Abstract
Increased oxidative stress and decrease in antioxidant enzymes have been suggested to be involved in the pathophysiology of myocardial infarction (MI). In this study, treadmill exercise training and losartan treatment began 1 week post-MI and lasted 8 weeks. We evaluated the changes in the mRNA and protein expressions for the enzymatic antioxidants-superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase after exercise and losartan treatment in post-MI rats. Our results demonstrated that GPx and catalase mRNA levels were comparable among all the groups, while the mRNA level for manganese SOD (MnSOD) was significantly increased in exercise training with/without losartan treatment as compared to the sedentary MI group. Moreover, the mRNA level for gp91phox was dramatically decreased by a combination of exercise and losartan treatment. The protein levels for MnSOD were significantly elevated by exercise training in combination with losartan treatment. The protein levels for catalase were significantly increased in response to exercise, and it was further augmented by exercise together with losartan treatment. Thiobarbituric acid-reactive substances in plasma were significantly increased in the MI rats, but were decreased by exercise or losartan treatment, indicating that both exercise and losartan may reduce lipid oxidative damage. In addition, catalase and SOD enzymatic activities were significantly enhanced by exercise combined with losartan treatment. Our results suggest that exercise training improves catalase and MnSOD expression and attenuates oxidative stress. These effects are potentiated when combining exercise with angiotensin II receptor blockade.
Keywords: oxidative stress, losartan, myocardial infarction, free radicals, exercise
It is widely accepted that exercise training may lead to beneficial cardiovascular adaptations. Although its precise mechanisms on heart health are not fully defined yet, exercise training is believed to influence many parameters, such as improving blood-lipid profile, reducing sympathetic tone and enhancing endogenous defense systems (Liu et al., 2000; Erikssen, 2001; Gao et al., 2007). Reactive oxygen species (ROS) and free radicals are mediators of several forms of tissue damage, such as ischemic injuries to different organs, inflammatory response, and injury (Husain & Hazelrigg, 2002). The cardiovascular system has a series of defense mechanisms including antioxidant enzymes and other free radical scavengers, such as beta-carotene, alpha-tocopherol, ascorbic acid, alpha-lipoic acid, and glutathione (GSH), to protect cells against cytotoxic ROS including superoxide anion (O2−) and hydrogen peroxide (H2O2). There are three primary antioxidant enzymes: superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx). SOD promotes the dismutation of O2− and forms H2O2 and oxygen, while catalase converts H2O2 to water and oxygen. GPx utilizes reduced GSH as a reducing equivalent to reduce H2O2 to form oxidized glutathione and water. There are three isoforms of SOD including the cytosolic copper-zinc SOD (CuZnSOD), extracellular SOD (ecSOD), and mitochondrial manganese superoxide dismutase (MnSOD). Previous studies (Hamilton et al., 2001; Hamilton et al., 2003) reported that exercise induced MnSOD elevation, but not CuZnSOD, and MnSOD may play an important role in exercise-induced cardioprotection against ischemia-reperfusion injury (Yamashita et al., 1999; French et al., 2008). NAD(P)H oxidase, which consists of six subunits, including two plasma membrane-associated proteins, gp91phox and p22phox, is a major source of O2− in the heart. Increased NAD(P)H oxidase activity and expression have been found in experimental pressure-overload left ventricular (LV) hypertrophy and myocardial infarction (MI) (Fukui et al., 2001; Li et al., 2002). The degree of oxidative stress and the severity of subsequent myocardial damage might depend on the imbalance between excess ROS production and the antioxidant defense within the heart (Shiomi et al., 2004). Previous studies suggest that oxidative stress plays a major role in the pathogenesis of cardiac remodeling and progression of CHF following MI (Hill & Singal, 1996; Kinugawa et al., 2000; Sia et al., 2002b). Also, accumulating evidence indicates that exercise training promotes antioxidant capacity and attenuates oxidative stress-mediated tissue damages (Leeuwenburgh et al., 1997; Ennezat et al., 2001; Linke et al., 2005). In addition, exercise training has been established as adjuvant therapy in chronic heart failure; however, it is still unknown whether the training-mediated preservation of cardiac function is associated with restoration of the local enzymatic radical scavenger system.
Cardiac renin-angiotensin system (RAS) is activated during the process of post-MI remodeling remodeling (Sun & Weber, 1994). Many studies have demonstrated that inhibition of cardiac RAS with angiotensin II receptor blockers (ARBs) improves LV function, prevents geometric remodeling, and prolongs survival, suggesting that AngII plays an important role after MI (Raya et al., 1991; Schieffer et al., 1994; Liu et al., 1997; Pitt et al., 1997). An important effect of circulating angiotensin II (AngII) is to promote the activation of NAD(P)H oxidase, which increases ROS production (Lu et al., 2004). Indeed, exercise training may normalize circulating RAS in animals with MI (Liu et al., 2000; Wan et al., 2007), and patients with heart failure (Braith et al., 1999). Therefore, the present study was designed to assess the effect of a combination of AngII blockade and exercise training on oxidative stress after MI. We investigated the changes in the activity, mRNA abundance, and protein levels of the enzymatic antioxidants, MnSOD, catalase, and GPx by exercise training combined with AngII blockade after MI. Our aim was to test the hypothesis that post-MI exercise training combined with AngII receptor blockade reconstitutes the activity of SOD, catalase and GPx, thereby reducing oxidative stress in the non-infarcted myocardium in rats.
Methods
Animals and Induction of MI
Seven-week-old male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN) were housed at constant temperature (22 ± 2 °C) on a 12-h light/dark cycle. They were fed ad libitum on standard rat chow and had free access to tap water. MI was induced by ligation of the left anterior descending coronary artery (LAD). These rats were derived from the same animals utilized in our previous study (Xu et al., 2008). The study was performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the study protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas at San Antonio.
Experimental groups
One week after surgery, echocardiography was performed on the surviving rats. Rats were matched by cardiac function (fractional shortening, FS) using echocardiography and randomly assigned to the following experimental groups: Sham-operated control (Sham, n=10), sedentary MI (Sed, n=9), MI plus exercise (EX, n=10), MI plus losartan (Sed+Los, n=12), and MI plus exercise and losartan (EX+Los, n=13).
Drug treatment and exercise training
Losartan treatment (20mg·kg−1·d−1) was initiated 1 week post-MI. The drug administration was performed via gastric gavage twice a day for 8 weeks. The determination of losartan dosage was based on the previous studies (Wang et al., 2005; Dent et al., 2006), which demonstrated positive effect in improving cardiac function and attenuating cardiac hypertrophy without signs of side effects. Tap water was also given by gastric gavage to rats without losartan treatment to avoid the possible physiological alterations associated with gavage-induced stress. Rats assigned to the exercise groups started exercising at 1 week post-MI using a motorized rodent treadmill as described previously (Xu et al., 2008), while the sham and sedentary groups were placed on the treadmill but remain sedentary for the same period of time as the exercise trained rats. To allow gradual adaptation to exercise stress, exercise training was initiated at 10 m/min, 5° incline for 10 min per session. The speed and duration were gradually increased to 16 m/min and 50 min per session (including a 5 min warm-up at 10 m/min) and maintained constant throughout the experiment. Exercise training was performed 5 days per week for 8 weeks. Our previous study (Wan et al., 2007) demonstrated that this exercise regimen improved the aerobic capacity of MI rats as indicated by significant elevation of muscle citrate synthase activity.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was extracted from non-infarcted LV with TRIzol® reagent (Invitrogen). For determination of mRNA levels, 1 μg of total RNA was reverse-transcribed with oligo (dT) primers and MMLV reverse transcriptase (Promega, Madison, WI). The relative expression of catalase, MnSOD, gp91phox and GPx-1 was normalized to the amount of β-actin in the same cDNA using the standard curve method. The primers and probes used were as follows. 5′-TCACTGTCATCATAAGGCCATCAAA-3′ (forward primer), 5′-CCTAACTGCAGTAGAACAGGATTACAG-3′ (reverse primer) and 5′-CACAGGCCCCAACACA-3′ (TaqMan probe) for MnSOD (GenBank accession number NM_017051). 5 ′-GGTCCCATGTTCCTGTACCTTTG-3′ (forward primer), 5′-GTGATGACCACCTTCTGTTGAGA-3′ (reverse primer) and 5′-CCGGACCAACCTCTCG-3′ (TaqMan probe) for gp91phox (GenBank accession number AF298656). 5′-TCTTTACCTTCCTGCGGAATGC-3′ (forward primer), 5′-TCTCAAAGTTCCAGGAAATGTCGTT-3′ (reverse primer) and 5′-CCCAGTGACGATCCC-3′ (TaqMan probe) for GPx-1 (GenBank accession number NM_030826). Other primers and probes used in the study were Assay-on-Demand gene expression products (Applied Biosystems). Because of proprietary issues and the policy of Applied Biosystems, the exact primer sequences used for the real-time PCR experiments are not provided but can be requested from the company based on the information shown in Table 1.
Table 1.
Oligonucleotide primers used for real-time RT-PCR
| Gene | Assay ID | Reference Sequence* |
Amplicon Length |
Exon Boundary |
|---|---|---|---|---|
| catalase | Rn00560930_m1 | NM_012520.1 | 107 | 1-2 |
| β-actin | Rn00667869_m1 | NM_031144.2 | 91 | 4-5 |
Accession numbers in GenBank for the sequence used in designing the primers.
Western blot
Twenty micrograms of protein was separated by sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE), and then transferred to PVDF membranes (Bio-Rad, Hercules, CA). Membranes were incubated with primary antibodies overnight at 4 °C. Primary antibodies used were anti-MnSOD (BD Transduction Laboratories), anti-catalase (Sigma), and GAPDH (Santa Cruz). Membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies. The membranes were detected with enhanced chemiluminescence (Amersham, Little Chalfont, Buckinghamshire, UK) followed by exposure to X-ray film. The protein bands on the X-ray film were scanned, and band density was calculated by Quantity One software (Bio-Rad).
Antioxidant Activity
The antioxidant enzymatic activities of GPx, catalase, and SOD were measured in the noninfarcted LV using the following commercial kits (Cayman Chemical, Michigan): SOD assay kit for SOD activity (U/mg protein); catalase assay kit for catalase activity (nmol/min/mg protein); glutathione peroxidase assay kit for GPx activity (nmol/min/mg protein). The assays were performed according to the manufacturer’s instructions. In the assay system for total GPx activity, the oxidation of glutathione (GSH) was coupled to NADPH oxidation by GSH reductase (GR). Briefly, twenty microliters of heart homogenate were added to the reaction mixture (190 μl). The reaction was initiated by addition of 20 μl of 0.2 mM cumene hydroperoxide at room temperature. The decrease in absorbance at 340 nm was recorded at 60 s intervals for 6 min. The rate of decrease in the absorbance is directly proportional to the GPx activity in the sample. Each assay was performed in duplicates, and enzyme units were recorded as nmol NADPH oxidized/min/mg protein in the tissue sample.
Thiobarbituric Acid Reactive Substances
The degree of lipid peroxidation was determined in plasma and LV non-infarcted myocardial tissues through biochemical assay of thiobarbituric acid reactive substances (TBARS) using a commercial kit (Cayman Chemical, Ann Arbor, MI).
Statistical analyses
Data were compared by analysis of variance and Tukey’s post hoc tests to determine whether there were significant mean differences among the experiment groups. A P value of less than 0.05 was considered statistically significant. Values are expressed as mean ± S.E.M.
Results
General characteristics
The infarct sizes were similar in all four infarcted groups ranging from 40.80% to 41.67%, and there was no significant difference in body weight among all the experimental groups. Heart weight to body weight ratio was significantly higher in the EX and Sed groups (3.82 ± 0.09 and 3.93 ± 0.16, respectively) than in the Sham group (3.34 ± 0.05), whereas the ratio was dramatically decreased in the EX+Los group (3.56 ± 0.10) compared to the EX and Sed groups.
Gene expressions in the non-infarcted LV myocardium
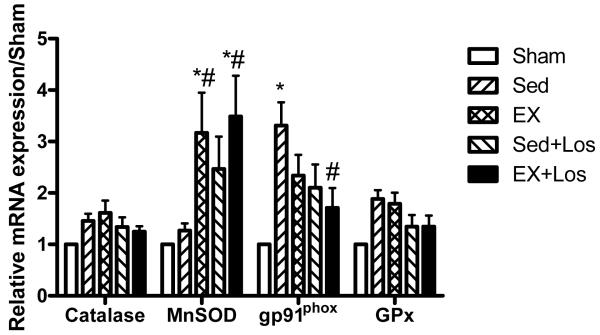
We investigated whether the increased O2− production after MI could be attributed to differences in the expression and/or abundance of GPx, catalase, MnSOD, and NAD(P)H oxidase. As seen in Figure 1, GPx and catalase mRNA levels were comparable among all the groups, while the mRNA level for MnSOD was significantly increased in exercise training with/without losartan treatment (3.49 ± 0.79 and 3.17 ± 0.78, respectively) as compared to the Sed group (1.27 ± 0.14, P<0.05). The gp91phox is one of the NAD(P)H oxidase subunits, which is a major source of O2− in the heart. Figure 1 showed that the mRNA level for gp91phox was significantly decreased by combination of exercise training with losartan treatment when compared to the Sed group (P<0.05).
Figure 1.
Results of real-time PCR for catalase, MnSOD, gp91phox, and GPx. *P<0.05 vs. the Sed group. *P<0.05 vs. Sham group; #P<0.05 vs. the Sed group.
Protein expressions and abundance of catalase and MnSOD in the non-infarcted LV myocardium
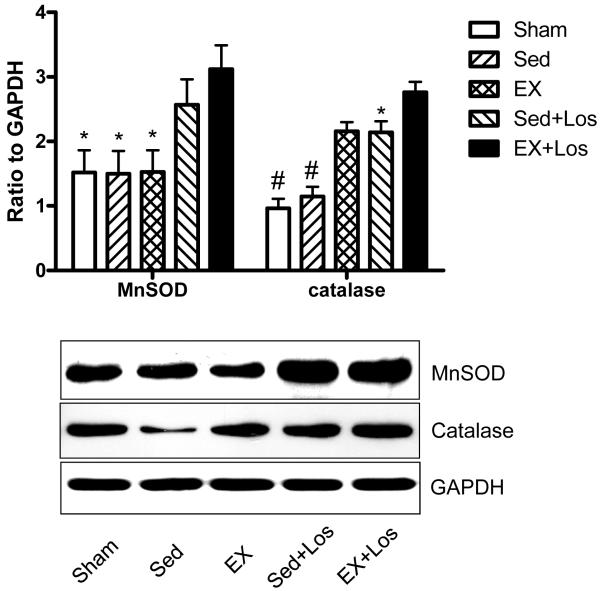
As shown in Figure 2, the protein levels for MnSOD were significantly higher in the EX+Los group than the Sed and EX groups. Furthermore, the protein level for catalase was significantly increased in both the exercise training (2.16 ± 0.14) and in the exercise training combined with losartan treatment groups (2.76 ± 0.16) compared with the sham and Sed groups (0.96 ± 0.15 and 1.15 ± 0.15, respectively).
Figure 2.
The results of western blot for MnSOD and catalase. #P<0.001 vs. the Sed+Los and EX+Los groups; *P<0.05 vs. the EX+Los group.
Antioxidant enzyme activities
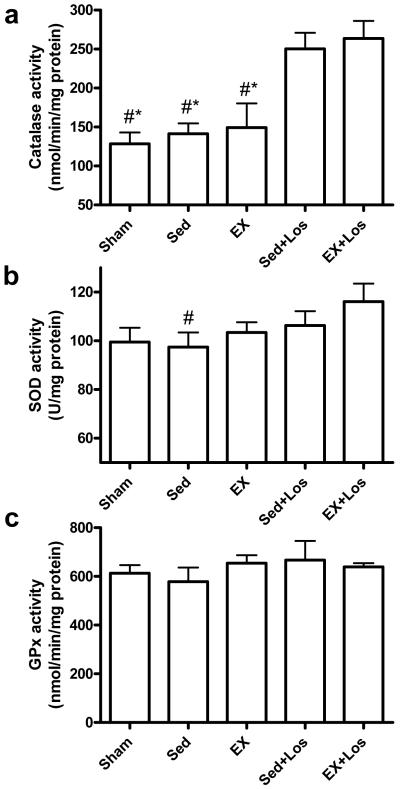
As shown in Figure 3, the catalase activity was significantly increased by losartan treatment, although it was not further enhanced when combined with exercise. The SOD activity was significantly elevated by exercise combined with losartan treatment when compared to the sedentary MI group. There was no significant difference in the GPx activity among all the groups.
Figure 3.
Cardiac catalase, SOD, and GPx activity. a) Catalase activity (nmol/min/mg protein); b) SOD activity (U/mg protein); c) GPx activity (nmol/min/mg protein). *P<0.05 vs. the Sed+Los group; #P<0.05 vs. the EX+Los group.
TBARS in plasma and myocardial tissue
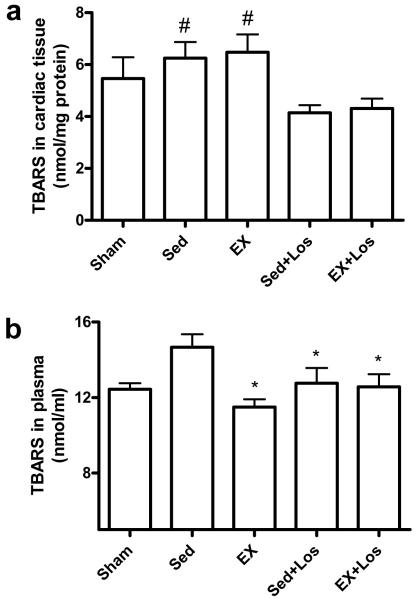
As seen in Figure 4, myocardial thiobarbituric acid-reactive substances (TBARS) in the cardiac tissue was significantly lower in the groups treated with losartan than the EX alone and Sed groups. In addition, TBARS in plasma were significantly increased in the Sed rats whereas both exercise training and losartan treatment significantly attenuated the MI-induced TBARS elevation (P<0.05).
Figure 4.
Free radical production, as measured by TBARS generation, in cardiac tissue (a) and plasma (b). *P<0.05 vs. the Sed group; #P<0.05 vs. the EX+Los and Sed+Los group.
Discussion
Heart failure subsequent to MI has been reported to be associated with an antioxidant deficit as well as increased oxidative stress (Hill & Singal, 1996). Our previous study demonstrated that exercise training may enhance cardiac function and reduce cardiac remodeling when combined with angiotensin II receptor blockade (Xu et al., 2008). Nevertheless, whether these beneficial effects are associated with restoration of the local enzymatic radical scavenger system is yet to be investigated. Data from this study provide further insights into the mechanisms underlying the improvement in morbidity and mortality produced by exercise training in patients with MI. There are three major findings in the current study. First, early post-MI exercise training induced the increase in MnSOD gene expression, while the gp91phox gene expression was attenuated by exercise in combination with losartan treatment. Second, exercise training combined with losartan treatment upregulated the catalase and MnSOD protein expression, and increased the enzymatic activity of catalase. Third, exercise training combined with losartan treatment reduced oxidative stress as indicated by the decreased lipid peroxidation.
Accumulating evidence suggests that ROS play a major role in the development and progression of LV remodeling and failure, whereas antioxidants exert protective and beneficial effects in the failing heart (Dhalla et al., 1996; Nakamura et al., 2002; Sia et al., 2002a; Shiomi et al., 2004). Hill and Signal (Hill & Singal, 1996, 1997) have shown evidence of a progressive decrease in SOD, catalase, and GPx activity in the infarcted heart (Fukui et al., 2001; Lu et al., 2004). Several studies reported that exercise training leads to an increase in myocardial SOD content along with improved recovery from ischemia-reperfusion injury (Yamashita et al., 1999; Brown et al., 2003). Others, however, reported that exercise training increased cardioprotection without amplifying myocardial SOD content (Lennon et al., 2004; Brown et al., 2005) and only certain cardiac antioxidant enzyme activities (i.e. SOD) were enhanced in the exercise trained animals (Powers et al., 1993; Somani et al., 1995; Yamashita et al., 1999). The variation in the findings of these studies may be due to the differences in the intensity and duration of exercise regimens. In the present study, our results showed that exercise training increased MnSOD gene expression after MI regardless of losartan treatment. In addition, exercise training together with losartan treatment remarkably enhanced the enzymatic activity of catalase, suggesting an additive effect of exercise training and angiotensin II receptor blockade treatment. Khaper et al (Khaper et al., 2003) reported that myocardial infarction led to decreases in the mRNA levels of SOD and catalase, while losartan treatment significantly increased protein levels for catalase at 16 weeks post-MI, which is in line with our findings in this study.
GPx is a selenium-containing enzyme that catalyzes the removal of H2O2 through oxidation of reduced GSH, which is recycled from oxidized glutathione by glutathione reductase. In the current study, we did not find any significant difference in GPx expression or its enzymatic activity among all the infarcted groups. However, the protein level of catalase and its activity were both dramatically increased after 8 weeks of exercise training, and these effects were further augmented by the addition of losartan treatment. Because catalase mRNA expression was not changed by exercise training and/or losartan in MI rats, the increase in catalase activity and protein expression may be the result of either enhanced catalase mRNA stability or posttranslational modification of catalase protein.
It has been reported that NAD(P)H oxidase expression dramatically increases in the infarcted myocardium (Fukui et al., 2001; Lu et al., 2004). Linke et al (Linke et al., 2005) and Adams et al (Adams et al., 2005) have revealed that exercise training may induce an antioxidant effect via reduction of expression of NAD(P)H oxidase and beneficial augmentation of the activity of radical scavenger enzymes. In this study, although losartan and exercise training alone did not significantly attenuate gp91phox gene expression, gp91phox was significantly reduced by the combination of exercise training and losartan treatment, indicating that this combination has an additive effect on the attenuation of NAD(P)H oxidase gene expression. Since NAD(P)H oxidase is a major source of O2− in the heart, the reduction of NAD(P)H oxidase may decrease the MI-induced oxidative stress. On the other hand, myocardial infarction resulted in an increase in oxidative stress in the non-infarcted myocardium which was evidenced by a significant increase in TBARS, whereas exercise training and/or losartan treatment dramatically reduced lipid peroxidation, suggesting that both exercise training and losartan may reduce lipid oxidative damage.
In summary, this study demonstrates that myocardial infarction is associated with a reduction in antioxidants as well as an increase in lipid peroxidation. Early post-MI exercise training increases MnSOD gene expression, decreases NAD(P)H oxidase and lipid peroxidation, and enhances catalase protein and activity. These effects are further augmented when combined with angiotensin II receptor blockade.
Acknowledgements
This study was supported by a grant from National Heart, Lung, and Blood Institute (R01-HL074273).
Footnotes
Disclosure
The tissue and blood samples used in this study were from the same animals used in a paper published in Cardiovascular Research (Xu et al., 2008). The data presented in this paper are original and have not been published elsewhere.
References
- Adams V, Linke A, Krankel N, Erbs S, Gielen S, Mobius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation. 2005;111:555–562. doi: 10.1161/01.CIR.0000154560.88933.7E. [DOI] [PubMed] [Google Scholar]
- Braith RW, Welsch MA, Feigenbaum MS, Kluess HA, Pepine CJ. Neuroendocrine activation in heart failure is modified by endurance exercise training. J Am Coll Cardiol. 1999;34:1170–1175. doi: 10.1016/s0735-1097(99)00339-3. [DOI] [PubMed] [Google Scholar]
- Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–2518. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS, Moore RL. Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol. 2005;564:619–630. doi: 10.1113/jphysiol.2004.081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent MR, Aroutiounova N, Dhalla NS, Tappia PS. Losartan attenuates phospholipase C isozyme gene expression in hypertrophied hearts due to volume overload. J Cell Mol Med. 2006;10:470–479. doi: 10.1111/j.1582-4934.2006.tb00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- Ennezat PV, Malendowicz SL, Testa M, Colombo PC, Cohen-Solal A, Evans T, LeJemtel TH. Physical training in patients with chronic heart failure enhances the expression of genes encoding antioxidative enzymes. J Am Coll Cardiol. 2001;38:194–198. doi: 10.1016/s0735-1097(01)01321-3. [DOI] [PubMed] [Google Scholar]
- Erikssen G. Physical fitness and changes in mortality: the survival of the fittest. Sports Med. 2001;31:571–576. doi: 10.2165/00007256-200131080-00001. [DOI] [PubMed] [Google Scholar]
- French JP, Hamilton KL, Quindry JC, Lee Y, Upchurch PA, Powers SK. Exercise-induced protection against myocardial apoptosis and necrosis: MnSOD, calcium-handling proteins, and calpain. Faseb J. 2008;22:2862–2871. doi: 10.1096/fj.07-102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T, Yoshiyama M, Hanatani A, Omura T, Yoshikawa J, Abe Y. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem Biophys Res Commun. 2001;281:1200–1206. doi: 10.1006/bbrc.2001.4493. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115:3095–3102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, Mehta JL. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol. 2001;281:H1346–1352. doi: 10.1152/ajpheart.2001.281.3.H1346. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Staib JL, Phillips T, Hess A, Lennon SL, Powers SK. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med. 2003;34:800–809. doi: 10.1016/s0891-5849(02)01431-4. [DOI] [PubMed] [Google Scholar]
- Hill MF, Singal PK. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol. 1996;148:291–300. [PMC free article] [PubMed] [Google Scholar]
- Hill MF, Singal PK. Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation. 1997;96:2414–2420. doi: 10.1161/01.cir.96.7.2414. [DOI] [PubMed] [Google Scholar]
- Husain K, Hazelrigg SR. Oxidative injury due to chronic nitric oxide synthase inhibition in rat: effect of regular exercise on the heart. Biochim Biophys Acta. 2002;1587:75–82. doi: 10.1016/s0925-4439(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Khaper N, Kaur K, Li T, Farahmand F, Singal PK. Antioxidant enzyme gene expression in congestive heart failure following myocardial infarction. Mol Cell Biochem. 2003;251:9–15. [PubMed] [Google Scholar]
- Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ Res. 2000;87:392–398. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths M, Gore M, Ji LL. Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol. 1997;272:R363–369. doi: 10.1152/ajpregu.1997.272.1.R363. [DOI] [PubMed] [Google Scholar]
- Lennon SL, Quindry JC, Hamilton KL, French JP, Hughes J, Mehta JL, Powers SK. Elevated MnSOD is not required for exercise-induced cardioprotection against myocardial stunning. Am J Physiol Heart Circ Physiol. 2004;287:H975–980. doi: 10.1152/ajpheart.01208.2003. [DOI] [PubMed] [Google Scholar]
- Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, Mobius-Winkler S, Schubert A, Schuler G, Hambrecht R. Antioxidative effects of exercise training in patients with chronic heart failure: increase in radical scavenger enzyme activity in skeletal muscle. Circulation. 2005;111:1763–1770. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: A role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Quinn MT, Sun Y. Oxidative stress in the infarcted heart: role of de novo angiotensin II production. Biochem Biophys Res Commun. 2004;325:943–951. doi: 10.1016/j.bbrc.2004.10.106. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Egashira K, Machida Y, Hayashidani S, Takeya M, Utsumi H, Tsutsui H, Takeshita A. Probucol attenuates left ventricular dysfunction and remodeling in tachycardia-induced heart failure: roles of oxidative stress and inflammation. Circulation. 2002;106:362–367. doi: 10.1161/01.cir.0000021430.04195.51. [DOI] [PubMed] [Google Scholar]
- Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB, Chang PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE) Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Lawler J, Martin D, Lieu FK, Ji LL, Herb RA. Rigorous exercise training increases superoxide dismutase activity in ventricular myocardium. Am J Physiol. 1993;265:H2094–2098. doi: 10.1152/ajpheart.1993.265.6.H2094. [DOI] [PubMed] [Google Scholar]
- Raya TE, Fonken SJ, Lee RW, Daugherty S, Goldman S, Wong PC, Timmermans PB, Morkin E. Hemodynamic effects of direct angiotensin II blockade compared to converting enzyme inhibition in rat model of heart failure. Am J Hypertens. 1991;4:334S–340S. doi: 10.1093/ajh/4.4.334s. [DOI] [PubMed] [Google Scholar]
- Schieffer B, Wirger A, Meybrunn M, Seitz S, Holtz J, Riede UN, Drexler H. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. doi: 10.1161/01.cir.89.5.2273. [DOI] [PubMed] [Google Scholar]
- Shiomi T, Tsutsui H, Matsusaka H, Murakami K, Hayashidani S, Ikeuchi M, Wen J, Kubota T, Utsumi H, Takeshita A. Overexpression of glutathione peroxidase prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2004;109:544–549. doi: 10.1161/01.CIR.0000109701.77059.E9. [DOI] [PubMed] [Google Scholar]
- Sia YT, Lapointe N, Parker TG, Tsoporis JN, Deschepper CF, Calderone A, Pourdjabbar A, Jasmin JF, Sarrazin JF, Liu P, Adam A, Butany J, Rouleau JL. Beneficial effects of long-term use of the antioxidant probucol in heart failure in the rat. Circulation. 2002a;105:2549–2555. doi: 10.1161/01.cir.0000016721.84535.00. [DOI] [PubMed] [Google Scholar]
- Sia YT, Parker TG, Liu P, Tsoporis JN, Adam A, Rouleau JL. Improved post-myocardial infarction survival with probucol in rats: effects on left ventricular function, morphology, cardiac oxidative stress and cytokine expression. J Am Coll Cardiol. 2002b;39:148–156. doi: 10.1016/s0735-1097(01)01709-0. [DOI] [PubMed] [Google Scholar]
- Somani SM, Frank S, Rybak LP. Responses of antioxidant system to acute and trained exercise in rat heart subcellular fractions. Pharmacol Biochem Behav. 1995;51:627–634. doi: 10.1016/0091-3057(94)00427-k. [DOI] [PubMed] [Google Scholar]
- Sun Y, Weber KT. Angiotensin II receptor binding following myocardial infarction in the rat. Cardiovasc Res. 1994;28:1623–1628. doi: 10.1093/cvr/28.11.1623. [DOI] [PubMed] [Google Scholar]
- Wan W, Powers AS, Li J, Ji L, Erikson JM, Zhang JQ. Effect of post-myocardial infarction exercise training on the renin-angiotensin-aldosterone system and cardiac function. Am J Med Sci. 2007;334:265–273. doi: 10.1097/MAJ.0b013e318068b5ed. [DOI] [PubMed] [Google Scholar]
- Wang X, Sentex E, Saini HK, Chapman D, Dhalla NS. Upregulation of beta-adrenergic receptors in heart failure due to volume overload. Am J Physiol Heart Circ Physiol. 2005;289:H151–159. doi: 10.1152/ajpheart.00066.2005. [DOI] [PubMed] [Google Scholar]
- Xu X, Wan W, Ji L, Lao S, Powers AS, Zhao W, Erikson JM, Zhang JQ. Exercise training combined with angiotensin II receptor blockade limits post-infarct ventricular remodelling in rats. Cardiovasc Res. 2008;78:523–532. doi: 10.1093/cvr/cvn028. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189:1699–1706. doi: 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]