Abstract
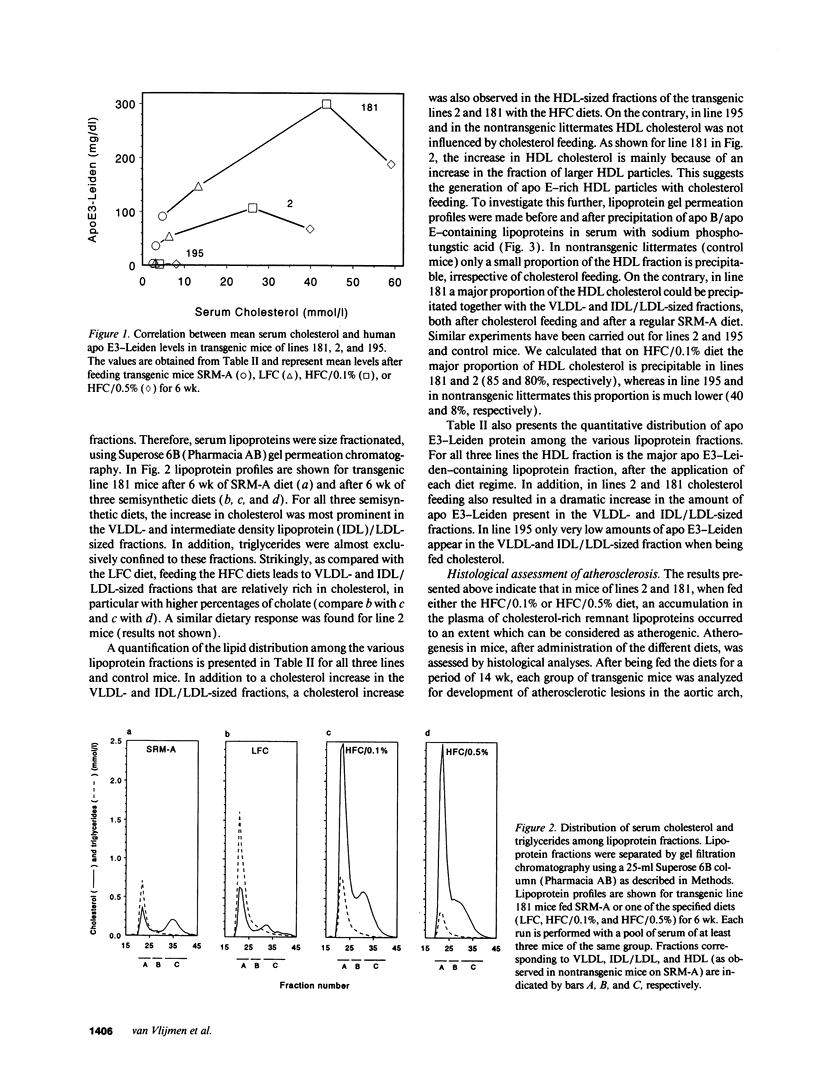
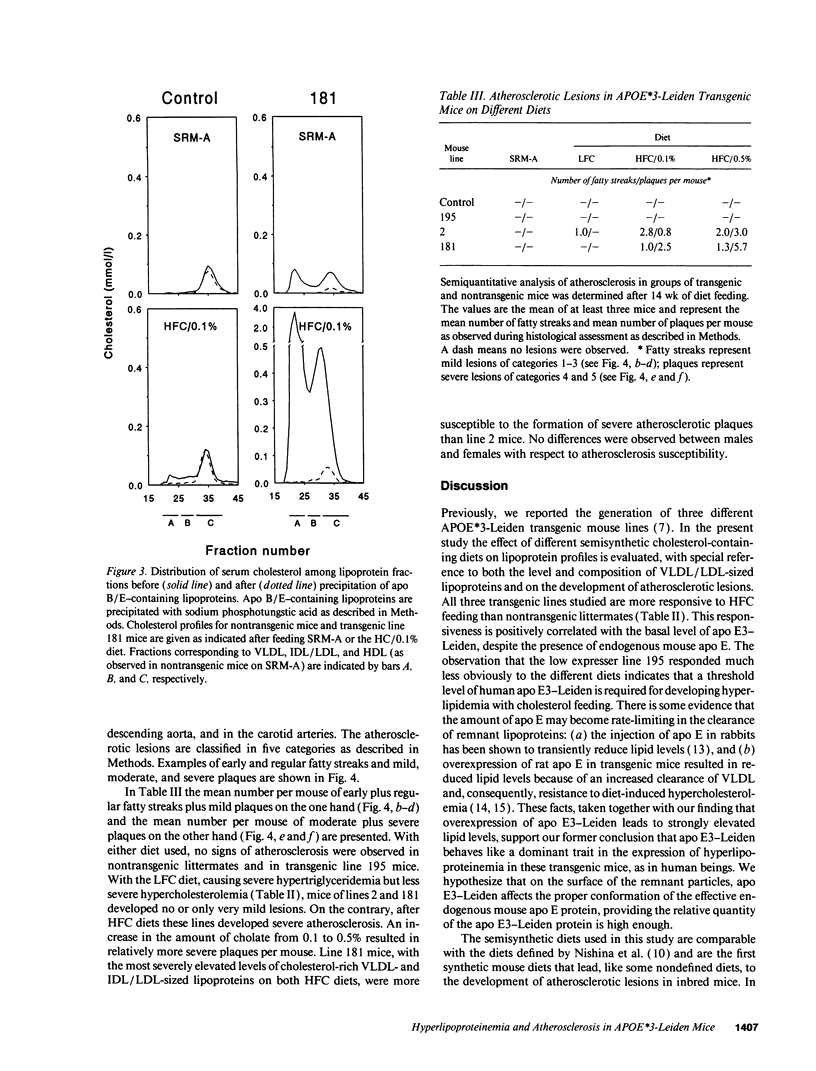
Apolipoprotein E3-Leiden (APOE*3-Leiden) transgenic mice have been used to study the effect of different cholesterol-containing diets on the remnant lipoprotein levels and composition and on the possible concurrent development of atherosclerotic plaques. On high fat/cholesterol (HFC) diet, the high expressing lines 2 and 181 developed severe hypercholesterolemia (up to 40 and 60 mmol/liter, respectively), whereas triglyceride levels remained almost normal when compared with regular mouse diet. The addition of cholate increased the hypercholesterolemic effect of this diet. In lines 2 and 181, serum levels of apo E3-Leiden also increased dramatically upon cholesterol feeding (up to 107 and 300 mg/dl, respectively). In these high expressing APOE*3-Leiden transgenic mice, the increase in both serum cholesterol and apo E3-Leiden occurred mainly in the VLDL/LDL-sized fractions, whereas a considerable increase in large, apo E-rich HDL particles also occurred. In contrast to the high expressing lines, the low expressing line 195 reacted only mildly upon HFC diet. On HFC diets, the high expresser APOE*3-Leiden mice developed atherosclerotic lesions in the aortic arch, the descending aorta, and the carotid arteries, varying from fatty streaks containing foam cells to severe atherosclerotic plaques containing cholesterol crystals, fibrosis, and necrotic calcified tissue. Quantitative evaluation revealed that the atherogenesis is positively correlated with the serum level of cholesterol-rich VLDL/LDL particles. In conclusion, with APOE*3-Leiden transgenic mice, factors can be studied that influence the metabolism of remnant VLDL and the development of atherosclerosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agellon L. B., Walsh A., Hayek T., Moulin P., Jiang X. C., Shelanski S. A., Breslow J. L., Tall A. R. Reduced high density lipoprotein cholesterol in human cholesteryl ester transfer protein transgenic mice. J Biol Chem. 1991 Jun 15;266(17):10796–10801. [PubMed] [Google Scholar]
- Grundy S. M., Denke M. A. Dietary influences on serum lipids and lipoproteins. J Lipid Res. 1990 Jul;31(7):1149–1172. [PubMed] [Google Scholar]
- Havel R. J., Chao Y., Windler E. E., Kotite L., Guo L. S. Isoprotein specificity in the hepatic uptake of apolipoprotein E and the pathogenesis of familial dysbetalipoproteinemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4349–4353. doi: 10.1073/pnas.77.7.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X. C., Agellon L. B., Walsh A., Breslow J. L., Tall A. Dietary cholesterol increases transcription of the human cholesteryl ester transfer protein gene in transgenic mice. Dependence on natural flanking sequences. J Clin Invest. 1992 Oct;90(4):1290–1295. doi: 10.1172/JCI115993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBoeuf R. C., Puppione D. L., Schumaker V. N., Lusis A. J. Genetic control of lipid transport in mice. I. Structural properties and polymorphisms of plasma lipoproteins. J Biol Chem. 1983 Apr 25;258(8):5063–5070. [PubMed] [Google Scholar]
- Lopes-Virella M. F., Stone P., Ellis S., Colwell J. A. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977 May;23(5):882–884. [PubMed] [Google Scholar]
- Lusis A. J., Taylor B. A., Quon D., Zollman S., LeBoeuf R. C. Genetic factors controlling structure and expression of apolipoproteins B and E in mice. J Biol Chem. 1987 Jun 5;262(16):7594–7604. [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Hussain M. M., Greenman B., Fisher M., Vogel T., Gorecki M. Intravenous infusion of apolipoprotein E accelerates clearance of plasma lipoproteins in rabbits. J Clin Invest. 1989 Jun;83(6):2125–2130. doi: 10.1172/JCI114126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Nishina P. M., Verstuyft J., Paigen B. Synthetic low and high fat diets for the study of atherosclerosis in the mouse. J Lipid Res. 1990 May;31(5):859–869. [PubMed] [Google Scholar]
- Paigen B., Holmes P. A., Mitchell D., Albee D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis. 1987 Apr;64(2-3):215–221. doi: 10.1016/0021-9150(87)90249-8. [DOI] [PubMed] [Google Scholar]
- Paigen B., Morrow A., Brandon C., Mitchell D., Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985 Oct;57(1):65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- Paigen B., Morrow A., Holmes P. A., Mitchell D., Williams R. A. Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis. 1987 Dec;68(3):231–240. doi: 10.1016/0021-9150(87)90202-4. [DOI] [PubMed] [Google Scholar]
- Piedrahita J. A., Zhang S. H., Hagaman J. R., Oliver P. M., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Shimano H., Yamada N., Katsuki M., Shimada M., Gotoda T., Harada K., Murase T., Fukazawa C., Takaku F., Yazaki Y. Overexpression of apolipoprotein E in transgenic mice: marked reduction in plasma lipoproteins except high density lipoprotein and resistance against diet-induced hypercholesterolemia. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1750–1754. doi: 10.1073/pnas.89.5.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H., Yamada N., Katsuki M., Yamamoto K., Gotoda T., Harada K., Shimada M., Yazaki Y. Plasma lipoprotein metabolism in transgenic mice overexpressing apolipoprotein E. Accelerated clearance of lipoproteins containing apolipoprotein B. J Clin Invest. 1992 Nov;90(5):2084–2091. doi: 10.1172/JCI116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet W. S., Bucay N., Lauer S. J., Wirak D. O., Stevens M. E., Weisgraber K. H., Pitas R. E., Taylor J. M. In the absence of a downstream element, the apolipoprotein E gene is expressed at high levels in kidneys of transgenic mice. J Biol Chem. 1990 Jul 5;265(19):10809–10812. [PubMed] [Google Scholar]
- Simonet W. S., Bucay N., Pitas R. E., Lauer S. J., Taylor J. M. Multiple tissue-specific elements control the apolipoprotein E/C-I gene locus in transgenic mice. J Biol Chem. 1991 May 15;266(14):8651–8654. [PubMed] [Google Scholar]
- Stewart-Phillips J. L., Lough J. Pathology of atherosclerosis in cholesterol-fed, susceptible mice. Atherosclerosis. 1991 Oct;90(2-3):211–218. doi: 10.1016/0021-9150(91)90117-l. [DOI] [PubMed] [Google Scholar]
- Warden C. H., Hedrick C. C., Qiao J. H., Castellani L. W., Lusis A. J. Atherosclerosis in transgenic mice overexpressing apolipoprotein A-II. Science. 1993 Jul 23;261(5120):469–472. doi: 10.1126/science.8332912. [DOI] [PubMed] [Google Scholar]
- Witztum J. L., Schonfeld G. Carbohydrate diet-induced changes in very low density lipoprotein composition and structure. Diabetes. 1978 Dec;27(12):1215–1229. doi: 10.2337/diab.27.12.1215. [DOI] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- de Knijff P., van den Maagdenberg A. M., Stalenhoef A. F., Leuven J. A., Demacker P. N., Kuyt L. P., Frants R. R., Havekes L. M. Familial dysbetalipoproteinemia associated with apolipoprotein E3-Leiden in an extended multigeneration pedigree. J Clin Invest. 1991 Aug;88(2):643–655. doi: 10.1172/JCI115349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Maagdenberg A. M., Hofker M. H., Krimpenfort P. J., de Bruijn I., van Vlijmen B., van der Boom H., Havekes L. M., Frants R. R. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J Biol Chem. 1993 May 15;268(14):10540–10545. [PubMed] [Google Scholar]




