Summary
Exophiala spinifera has been reported as an agent of cutaneous disease eighteen times in the literature. Clinical presentations of cutaneous lesions vary widely, including erythematous papules, verrucous plaques, and deep subcutaneous abscesses. The clinical distribution and course of disease are also variable, depending on the age and immune competency of the patient. Histologic appearance occurs in one of two patterns – phaeohyphomycosis or chromoblastomycosis. While E. spinifera appears to be susceptible to multiple antimicrobial agents in vitro, clinical experience with treatment modalities has been variable. Prior to the availability of sequencing methods for speciation, identification was made based on the histopathologic presentation in tissue and morphologic features in culture. It is likely that E. spinifera cutaneous infection is underreported due to incorrect species identification based on earlier methods. We report an additional case of E. spinifera phaeohyphomycosis, the first to be definitively identified by sequencing, and summarize the variable clinical, histopathologic, and morphologic features, as well as treatment responses in previously reported cutaneous infections by E. spinifera.
Keywords: Phaeohyphomycosis, Exophiala spinifera, itraconazole, chromoblastomycosis
Introduction
Over one hundred species of dematiaceous fungi have been reported to cause infections in humans [1]. These darkly-pigmented fungi contain melanin in their cell walls and are widely distributed throughout the environment. Members of the order Chaetothyriales are particularly significant as recurrent agents of infection. These fungi are oligotrophic, particularly occurring at low frequency in low-nutrient or hydrocarbon-polluted environments [2]. Black moulds are etiologic agents of both chromoblastomycosis and phaeohyphomycosis. Chromoblastomycosis occurs most commonly in tropical environments and is a slow, progressive disease, often developing over several years. It is characterized clinically by verrucous lesions, usually on the lower extremities, and appears histopathologically as sclerotic bodies in tissue. Phaeohyphomycosis occurs worldwide, usually develops over a shorter duration, and presents in variable forms in tissue, including phaeoid yeastlike cells, hyphal elements, swollen cells, pseudohyphal elements, and/or moniliform hyphae.
The clinical syndromes of phaeohyphomycosis include allergic conditions, such as fungal allergic sinusitis [3,4], cutaneous disease (superficial, dermal or subcutaneous), as well as life-threatening disseminated infection [5], often characterized by brain abscess formation [6]. Acquisition of the organism varies among syndromes. Cutanous disease presumably occurs through traumatic implantation of colonized material, while allergic sinusitis and disseminated infections including central nervous system involvement are likely acquired through inhalation.
Until recently, identification of various genera to the species level has been based upon recognition of macroscopic and microscopic features formed in culture, temperature studies, and a limited number of physiologic assays. These methods, however, have proved unreliable in differentiating very similar species, particularly in the genus Exophiala [7]. Definitive molecular techniques employing ribosomal DNA sequencing are now available for species identification [2]. This will become increasingly important, as treatment modalities have variable effectiveness across species [8]. Primarily based on morphology, E. spinifera has previously been implicated in eleven cases of phaeohyphomycosis and three cases of chromoblastomycosis in the English literature. We report an additional case of phaeohyphomycosis caused by E. spinifera and review the literature to summarize patterns in clinical presentation, histologic appearance, morphologic features, molecular characterization, genetic variation, and treatment strategies.
Case Report
A forty-nine-year-old Caucasian man presented to our outpatient clinic for evaluation of a non-healing ulcer on his right shin that had enlarged over the previous four months. Eleven years prior to presentation, he underwent a bilateral lung transplant for α1-antitrypsin deficiency, and his transplant was maintained with prednisone (10 mg/day), tacrolimus (2.5 mg twice/day) and mycophenolate mofetil (500 mg twice/day). He denied any systemic signs or symptoms, including other skin lesions, difficulty breathing, headache, or neurologic symptoms. The patient is a building contractor who works outdoors doing physical labor.
Physical exam revealed a cushingoid man with an edematous right lower leg and a large ulcer with an indurated border on the pretibial aspect. Two erythematous nodules were present in close proximity to the ulceration (Fig. 1). There was no palpable lymphadenopathy.
Figure 1.
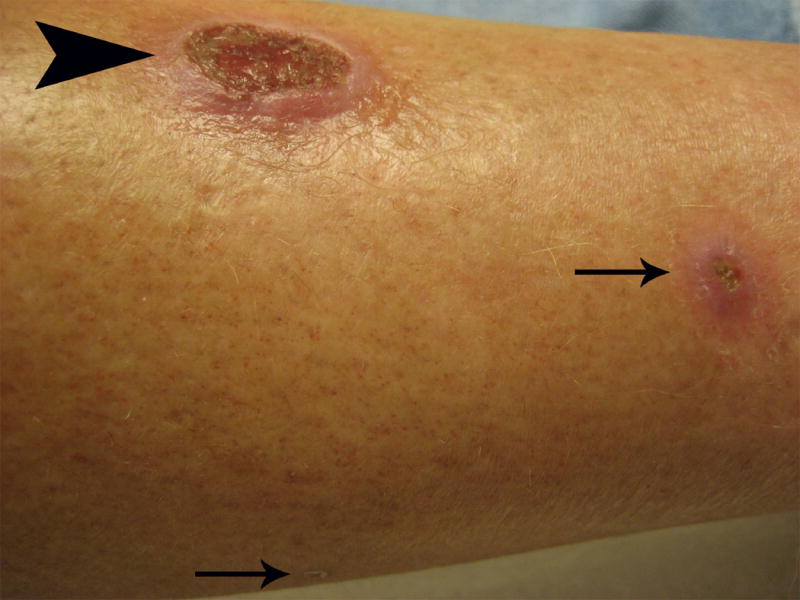
Large ulcer (large arrow head) located on the right lower extremity with two smaller erythematous nodules (small arrows) in close proximity.
Two separate punch biopsies, one from the indurated ulcer border and another from a nearby nodule, each demonstrated pseudoepitheliomatous hyperplasia associated with a dense neutrophilic infiltrate. Pigmented yeast-like and pseudohyphal elements were noted on routine hematoxylin and eosin staining and were highlighted with GMS, PAS and Fontana-Masson stains (Fig. 2).
Figure 2.
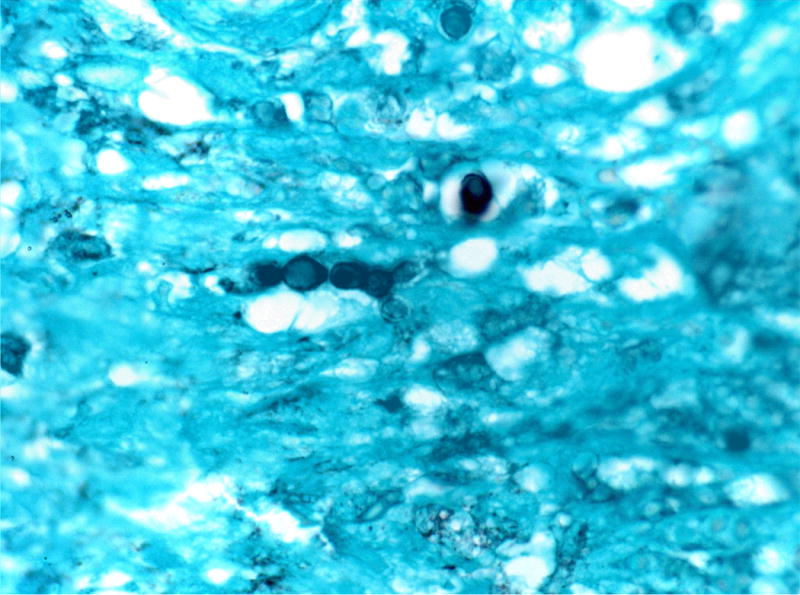
GMS staining of a representative lesional skin biopsy highlights yeast-like fungal elements present both singly and forming pseudohyphae (GMS staining, 1000 x).
A portion of the biopsied tissue and a separate wound swab were inoculated onto blood agar. Within one week, identical pigmented moulds were observed growing in these specimens. The isolate was referred to the Fungus Testing Laboratory in the Department of Pathology at the University of Texas Health Science Center for further identification and accessioned into their stock collection as UTHSC 06-3355. Colonies were identified on potato flakes agar (PFA) [9] in ambient air with an on and off light cycle at 25°C. They measured 26mm after 14 days and were moist initially but became more filamentous at maturity (Fig. 3). Microscopic features included long, brown, septate, annellophores with obovoidal annelloconidia (2-2.5 × 3-4 μm) produced at the apices of the conidiogenous cells as well as from intercalary loci (Fig. 4). A capsular black yeast synanamorph was also present. The isolate assimilated nitrate [10] and grew at 35°C but failed to grow at 40°C. Based upon the features noted, the isolate was identified as an Exophiala species, not E. dermatitidis.
Figure 3.
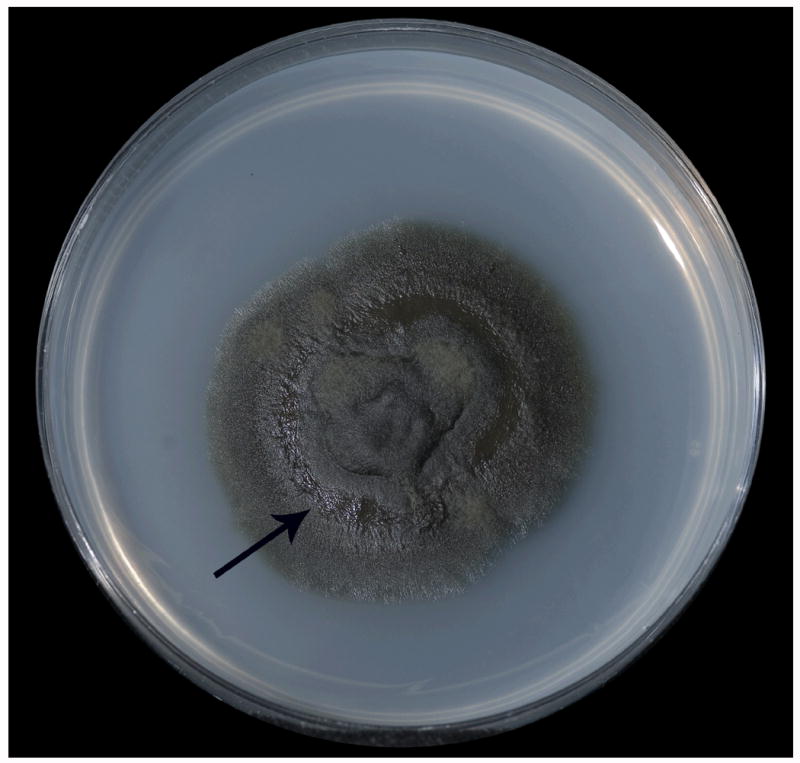
Colonial morphology of E. spinifera after 14 days incubation at 25°C on potato flakes agar. Note moist area (arrow) of the yeast synanamorph.
Figure 4.
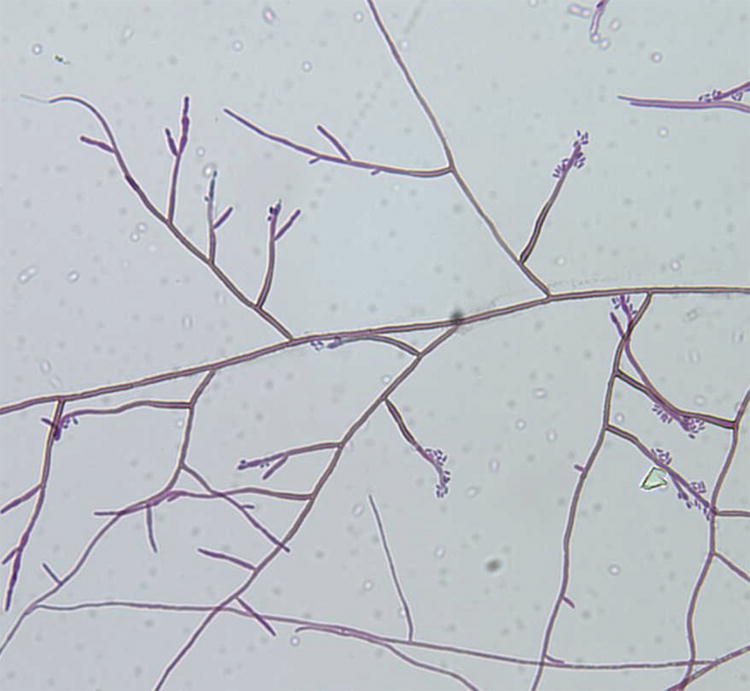
Microscopic morphology of E. spinifera showing long annellophores and annelloconidia, approximately 460X.
The isolate was subsequently sequenced. After incubation overnight on potato dextrose agar at 30°C, a small amount of material was scraped off the plate and inoculated into 50 μl of Prepman Ultra reagent (Applied Biosystems, Inc., Foster City, CA). The cells were lysed according to the manufacturer’s instructions and the suspension pelleted in a microfuge at 14000 × g for 10 minutes. PCR was performed on 5 μl of the supernatant in a 0.5 ml microfuge tube in a 50 μl reaction volume using Triple Master Taq DNA polymerase (Eppendorf/Brinkmann Instruments, Inc., Westbury, NY) according to the manufacturer’s instructions. Two primer pairs were used to amplify two regions of the ribosomal repeat from the genomic DNA template. The ITS region (ITS1-5.8S-ITS2) was amplified using ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC-3’) as described [11]. The D1/D2 region of the large ribosomal DNA subunit was amplified with primers NL-1 (5’-GCATATCAATAAGCGGAGGAAAAG-3) and NL-4 (5’-GGTCCGTGTTTCAAGACGG-3) as described previously [12]. All PCR reactions were performed in a PTC-100 thermocycler (MJ Research, Watertown, MA) using the preprogrammed three-step protocol as the standard program for all reactions. PCR reactions were electrophoresed through a 0.7% agarose gel to confirm amplification. The remaining template DNA was then cleaned using a Qiaquick PCR purification kit (Qiagen, Inc., Valencia, CA). Sequencing was performed on both strands at the UTHSCSA Advanced Nucleic Acids Core Facility using all four PCR primers. Both ITS and D1/D2 sequences were used to perform nucleotide-nucleotide searches using the BLASTn algorithm at the NCBI website (http://www.ncbi.nlm.nih.gov/BLAST/). Identifications were made when BLAST searches yielded ≥98% identity. A definitive identification of Exophiala spinifera was made based upon a 100% and 99% identity with D1/D2 and ITS sequences, respectively, when compared with GenBank nucleotide deposits for this species and the Centraalbureau voor Schimmelcultures (CBS) Exophiala database. Both the ITS and D1/D2 sequences were submitted to Genbank under accession numbers EU257701 (ITS), and EU257702 (D1/D2).
The patient received oral itraconazole at an initial dose of 200 mg twice/day that was later tapered to 100 mg twice/day. Importantly, itraconazole inhibits the cytochrome P450 enzyme CYP3A4 and thus typically results in increased plasma levels of tacrolimus, part of the immunosuppressive regimen in our patient. Previous reports have shown that a reduction in tacrolimus dose of 50-66% was necessary in transplant patients started on itraconazole [13]. In anticipation of this interaction, our patient’s tacrolimus dose was initially decreased by approximately 50%, from 2.5 mg (twice/day) to 1.5 mg (twice/day). Elevated plasma levels of tacrolimus on follow-up testing required temporary discontinuation, restarting at a low dose (0.5 mg twice/day), and close monitoring in follow-up while on itraconazole. The patient experienced rapid improvement and resolution of the lesions. He completed a total of six months antifungal therapy and to date has not experienced recurrence or new lesions.
Discussion and review of the literature
Summary of cases
Until recently, species identification of dematiaceous fungi has been based on appearance in tissue and in culture. By these criteria, Exophiala spinifera has been implicated in a total of eighteen cases of clinical infections from ten different countries, fourteen cases in the English literature. The initial case was reported in 1954 in a boy from India who succumbed to the infection. The pathogen was misclassified as Hormodendrum dermatitidis but was later reclassified based on modern morphologic criteria [14].
Of the fourteen reported cases of E. spinifera infection in the English literature, eleven had histologic characteristics of phaeohyphomycosis and three of chromoblastomycosis (Table 1). Six cases were reported in children 13 years of age or younger – all had disseminated infection and two resulted in death. While both deaths occurred in healthy children, their clinical courses were marked by delayed diagnosis and ineffective treatment for a prolonged period prior to appropriate therapy. The remaining nine cases (including our report) were localized infections in adults and were easily treated in all but one. Clinical presentations were variable, including erythematous papules, verrucous plaques, deep subcutaneous abscesses, and an ulcer in our case. The site of initial infection (by history or exam) was on the face in four children but on an extremity in seven of nine adults. Cases with dissemination from E. spinifera described various distant sites including skin, lymph nodes, bone, lung, and possibly the eye. Unlike disseminated phaeohyphomycosis from other species [6], none involved the central nervous system.
Table 1.
Summary of cases of cutaneous E. spinifera infection reported in the English literature. The first six cases listed are children, followed by six adult phaeohyphomycosis patients, followed by three adult chromoblastomycosis patients.
| Year/age/sex | Phaeo/Chromo | Immunosuppression | Initial Site | Additional sites | Country | Ref |
|---|---|---|---|---|---|---|
| 1954/7/M* | P | N | L cheek | face, trunk, extremities | India | 14 |
| 2000/12/F | P | N | L forehead | face, chest, arms, thighs; LAD | India | 14 |
| 1980/6/M | P | N | R cheek | face, earlobe, trunk, arm, lung | El Salvador | 15 |
| 1994/12/M* | P | N | R face | finger, elbow, toe; bone, regional LAD | Brazil | 16 |
| 1993/13/M | P | N | unknown | “disseminated” | Pakistan | 17 |
| 2008/10/M | P | N | legs | face, arms, upper back; LAD | India | 18 |
| 1968/72/F | P | N | Nose | none | USA | 19 |
| 1983/60/M | P | Y - pred | R forearm | R hand, thigh | USA | 20 |
| 1988/62/F | P | Y - gold + pred | L 4th finger | none | USA | 21 |
| 1990/32/F | P | N† | R forehead | forehead, inguinal folds, elbow; knee (bone), eyes, dissem LAD | Argentina | 22 |
| 2005/85/F | P | Y - prednisol + aza | L arm | none | Japan | 23 |
| 2008/49/M | P | Y - pred, tac, myco mof | R leg | none | USA | this case |
| 1992/49/M | C | N | dorsal R 4th finger | none | Mexico | 24 |
| 1993/62/M | C | Y - aza + pred (>16) | R 2nd finger | R forearm, elbow | USA | 25 |
| 2002/78/M | C | N | L forearm | none | UK/Pakistan | 26 |
Abbreviations: P – phaeohyphomycosis, C – chromoblastomycosis, pred – prednisone, prednisol – prednisolone, aza – azathioprine, tac – tacrolimus, myco mof – mycophenylate mofetil, LAD – lymphadenopathy.
Patient died.
Patient had a remote history of oral corticosteroids and disease was exacerbated during pregnancy.
Five of the nine adults were immunosuppressed, and all suppressive regimens included prednisone or prednisolone in doses from 2.5 to 16 mg/day. One adult patient who was not on an immunosuppressive regimen during infection had a remote history of oral corticosteroids for asthma and her disease was exacerbated during pregnancy, a physiologically immunosuppressed state [14-27]. Our case is the fifteenth case of clinical infection with E. spinifera reported in the English literature, and the first case to be definitively identified by molecular characterization.
Histology
The histologic features of cutaneous infection with E. spinifera are typical for a cutaneous deep fungal infection, including epidermal hyperkeratosis, acanthosis, pseudoepitheliomatous hyperplasia, and intraepidermal pustule formation. Dermal findings have included a dense mixed infiltrate as well as suppurative and granulomatous infiltrates. Multinucleated giant cells are a common feature. Pigmented fungal elements can be detected most often in areas of inflammation, within or adjacent to multinucleate giant cells. GMS, PAS, and Fontana stains can be employed to highlight the fungal elements for lightly pigmented organisms. The fungal elements display one of two distinct histologic morphologies: a phaeohyphomycotic form or a chromoblastomycotic form. Three of the fourteen previously reported cases were classified as chromoblastomycosis and the remaining cases as phaeohyphomycosis. Our case fulfills criteria for phaeohyphomycosis.
Specifically, phaeohyphomycosis is defined by the presence of dematiaceous fungi in tissue with a yeast-like, hyphal, or pseudohyphal morphology. These morphologies can be present uniformly or mixed. In culture-proven E. spinifera phaeohyphomycosis, the fungal elements have been described either as solitary forms or clusters of budding cells, thick-walled cells, or branched or septated hyphae. Other histologic descriptions have included budding yeast-like cells, thick walled chlamydospores, branched hyphae that were constricted at prominent septations, toruloid hyphal elements, and hyphae without prominent swellings. Some have observed hyaline to pale brown yeast cells with variable budding and the formation of pseudohyphae. In a case where the phaeohyphomycosis infection spread to an axillary lymph node, epithelioid cell granulomas with giant cells containing pigmented fungal elements were seen [14,15,20].
Chromoblastomycosis is defined by the presence of pigmented fungal elements in the form of muriform cells, thought to be an intermediate fungal form in the transition between the yeast and hyphal morphology. Other common terms for these elements include sclerotic bodies, fumagoid cells, copper pennies, Medlar bodies, and chlamydospores. In cases of E. spinifera, these elements have been described as round, chestnut brown or brownish in color, having a central pale area, and thick walls. Padhye et al. described the muriform cells as round-to-oval and ranging from 5 to 12 micrometers in diameter. They also described the presence of dematiaceous sporangium-like cells, which measured 12-20 micrometers in diameter and contained endoconidia. Propagation of the muriform cells in tissue was demonstrated by planate division [24-26].
Cases of E. spinifera cutaneous infection with concurrent chromoblastomycosis and phaeohyphomycosis forms have not been reported to date. It is likely, therefore, that these diagnoses represent two poles of a spectrum of disease and that the development of either form in a particular patient may depend on a specific strain-individual-environmental interaction [24].
Mycology
The black yeast genus Exophiala contains several species capable of inciting human disease, and differentiation of these species remains problematic for routine clinical laboratories. Features contributing to difficulty in identification include the pleomorphic nature of the genus, i.e., the ability of several species to form a yeast phase as well as a filamentous phase [28], limited physiologic differences, and the very similar macroscopic and microscospic morphology between species. Prior to the development of molecular techniques to characterize these agents, the most common species reported were E. jeanselmei and E. dermatitidis. Our understanding of the genus greatly expanded with the advent of sequencing methods and the analysis of sequence data of the ribosomal DNA (rDNA) Internal Transcribed Spacer (ITS) regions permitting differentiation of the genus into specific “clades” or clusters of taxa, including the E. spinifera clade [8,29,30]. The common clinical species Exophiala jeanselmei, long known to be a heterogeneous collection of yeast-like fungi, was also further defined and shown to consist of several species including E. heteromorpha, E. lecanii-corni, E. oligosperma and E. xenobiotica [31], as well as E. jeanselmei in the restricted sense. In a recent study of the distribution of Exophiala species recovered from clinical specimens in the United States using ITS sequence data, the order of frequency for the most common isolates was as follows: E. dermatitidis (29.3%), E. xenobiotica (19.7%), E. oligosperma (18.6%), E. lecanii-corni (6.9%), and E. phaeomuriformis (6.4%). The recovery of E. jeanselmei and E. spinifera were at 3.7% and 2.7%, respectively [7]. Although Exophiala spinifera is infrequently recovered and/or reported due either to misidentification or lack of species identification, this organism is a cause of significant cutaneous disease.
Treatment
Definitive identification of the causative agent of phaeohyphomycosis is clinically important, as different genera/species may vary in their susceptibility to antimicrobial agents [8]. In vitro studies suggest that E. spinifera is most highly susceptible to itraconazole but poorly responsive to amphotericin B [8,32]. In a more recent study evaluating the spectrum of clinically relevant Exophiala species in the U.S. and determining their in vitro susceptibility patterns to the currently available antifungal agents, no significant differences were noted between species, except for amphotericin B resistance in three isolates of E. attenuata [7]. A summary of the in vitro susceptibilities of E. spinifera to various drugs is listed in Table 2. One study showed that the addition of quinolones to itraconazole or amphotericin B improved antimicrobial responses of E. spinifera, however this has not yet been tested clinically [33]. Importantly, it has been noted that in vitro susceptibility data seem to correlate poorly with in vivo efficacy [32]. Multiple modalities have been attempted to treat infection with E. spinifera, including the antifungals amphotericin B, ketoconazole, fluconazole, itraconazole, voriconazole, 5-fluorocytosine, terbinafine, griseofulvin, posaconazole, streptomycin, and the physical modalities heat, cryosurgery and excision [8,14-26]. However, as detailed below, few of these treatments were ultimately found to be effective. Seven of the twelve patients who recovered from their disease, including our patient, were receiving itraconazole as part of their treatment regimen, and five were taking this medication as monotherapy. Four patients received amphotericin B in their initial treatment regimens; however, two patients required additional antifungal agents to obtain an adequate clinical response and one patient died, while one showed early improvement in short-term follow-up [15-17,22]. Two cases reported increasing resistance to itraconazole over the course of treatment that was verified by in vitro testing [15,25], and one report noted decreasing efficacy of multiple antifungal treatments over a prolonged course [22], suggesting that E. spinifera possesses the ability to develop resistance to antifungal therapy. A recent report found a panel of treatments that included itraconazole, terbinafine, fluconazole, and cryosurgery to be ineffective [18]. Other treatments that were used in the remaining patients who recovered without itraconazole treatment included posoconazole, ketoconazole, and 5-fluorocytosine antifungal therapies, liquid nitrogen cryotherapy, and surgical excision without additional therapy.
Table 2.
Minimal inhibitory concentrations in μg/ml of drugs tested against E. spinifera. MIC listed is the geometric mean of multiple strains tested in each reference.
| Ref | ITZ | VCZ | FCZ | TBF | 5-FC | AMB | POS |
|---|---|---|---|---|---|---|---|
| 7* | 0.03 | 0.125 | nd | nd | nd | 0.5 | <0.015 |
| 8 | 0.05 | 0.3 | 55.05 | 0.26 | 3.44 | 0.7 | nd |
| 32† | 0.06 | 0.27 | 45.25 | 0.3 | 5.66 | 0.92 | nd |
Abbreviations: ITZ – itraconazole, VCZ – voriconazole, FCZ – fluconazole, TBF – terbinafine, 5-FC – 5 fluorocytosine, AMB – amphotericin B, POS – posaconazole, nd – not done.
This reference determined MIC50 and MIC90. The MIC listed is for MIC50, consistent with the other references.
This reference tested susceptibility of environmental and clinical strains at various temperatures. The MIC listed is for clinical isolates grown at 35°C, consistent with the other references.
Conclusions
In summary, phaeohyphomycosis incited by E. spinifera: 1) typically presents in children as a lesion on the face, usually from an unidentified exposure, whereas in adults it primarily occurs on an extremity and is secondary to traumatic implantation; 2) may develop into disseminated disease, which is more common in children than in adults and has a higher rate of mortality; 3) usually occurs in healthy children; however, more than half of reported adult patients were on an immunosuppressive regimen which included daily prednisone; 4) may present histologically with either a phaeohyphomycosis or chromoblastomycosis morphology; and 5) has been most successfully eradicated when treatment protocols included itraconazole.
Acknowledgments
The authors thank Elizabeth Thompson and Anna M. Romanelli for the morphologic identification and sequencing of the isolate, respectively.
Footnotes
Conflict of Interest: None.
Contributor Information
John E. Harris, Dept. of Dermatology (patient care), University of Pennsylvania School of Medicine, Philadelphia, PA 19104
Deanna A. Sutton, Dept. of Pathology (morphologic identification), University of Texas Health Science Center San Antonio, TX 78229
Adam Rubin, Dept. of Dermatology (histology), University of Pennsylvania School of Medicine, Philadelphia, PA 19104.
Brian Wickes, Dept. of Microbiology (sequencing), University of Texas Health Science Center San Antonio, TX 78229.
G.S. de Hoog, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands (CBS database matching)
Carrie Kovarik, Dept. of Dermatology (patient care and histology), University of Pennsylvania School of Medicine, Philadelphia, PA 19104.
References
- 1.Revankar SG. Phaeohyphomycosis. Infect Dis Clin North Am. 2006;20:609–620. doi: 10.1016/j.idc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Prenafeta-Boldú FX, Summerbell RC, de Hoog GS. Fungi growing on aromatic hydrocarbons: biotechnology’s unexpected encounter with biohazard. FEMS Microbiol Rev. 2006;30:109–130. doi: 10.1111/j.1574-6976.2005.00007.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson BJ. Definitions of fungal rhinosinusitis. Otolaryngol Clin N Am. 2000;33:227–235. doi: 10.1016/s0030-6665(00)80002-x. [DOI] [PubMed] [Google Scholar]
- 4.Schubert MS. Allergic fungal sinusitis: pathogenesis and management strategies. Drugs. 2004;64:363–374. doi: 10.2165/00003495-200464040-00002. [DOI] [PubMed] [Google Scholar]
- 5.de Hoog GS, Guarro J, Gené J, Figueras MJ, editors. Atlas of Clinical Fungi. 2. Utrecht, The Netherlands and Universitat Rovira i Virgili Reus, Spain: Centraalbureau voor Schimmelcultures; 2000. [Google Scholar]
- 6.Revankar SG, Sutton DA, Rinaldi MG. Primary central nervous system phaeohyphomycosis: a review of 101 cases. Clin Infect Dis. 2004;38:206–216. doi: 10.1086/380635. [DOI] [PubMed] [Google Scholar]
- 7.Zeng JS, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, de Hoog GS. Spectrum of clinically relevant Exophiala species in the U.S.A. J Clin Microbiol. 2007;45:3713–3720. doi: 10.1128/JCM.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitale RG, de Hoog GS. Molecular diversity, new species and antifungal susceptibilities in the Exophiala spinifera clade. Med Mycol. 2002;40:545–556. doi: 10.1080/mmy.40.6.545.556. [DOI] [PubMed] [Google Scholar]
- 9.Rinaldi MG. Use of potato flakes agar in clinical mycology. J Clin Microbiol. 1982;15:1159–1160. doi: 10.1128/jcm.15.6.1159-1160.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pincus DH, Salkin IF, Hurd NJ, Levy IL, Kemna MA. Modification of potassium nitrate assimilation test for identification of clinically important yeasts. J Clin Microbiol. 1988;26:366–368. doi: 10.1128/jcm.26.2.366-368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White TJ, Bruns TD, Lee SB, Taylor JW. PCR-Protocols and Applications-A Laboratory Manual. Academic Press; New York: 1990. Amplification and sequencing of fungal ribosomal RNA genes for phylogenetics; pp. 315–322. [Google Scholar]
- 12.Kurtzman CP, Robnett CJ. Identification of clinically important ascomycetous yeasts based on nucleotide divergence in the 5’ end of the large-subunit (26S) ribosomal DNA gene. J Clin Microbiol. 1997;35:1216–1223. doi: 10.1128/jcm.35.5.1216-1223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saad AH, DePestel DD, Carver PL. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy. 2006;26:1730–1744. doi: 10.1592/phco.26.12.1730. [DOI] [PubMed] [Google Scholar]
- 14.Rajendran C, Khaitan BK, Mittal R, et al. Phaeohyphomycosis caused by Exophiala spinifera in India. Med Mycol. 2003;41:437–441. doi: 10.1080/1369378031000153820. [DOI] [PubMed] [Google Scholar]
- 15.Padhye AA, Ajello L, Chandler FW, et al. Phaeohyphomycosis in El Salvador caused by Exophiala spinifera. Am J Trop Med Hyg. 1983;32:799–803. doi: 10.4269/ajtmh.1983.32.799. [DOI] [PubMed] [Google Scholar]
- 16.Campos-Takaki GM, Jardim ML. Report of chronic subcutaneous abscesses caused by Exophiala spinifera. Mycopathologia. 1994;127:73–76. doi: 10.1007/BF01103061. [DOI] [PubMed] [Google Scholar]
- 17.Mirza SH, Hannan A, Ahmad A, Ahmad M. Subcutaneous phaeohyphomycosis. J Infect. 1993;27:75–78. doi: 10.1016/0163-4453(93)93908-m. [DOI] [PubMed] [Google Scholar]
- 18.Singal A, Pandhi D, Bhattacharya SN, et al. Pheohyphomycosis caused by Exophiala spinifera: a rare occurrence. Int J Dermatol. 2008;47:44–47. doi: 10.1111/j.1365-4632.2007.03430.x. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen HS, Conant NF. A new human pathogenic Phialophora. Saboraudia. 1967-1968;6:228–231. [PubMed] [Google Scholar]
- 20.Padhye AA, Kaplan W, Neuman MA, Case P, Radcliffe GN. Subcutaneous Phaeohyphomycosis caused by Exophiala spinifera. Sabouraudia. 1984;22:493–500. [PubMed] [Google Scholar]
- 21.Kotylo PK, Israel KS, Cohen JS, Bartlett MS. Subcutaneous Phaeohyphomycosis of the finger caused by Exophiala spinifera. Am J Clin Path. 1989;91:624–627. doi: 10.1093/ajcp/91.5.624. [DOI] [PubMed] [Google Scholar]
- 22.Negroni R, Helou SH, Petri N, et al. Case study: posaconazole treatment of disseminated Phaeohyphomycosis due to Exophiala spinifera. Clin Infect Dis. 2004;38:e15–e20. doi: 10.1086/380840. [DOI] [PubMed] [Google Scholar]
- 23.Takahara M, Imafuku S, Matsuda T, et al. Concurrent double infections of the skin: Phaeohyphomycosis and nocardiosis in a patient with idiopathic thrombocytopenic purpura. J Am Acad Dermatol. 2005;53:S277–S280. doi: 10.1016/j.jaad.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 24.Barba-Gomez JF, Mayorga J, McGinnis MR, et al. Chromoblastomycosis caused by Exophiala spinifera. J Am Acad Dermatol. 1992;26:367–370. doi: 10.1016/0190-9622(92)70058-n. [DOI] [PubMed] [Google Scholar]
- 25.Padhye AA, Hampton AA, Hampton MT, et al. Chromoblastomycosis caused by Exophiala spinifera. Clin Infect Dis. 1996;22:331–335. doi: 10.1093/clinids/22.2.331. [DOI] [PubMed] [Google Scholar]
- 26.Tomson N, Abdullah A, Maheshwari MB. Chromomycosis caused by Exophiala spinifera. Clin Exp Dermatol. 2006;31:239–241. doi: 10.1111/j.1365-2230.2005.02006.x. [DOI] [PubMed] [Google Scholar]
- 27.Hunt JS. Stranger in a strange land. Immunol Rev. 2006;213:36–47. doi: 10.1111/j.1600-065X.2006.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.deHoog GS, Takeo K, Yoshida S, et al. Pleoanamorphic life cycle of Exophiala (Wangiella) dermatitidis. Antonie Leeuwenhoek. 1994;65:143–153. doi: 10.1007/BF00871755. [DOI] [PubMed] [Google Scholar]
- 29.deHoog GS, Poonwan N, Gerrits van den Ende AHG. Taxonomy of Exophiala spinifera and its relationship to E. jeanselmei. Stud Mycol. 1999;43:133–142. [Google Scholar]
- 30.deHoog GS, Vicente V, Caligiorne RB, et al. Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. J Clin Microbiol. 2003;41:4767–4778. doi: 10.1128/JCM.41.10.4767-4778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Hoog GS, Zeng JS, Harrak MJ, Sutton DA. Exophiala xenobiotica sp. nov., an opportunistic black yeast inhabiting environments rich in hydrocarbons. Antonie van Leeuwenhoek. 2007;90:257–268. doi: 10.1007/s10482-006-9080-z. [DOI] [PubMed] [Google Scholar]
- 32.Vitale RG, de Hoog GS, Verweij PE. In vitro activity of amphotericin B, itraconazole, terbinafine, and 5-fluocytosine against Exophiala spinifera and evaluation of post-antifungal effects. Med Mycol. 2003;41:301–307. doi: 10.1080/13693780310001600822. [DOI] [PubMed] [Google Scholar]
- 33.Vitale RG, Afeltra J, de Hoog GS, Rijs AJ, Verweij PE. In vitro activity of amphotericin B and itraconazole in combination with flucytosine, sulfadiazine and quinolones against Exophiala spinifera. J Antimicrob Chemother. 2003;51:1297–1300. doi: 10.1093/jac/dkg218. [DOI] [PubMed] [Google Scholar]


