, some of them being closely opposed to the melanoma cells. Together, these studies demonstrate the existence of a local adaptive immune response clinically associated to tumor regression, thus strongly supporting the validity of the immunosurveillance concept in certain human tumors.
Full text
PDF
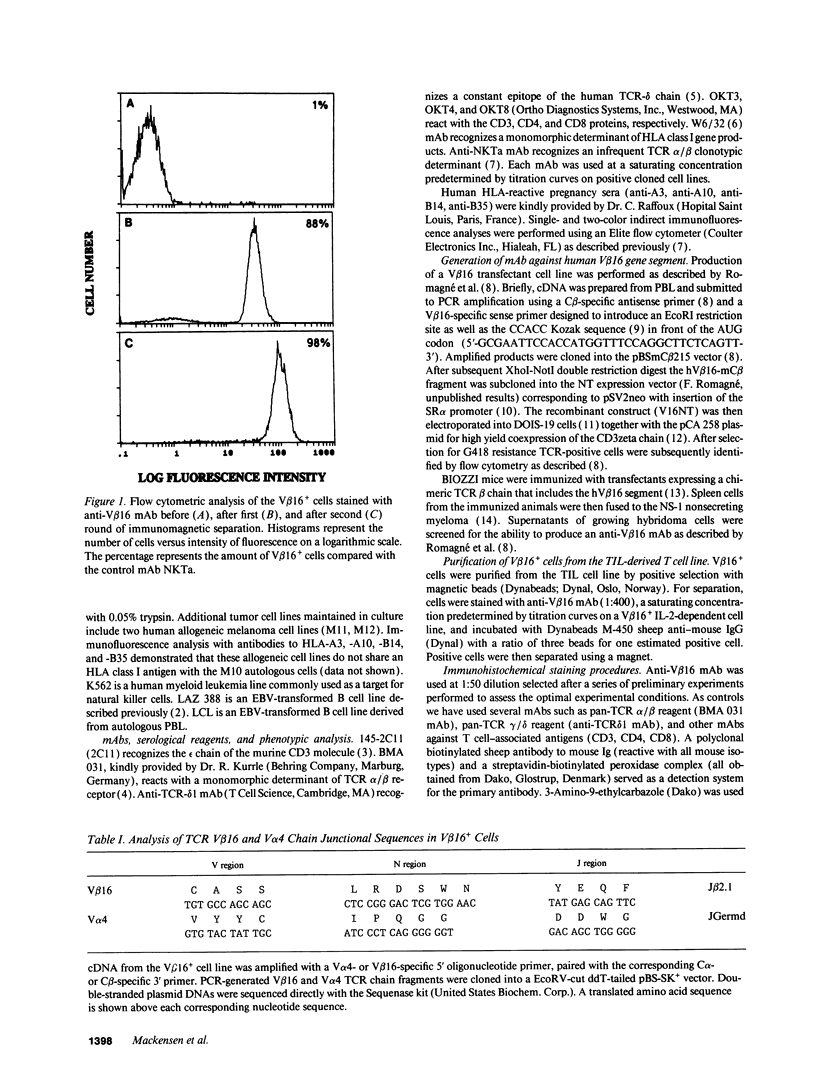
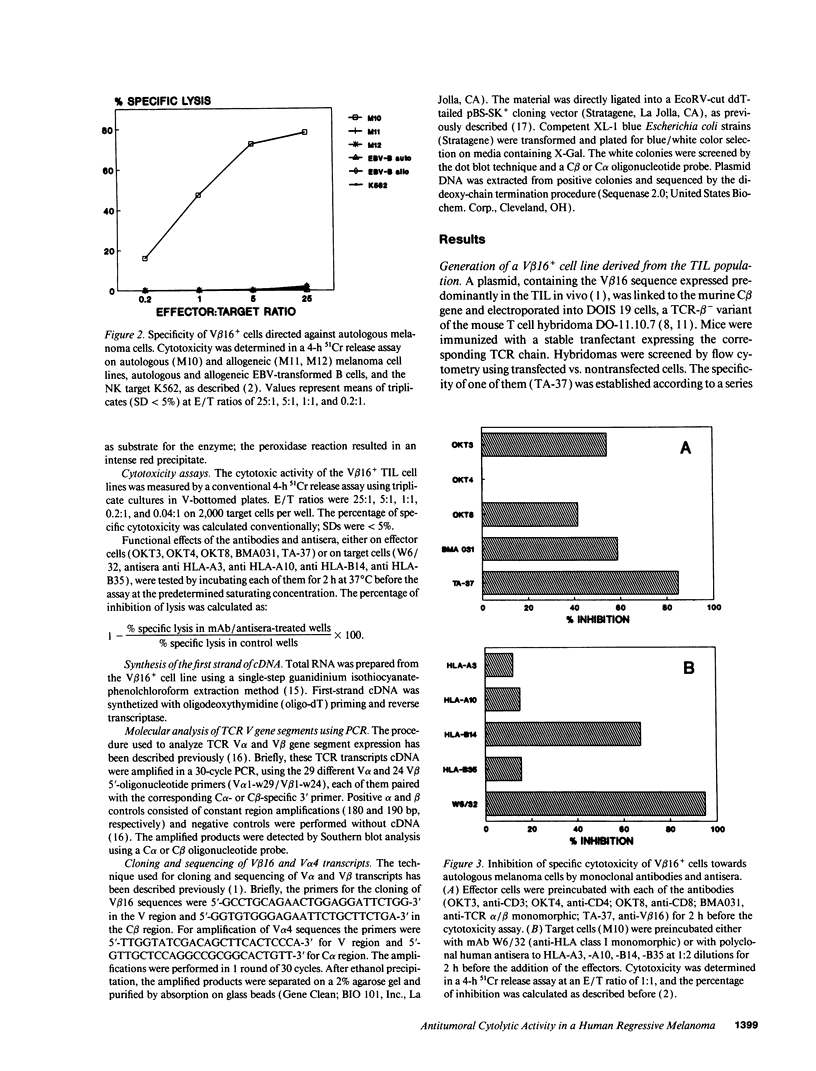



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M. S., Brenner M. B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor delta subunit. Science. 1987 Oct 30;238(4827):682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Barnhill R. L., Mihm M. C., Jr The histopathology of cutaneous malignant melanoma. Semin Diagn Pathol. 1993 Feb;10(1):47–75. [PubMed] [Google Scholar]
- Belyavsky A., Vinogradova T., Rajewsky K. PCR-based cDNA library construction: general cDNA libraries at the level of a few cells. Nucleic Acids Res. 1989 Apr 25;17(8):2919–2932. doi: 10.1093/nar/17.8.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Burnet F. M. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- Diu A., Romagné F., Genevée C., Rocher C., Bruneau J. M., David A., Praz F., Hercend T. Fine specificity of monoclonal antibodies directed at human T cell receptor variable regions: comparison with oligonucleotide-driven amplification for evaluation of V beta expression. Eur J Immunol. 1993 Jul;23(7):1422–1429. doi: 10.1002/eji.1830230703. [DOI] [PubMed] [Google Scholar]
- Ferradini L., Mackensen A., Genevée C., Bosq J., Duvillard P., Avril M. F., Hercend T. Analysis of T cell receptor variability in tumor-infiltrating lymphocytes from a human regressive melanoma. Evidence for in situ T cell clonal expansion. J Clin Invest. 1993 Mar;91(3):1183–1190. doi: 10.1172/JCI116278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevée C., Diu A., Nierat J., Caignard A., Dietrich P. Y., Ferradini L., Roman-Roman S., Triebel F., Hercend T. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol. 1992 May;22(5):1261–1269. doi: 10.1002/eji.1830220522. [DOI] [PubMed] [Google Scholar]
- Hercend T., Reinherz E. L., Meuer S., Schlossman S. F., Ritz J. Phenotypic and functional heterogeneity of human cloned natural killer cell lines. Nature. 1983 Jan 13;301(5896):158–160. doi: 10.1038/301158a0. [DOI] [PubMed] [Google Scholar]
- Holton T. A., Graham M. W. A simple and efficient method for direct cloning of PCR products using ddT-tailed vectors. Nucleic Acids Res. 1991 Mar 11;19(5):1156–1156. doi: 10.1093/nar/19.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M. M., Gale R. P., Sondel P. M., Goldman J. M., Kersey J., Kolb H. J., Rimm A. A., Ringdén O., Rozman C., Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990 Feb 1;75(3):555–562. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F., Malissen B. Derivation of a T cell hybridoma variant deprived of functional T cell receptor alpha and beta chain transcripts reveals a nonfunctional alpha-mRNA of BW5147 origin. Eur J Immunol. 1989 Dec;19(12):2269–2274. doi: 10.1002/eji.1830191214. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Rubin J. T., Zeh H. J. New biologic agents come to bat for cancer therapy. Curr Opin Oncol. 1992 Dec;4(6):1116–1123. [PubMed] [Google Scholar]
- Mackensen A., Ferradini L., Carcelain G., Triebel F., Faure F., Viel S., Hercend T. Evidence for in situ amplification of cytotoxic T-lymphocytes with antitumor activity in a human regressive melanoma. Cancer Res. 1993 Aug 1;53(15):3569–3573. [PubMed] [Google Scholar]
- Malissen B., Rebai N., Liabeuf A., Mawas C. Human cytotoxic T cell structures associated with expression of cytolysis. I. Analysis at the clonal cell level of the cytolysis-inhibiting effect of 7 monoclonal antibodies. Eur J Immunol. 1982 Sep;12(9):739–747. doi: 10.1002/eji.1830120908. [DOI] [PubMed] [Google Scholar]
- Mittelman A., Chen Z. J., Yang H., Wong G. Y., Ferrone S. Human high molecular weight melanoma-associated antigen (HMW-MAA) mimicry by mouse anti-idiotypic monoclonal antibody MK2-23: induction of humoral anti-HMW-MAA immunity and prolongation of survival in patients with stage IV melanoma. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):466–470. doi: 10.1073/pnas.89.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platsoucas C. D. Human autologous tumor-specific T cells in malignant melanoma. Cancer Metastasis Rev. 1991 Jun;10(2):151–176. doi: 10.1007/BF00049412. [DOI] [PubMed] [Google Scholar]
- Poppema S., Bröcker E. B., de Leij L., Terbrack D., Visscher T., Ter Haar A., Macher E., Thé T. H., Sorg C. In situ analysis of the mononuclear cell infiltrate in primary malignant melanoma of the skin. Clin Exp Immunol. 1983 Jan;51(1):77–82. [PMC free article] [PubMed] [Google Scholar]
- Romagné F., Besnardeau L., Malissen B. A versatile method to produce antibodies to human T cell receptor V beta segments: frequency determination of human V beta 2+ T cells that react with toxic-shock syndrome toxin-1. Eur J Immunol. 1992 Oct;22(10):2749–2752. doi: 10.1002/eji.1830221043. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traversari C., van der Bruggen P., Luescher I. F., Lurquin C., Chomez P., Van Pel A., De Plaen E., Amar-Costesec A., Boon T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992 Nov 1;176(5):1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]




