Abstract
Objectives
The purpose of this review is to summarize physiological and psychological characteristics that are common among women diagnosed with polycystic ovary syndrome (PCOS) and provide evidence suggesting that addressing psychological disturbances can reduce or alleviate physical symptoms of PCOS via behavioral pathways and physiological pathways.
Methods
Empirical studies and expert consensuses pertaining to physiological, psychological and medical management aspects of PCOS were identified and presented in this review. Papers were identified via searching Pubmed, PsycInfo, Medline ISI, CINAHL, or a web browser (i.e., Google) using numerous combinations of terms pertaining to physiological, psychological, and medical management aspects of PCOS. A paper was chosen to be included in this review if it reported findings and/or provided information that related to and helped support the main purpose(s) of this review paper.
Results
Available literature on the physiological (i.e., hyperandrogenism, central obesity, inflammation, insulin resistance) and psychological (i.e., depression, anxiety, eating disorders) factors among women with PCOS provides evidence that these various aspects of PCOS are strongly inter-related.
Discussion
The existence of these relationships among physiological and psychological factors strongly suggests that medical management of PCOS would greatly benefit from inclusion of psychological and behavioral approaches.
Keywords: polycystic ovary syndrome, inflammation, insulin resistance, hyperandrogenism, depression, stress management, visceral fat
Polycystic ovary syndrome characteristics
Polycystic ovary syndrome (PCOS) is a relatively common endocrine disorder among women of reproductive age and one of the most common causes of female infertility. Prevalence of PCOS in the United States as defined by the 1990 NIH criteria is between 6.5% and 8% (1). In addition to infertility, several physiological (i.e., insulin resistance, inflammation, visceral fat, infertility) and psychological (i.e., depression, anxiety, body image, social anxieties) abnormalities are common among women with PCOS, all of which will be discussed herein. Further, physiological mechanisms which connect these phenomena will be explained. This paper will summarize evidence suggesting that addressing psychological disturbances can reduce or alleviate physical symptoms of PCOS via not only behavioral pathways (i.e., increased exercise, smoking cessation), but also mood- and stress-related physiological pathways. The articles included in this review were identified in Pubmed, Medline ISI, PsycInfo, CINAHL, or by searching via a web browser (i.e., Google). Articles were reviewed if they were identified when searching using numerous combinations of two or more of the following terms: polycystic ovary syndrome, inflammation, heart disease, insulin resistance, psychological disorders, depression, anxiety, adolescents, intervention, trial, cognitive behavioral, exercise, physical activity, fertility, hyperandrogenism, testosterone, visceral fat, sleep disorders, cortisol, weight loss, diet, stress management, metformin, cost, psychotherapy. A paper was chosen to be included in this review if it reported findings and/or provided information which related to and/or helped support the main purpose(s) of this review paper.
Three key features characterize PCOS, including hyperandrogenism, chronic anovulation, and polycystic ovaries. Hyperandrogenism, perhaps the most consistent and obvious diagnostic feature of PCOS, is assessed clinically by acne, hirsutism, and alopecia, and also by biochemical indices, including free testosterone and sex hormone binding globulin (SHBG). These clinical characteristics and biochemical indices can vary greatly among individuals depending on many factors including ethnicity, body mass index (BMI), and age. Partly due to PCOS definition, 60% to 80% of women with PCOS have abnormally elevated circulating testosterone (2).
The direct cause of PCOS remains unknown; both environmental and genetic factors are implicated. Aggregate evidence suggests that hypothalamic-pituitary-adrenal (HPA) axis defects, insulin activity abnormalities [i.e., insulin resistance in skeletal muscle tissue but insulin sensitivity in adrenal and ovary tissue (3)], and genetic influences (4, 5) interact to set the stage for possible development of PCOS(6, 7). The fact that its clinical presentation varies so much among individuals is likely due to interactions of genetic and environmental factors.
Physiological Regulatory Problems and Diseases Common in PCOS
Insulin Resistance
Numerous studies have documented that insulin resistance is common in both obese and lean women with PCOS (8). In fact, 70% of women with PCOS are insulin resistant (9), and prospective clinical studies among women with PCOS in the U.S. have revealed a 7.5% to 10% prevalence of type 2 diabetes mellitus compared to a 0.7% prevalence of type 2 diabetes in healthy young women (10). Furthermore, the conversion rate from impaired glucose tolerance to frank diabetes is 5- to 10-fold higher in women with PCOS compared with non-PCOS women in both the U.S. and Australia (11).
The hyperinsulinemic state present in most women with PCOS appears to play a central role in PCOS development and is considered to be the cause rather than the result of hyperandrogenism (12). Studies supporting this hypothesis reveal that antiandrogen therapy does not improve insulin resistance (13), while insulin-sensitizing agents (i.e., metformin) not only improve insulin sensitivity but also improve menstrual abnormalities and reproductive outcomes in women with PCOS, providing indirect evidence of reduced hyperandrogenism (14). Insulin resistance appears to contribute to hyperandrogenism and other gonadotropin abnormalities via at least two mechanisms. First, high concentrations of insulin reduce circulating SHBG levels, resulting in increased bioavailable (free) testosterone, as less SHBG is available to bind with testosterone (15). Second, high insulin concentrations also stimulate androgen biosynthesis from ovaries (16).
Obesity
A common correlate of insulin resistance, obesity, is prevalent in women with PCOS. It is important to emphasize that visceral, rather than subcutaneous, fat is a defining characteristic of PCOS (17), as visceral fat plays an important role in the pro-inflammatory response (described herein). Further, utilizing body mass index (BMI) to determine health status may be misleading, as women with PCOS have greater total and proportion of lean muscle mass per height than do controls (18). Surprisingly, given that lean muscle mass may be higher in PCOS women and muscle tissue has relatively high caloric demands, women with PCOS appear to have a significantly lower basal metabolic rate than do age- and BMI-controls; 1446 kcal/day and 1841 kcal/day, respectively (19).
The presence of obesity substantially affects the development and expression of PCOS. For example, it is possible that a woman may have polycystic ovaries (PCO) but not meet diagnostic criteria for PCOS, as obesity often contributes to development of other aspects of the syndrome (i.e., menstrual irregularity, hirsutism) (20). In fact, PCO is present in approximately 20% of all women and many of these women do not display other syndrome features, (i.e., hirsutism, irregular menstrual cycles, and elevated testosterone), which likely would appear if a PCO woman were to become overweight or obese (21). Prevention of obesity, then, is paramount in order to prevent a commensurate increase in the incidence of PCOS and its associated physiological abnormalities, especially during childhood and pre-adolescent years.
Endocrine abnormalities
In addition to insulin, other hormones related to obesity are cholecystokinin, the “satiety hormone,” and cortisol. Interestingly, secretion of cholecystokinin is reduced among women with PCOS, which causes abnormal appetite regulation, possibly leading to overeating and subsequent obesity (22). Likely partially due to obesity but also seemingly inherent in women with PCOS are cortisol level abnormalities; specifically, an increase of peripheral cortisol metabolism due to abnormal levels of certain enzymes that metabolize cortisol (i.e., 5-alpha reductase, 11 beta-hydroxysteroid dehydrogenase) (23–25). This increase in cortisol metabolism may result in a decreased negative feedback signal to the HPA axis, thereby maintaining high production of ACTH and, consequently, increased production of adrenal androgens, which exacerbates PCOS symptoms (12). Evidence of HPA axis abnormalities among women with PCOS have been demonstrated in several studies, and these abnormalities cannot necessarily be attributed to body fat distribution, obesity, or androgen or insulin levels (26, 27).
Cardiovascular disease risk factors
Several studies have shown that women with PCOS and even PCO have increased cardiovascular disease (CVD) risk factors compared with age-matched controls, including hypertension, dyslipidemia, endothelial dysfunction, reduced vascular compliance, and atherosclerosis (8, 28–30). Christian et al. (31) showed that PCOS women had a 2-fold higher level of coronary artery calcification than obese controls, and Talbott et al. (32) showed that middle-aged women with PCOS had thicker carotid intima-media than controls; markers, respectively, of subclinical coronary artery disease and risk for future events.
Inflammation
The above CVD risk factors could be the result of increased inflammation among women with PCO or PCOS. One study found that C-Reactive Protein (CRP), an inflammatory marker that has been shown to predict cardiovascular events in previously healthy women (33), was significantly higher in women with PCOS and PCO than in controls and this difference could not be attributed to age, BMI, waist-to-hip ratio, and lipid profile (34). Another study found that IL-18 was higher in lean and obese PCOS participants compared to controls matched for BMI and smoking status (35).
Psychological Problems Common in PCOS
Several studies have shown that emotional disturbances are more common in women with PCOS than among women without PCOS. The prevalence of depression in women with PCOS has been reported to be as high as 40% (36), and a nationwide internet survey in Germany that included 448 women with PCOS revealed a clinical depression prevalence rate of 21% (37). Another study revealed that 23.9% and 25.2% of women with PCOS scored in the mild to moderate and clinically relevant ranges of depression on the Beck Depression Inventory (BDI), respectively. In this sample, the average BDI score was 12.7, significantly higher than scores for a representative normative sample (38). Similarly, another study found that the BDI score among women with PCOS was significantly higher than that of controls (11.69 versus 5.80, respectively) (39). Further, PCOS women also are more likely than non-PCOS women to suffer from social withdrawal (40), eating disorders (40), and anxiety disorders (41), with one study reporting that 34% of women with PCOS have clinically significant anxiety (37).
Since much research has shown that obesity and depression are positively associated in the general population (42) and given that women with PCOS generally are more obese than non-PCOS women, it could be hypothesized that obesity may account for emotional disturbances in women with PCOS, as suggested in a recent review of emotional disorders and their impact on quality of life in PCOS (43). One study that diagnosed depression based on a semi-structured clinical interview, however, revealed that the significantly higher number of single and recurrent major depressive episodes and suicide attempts among PCOS women versus controls was not accounted for by BMI (40). Similarly, another study found that obese women with PCOS had a substantially greater risk of developing depressive disorders than did obese controls (44% versus 7%), suggesting again that women with PCOS may be at greater risk for depression than non-PCOS women, regardless of obesity (41).
Further, it could be argued that other common aspects of PCOS, such as infertility, hirsutism, acne, and body dissatisfaction could account for emotional disturbances in women with PCOS. Some studies have shown associations between infertility and emotional disturbances (44, 45), whereas others have not (36, 38). Among these studies, Tan et al. (38) was the only one that utilized a valid questionnaire to assess feelings related to infertility. Similarly, some studies among both PCOS and healthy subjects have shown that hirsutism per se (46) and acne (47) are associated with anxiety and greater psychotic symptoms; other studies, however, have not found these characteristics to correlate with emotional distress (36, 48). These studies are quite different, as Mallon et al. (47) included male and female participants whose acne resulted from numerous varied endocrine abnormalities. Further, the average age of the participants greatly differed among Sonino et al. (46), Keegan et al.(48), and Kerchner et al. (36); 22, 30, and 32 years old, respectively, suggesting that younger women are more likely than older women to become emotionally affected by their appearance. Unfortunately, PCOS emerges just as young women become interested in dating, and body dissatisfaction may affect one's emotional development by interfering with healthy establishment of a social and dating life (49). At this juncture, it is not entirely clear exactly how, or if, clinical manifestations of PCOS affect emotional disturbances. It is very important, then, to be careful not to attribute depressed mood to clinical symptoms inherent in PCOS and to address depression regardless of clinical symptoms.
In summary, women with PCOS appear to be at elevated risk for depression, eating and anxiety disorders, and suicide attempts, which cannot necessarily be accounted for by obesity or distressing PCOS symptoms such as infertility, hirsutism, and acne.
Relationships Between Physiological and Psychological Phenomena in PCOS
As previously discussed, women with PCOS frequently are insulin resistant, have elevated inflammatory markers and endocrine abnormalities, and report higher rates and greater severity of depression and other emotional disturbances than women without PCOS. Insulin resistance, inflammation, endocrine abnormalities, and depression all appear to be related and, due to their interrelationships and sequelae (i.e., obesity, infertility, CVD), are especially problematic among women with PCOS. As the purpose of this paper is to propose that treatment of psychological disturbances can alleviate or reduce physical symptoms of PCOS via not only behavioral pathways but also physiological pathways, the following section will describe relationships among emotional disturbances, insulin resistance, inflammation, and endocrine abnormalities that are relevant to PCOS.
Emotional disturbances such as depression and chronic stress are associated with certain physiological changes such as increased immune system activity and pro-inflammatory markers, which increase one's risk for eventual development of chronic disorders such as cardiovascular disease, diabetes, and cancer (50). Correlational studies reveal consistent, robust associations between levels of Interleukin-6 (IL-6) and depression irrespective of the presence of any physical disease (50, 51). Some evidence suggests strong associations between depression and other proinflammatory cytokines such as Tumor Necrosis Factor-alpha (TNF-α) and Interleukin-1β (IL-1β) (52, 53). Prospective studies employing pharmaceutical treatment illustrate the relationship between mood and inflammation. For example, antidepressants have been shown to inhibit pro-inflammatory cytokine production both in vitro and in vivo (54). Conversely, COX-2 inhibitors, which are potent anti-inflammatory agents, have been shown to supplement the effects of the antidepressant reboxetine in treating patients with major depression (55), further illustrating the strong relationship between treatments directed at reducing both inflammation and depression. Prospective studies that test whether chronic psychological stress causes inflammation have yielded inconsistent results in humans (56), though in animals they have demonstrated that chronic mild stress induces depression-like syndromes and concomitant increases in proinflammatory cytokines (57).
The interactions among mood, behavior, and inflammation often manifest in a cluster of symptoms referred to as “sickness behavior” including depressed mood, anhedonia, fatigue, psychomotor retardation, decreased appetite, social withdrawal, sleep disturbances, cognitive dysfunction, and increased sensitivity to pain (58, 59). These symptoms generally are exacerbated when inflammation increases. The relations among these symptoms and inflammation are so robust that even exogenous administration of pro-inflammatory cytokines reliably produces sickness behavior symptoms (60). Possibly due to chronically elevated inflammatory markers, PCOS women exhibit some sickness behavior symptoms, including fatigue, depressed mood, social withdrawal (40), and sleep disturbances.
A mechanism by which inflammation might affect mood and induce “sickness behavior” is that cytokines have been found to spur degeneration of the blood-brain-barrier (61), thereby allowing easy entry of inflammatory cells into and cytokine production within the central nervous system (CNS). Once in the brain, evidence suggests that inflammatory cytokines may affect mood by changing the levels of certain neurotransmitters which are implicated in depression etiology; specifically, via causing a serotonin deficiency (62) and excessive and sustained norepinephrine secretion (63). A disease model that nicely illustrates this mechanism is multiple sclerosis. This disorder is characterized by CNS changes that may be exacerbated by proinflammatory cytokine perfusion of the CNS (64) and cytokine production by CNS microglia and astrocytes (65). Not so coincidentally, multiple sclerosis is also famously characterized by a high comorbidity of depression in humans (66) and in animal models, by numerous symptoms of “sickness behavior” (67). Elevated pro-inflammatory cytokines and other inflammatory markers may also explain why women with PCOS report a variety of “sickness behavior” symptoms, including increased fatigue and a greater number and variety of sleep disturbances compared to women without PCOS. Compared with controls, women with PCOS are much more likely to suffer from sleep disordered breathing, report more daytime sleepiness and fatigue (68) and report greater difficulty falling asleep, which may be due to a greater incidence of anxiety, known to interfere with sleep onset (69). Alleviating sleep disorders among women with PCOS might not only improve sleep quality but also alleviate daytime fatigue and depressed mood; thereby affecting health behavior. An emerging literature is revealing that improvement of sleep quality may also affect obesity and inflammation (70, 71), though a thorough discussion of this literature is beyond the scope of this paper.
In addition to being associated with increased inflammatory markers, it is well-documented that depression is related to abnormal (often elevated) cortisol secretion (72). Women with PCOS generally display cortisol secretion abnormalities regardless of mood. In one study, emotionally healthy women with and without PCOS were administered the Stroop Color Word Test, which causes a considerable amount of stress in healthy women (73). They found that although women with PCOS did not differ from controls in the cognitive response to the stressor, the Stroop test caused a rise in cortisol levels among women with PCOS that was not observed in the control group (74). Also during the Stroop test systolic blood pressure values were significantly higher among PCOS women versus controls, showing evidence that sympathetic nervous system activity is more adversely affected by stress in the PCOS woman. Another study that employed a stressful task (i.e., public speaking) similarly found no differences between PCOS women and controls in self-reported emotional response to the task. However, while cortisol, ACTH, and heart rate rose significantly in response to the stressor among all participants, cortisol and heart rate rose significantly more among PCOS women compared with controls (75). These findings were independent of whether women were receiving the insulin sensitizer metformin, suggesting that pharmacological management alone may not adequately treat some endocrine and cardiovascular abnormalities among women with PCOS.
Cortisol is secreted in response to stressful stimuli in all individuals, and contributes to increased visceral fat (76) and increased inflammation (77). This mechanism could be especially problematic in woman with PCOS since they have more visceral fat and higher inflammatory markers than normal women, and cortisol secretion contributes to hyperandrogenism. Further, a very provocative study reported that lean women with a high waist-to-hip ratio (WHR) secrete more cortisol following both novel and familiar cognitive laboratory stressors than do lean women with a low WHR (78). These results provide evidence that women with a high WHR, unlike those with a lower WHR, tend not to adapt even to familiar stressors, suggesting that they are much more likely to experience elevated cortisol—and, consequently, increased visceral fat—in response to commonly faced stressors. These researchers speculate that maladaptive psychological characteristics such as pessimism, negative affect, passive coping, and greater threat perception may play a role in the bidirectional relationship between cortisol and visceral fat among women with higher WHR.
Thus, the above studies not only illustrate cortisol secretion abnormalities among women with PCOS, but also underscore the importance of reducing stress and other maladaptive psychological characteristics in the PCOS woman so as to reduce, as much as possible, an elevated cortisol response and subsequent visceral fat and hyperandrogenism. Since treatment with metformin may not affect stress reactivity, stress reduction interventions may be important adjunctive approaches when treating women with PCOS.
Another hormone generally elevated among women with PCOS, testosterone, may contribute to increased sympathetic nervous system (SNS) activity. A very interesting study found that exogenous testosterone administration among healthy young women induced cardiac acceleration in response to images of angry faces (79). Since the majority of women with PCOS have elevated testosterone, it is likely that women with PCOS will have an exaggerated sympathetic nervous system response to anger and other negative affect, which may exacerbate inflammation (80). These findings underscore the importance of stress management interventions among PCOS women so as to reduce the adverse physiological changes which can result from psychological stress.
Persistent negative affect and exaggerated SNS activity, then, may exist in the PCOS woman partly due to elevated testosterone alone. The relationship between testosterone and mood, however, is not entirely clear, as one study demonstrated that there is a curvilinear relationship between testosterone levels and depression in women with and without PCOS such that the most severe depression was associated with levels below and above the normal female range of testosterone (81).
Negative affect and SNS activation may adversely affect these women by contributing to cortisol abnormalities and chronically elevated inflammation. Normally, inflammation is down-regulated by cortisol, but states of prolonged inflammation (likely to exist in PCOS) result in a phenomenon known as glucocorticoid resistance. In this state, glucocorticoids are no longer able to suppress the production of pro-inflammatory markers (82). Excess circulating glucocorticoids resulting from glucocorticoid resistance are associated with insulin resistance (83). Further, glucocorticoid resistance is a mechanism that may explain the well-established relationship between insulin resistance and depression (84, 85).
As previously discussed, it has been hypothesized that insulin resistance is the underlying cause in the complicated etiology of PCOS; that depression and sleep disorders are quite common among women with PCOS; and women with PCOS have seemingly-inherent chronic inflammation (thereby rendering them susceptible to glucocorticoid resistance). Further, women with PCOS display enhanced sympathetic nervous system and HPA axis activity in response to stressors, which may contribute to increased visceral fat and subsequent inflammation. Interrelations among these abnormalities may create a negative spiral leading to greater insulin resistance and subsequent hyperandrogenism, exacerbating clinical symptoms such as infertility, acne, and hirsutism, and possibly negative affect as well.
Standard Medical Care of PCOS
Current standard medical care of women with PCOS depends on the goal(s) of therapy. Often, the primary goal is ovulation induction and eventual successful pregnancy. Secondary goals include management of obesity and insulin resistance so as to prevent development of diabetes and CVD. According to the most recent Consensus on Infertility Treatment related to PCOS, counseling related to lifestyle changes (i.e., weight loss, exercise, smoking, and alcohol consumption) should precede pharmacological interventions (86). Following lifestyle counseling, the first-line treatment for ovulation induction is clomiphene citrate (CC); should this fail, second-line treatment is either exogenous gonadotrophins or laparoscopic ovarian surgery. Use of metformin as adjunctive to CC has been shown in some studies to be more effective than CC alone (87, 88), but its use as adjunctive therapy both prior to and during pregnancy continues to be debated, and it is not yet approved by the Food and Drug Administration for this purpose (89). The Consensus recommends that metformin use be restricted to those who are glucose intolerant and cautions that it should not be used routinely for ovulation induction (86). If the objectives of treatment include management of obesity, insulin resistance, and clinical symptoms (i.e., acne, irregular menses), metformin and/or estrogen-progestin contraceptives generally are effective (88).
Behavioral and Psychological Interventions as Adjunctive Treatment
Much empirical evidence shows that psychosocial factors are related to physiological changes among healthy individuals and women with PCOS, often affecting fertility, and some clinicians have asserted that attending to psychosocial health should be an aspect of managing PCOS (89). Giallauria and colleagues agree that a more comprehensive, long-term approach should be adopted so as to improve medical management of PCOS (90). The most recent publication on infertility treatment guidelines among women with PCOS, however, did not address psychosocial problems, even in the context of achieving weight loss (86). Further, treatment of psychosocial factors is not discussed on frequently accessed public websites such as mayoclinic.com or patient.co.uk (91, 92). It appears, then, despite mounting evidence that improving one's lifestyle and psychological well-being can improve several physiological parameters, guidelines related to lifestyle and psychological well-being in women with PCOS do not yet pervade private and public sectors. Evidence supporting suggestions for behavioral and psychological interventions as adjunctive treatment are discussed below.
Weight loss
Current treatment guidelines do indeed emphasize that weight loss is a crucial aspect of treating PCOS, not only to help induce ovulation and eventual pregnancy but also to reduce the possibility of developing chronic diseases associated with obesity that are prevalent in PCOS (i.e., diabetes, CVD) (89). Weight loss may indirectly affect mood as well, as even a small (i.e., 2% to 5%) reduction in weight greatly improves metabolic and menstrual cycle abnormalities (93, 94) and significantly reduces visceral fat mass (95). Such changes may reduce inflammation, which is associated with mood. Women who have lost weight in short-term weight loss studies show decreased abdominal fat, fewer symptoms of hyperandrogenism, decreased insulin resistance, decreased serum insulin levels, improved lipid profiles, and improvements in menstrual cycle regularity (89). Weight loss increases SHBG concentration, thus reducing bioavailable testosterone levels and some PCOS symptoms (96).
Exercise
Even in the absence of significant weight loss, regular exercise appears to engender improvements in ovulation rate and likelihood of pregnancy; the primary goals of PCOS management. In one study, a 6-month lifestyle program intervention including both diet and exercise counseling restored normal menstrual cycles in 60% of anovulatory women with PCOS (97). Interestingly, in this study, despite no significant change in BMI, participants whose cycles resumed after the intervention achieved a 71% improvement in insulin sensitivity, while insulin resistance did not improve in those participants who remained anovulatory following the intervention. There also is evidence that exercise alone (without concurrent changes in other lifestyle behaviors) improves these primary clinical goals. For example, menstrual frequency and ovulation rate improved significantly more in a group of PCOS women following a 24-week exercise intervention compared with those in a diet intervention group (98). Similarly to the previous study, insulin resistance decreased significantly more in the group for whom ovulation resumed compared to those who remained anovulatory following the intervention. Additionally in this study, insulin resistance improved significantly more in the exercise group compared with the diet group, but only among those for whom ovulation resumed. This change in insulin resistance was not accounted for by BMI, as BMI actually reduced significantly more in the diet group than the exercise group. Other studies show improvements in insulin resistance following exercise training in the absence of significant weight loss. For example, one recent study including women with PCOS reported that insulin resistance marginally significantly improved following a moderate-intensity 8- to 12-week exercise training intervention even though BMI did not change significantly (99).
Taking together the results of the above studies, researchers assert that improvements in insulin resistance alone pave the path toward the improved fertility observed in these studies. Exercise is related not only to overall weight loss but also, and more importantly, to visceral fat reduction even if weight loss is modest, thereby greatly affecting insulin resistance and other biological parameters since visceral fat is more metabolically active than subcutaneous fat. Further, increased exercise may improve insulin resistance via increased muscle mass, as muscle contractions stimulate glucose uptake in the absence of insulin (100). This mechanism is illustrated by a study that induced PCOS in rats and randomly assigned them to control and exercise groups (consisting of 4 to 5 weeks of free access to a wheel) and found that those in the exercise condition had significantly lower insulin resistance than the PCOS controls (101). Interestingly, the soleus muscle weight, relative to overall body weight, was higher in the PCOS exercise rats compared with the PCOS controls. One can speculate that this relative increase in muscle mass and subsequent increase in muscle contractions plays a role in the insulin resistance improvement observed in this study following the exercise intervention.
Given the established relationship between insulin resistance and hyperandrogenism, one might expect commensurate improvements in androgen levels in the above studies. Surprisingly, in one of the studies, none of total or free testosterone or SHBG levels differed between the group of women whose ovulation resumed and those who remained anovulatory (99). In another study, however, total and free testosterone were significantly reduced among those whose ovulation resumed versus the anovulatory participants, and percent free testosterone reduced significantly more in the exercise group than the diet group, suggesting that the insulin resistance improvements in the exercise group may be related to reduced free testosterone (98). At this juncture, given these conflicting results, the nature and strength of a relationship among androgens, fertility, and insulin resistance is unclear. Also unclear is the effect of reduced androgen levels on its clinical manifestations. Interestingly, even when testosterone decreased, hirsutism did not change significantly, regardless of ovulation status or type of intervention (i.e., exercise or diet) (98), suggesting that neither androgen levels nor insulin resistance is related to this particular clinical manifestation of hyperandrogenism. Also noteworthy is the finding that neither the diet nor exercise intervention affected any parameter (i.e., anthropometric, hormonal, or metabolic) for those who remained anovulatory (98), suggesting that advantageous changes in body composition, androgen levels, and/or metabolic profile are necessary in order to achieve ovulation in initially anovulatory women with PCOS.
Exercise also improves inflammatory profiles independently of improvements in obesity indices. Broadly among the general population, a large-scale study of over 700 healthy men (The Aerobics Center Longitudinal Study) reported a significant inverse relationship between CRP and fitness level, which was assessed by a maximal exercise test on a treadmill (102). This relationship was independent of BMI, percent body fat, and waist circumference. Another study including patients with stable coronary artery disease revealed significantly reduced CRP levels (by 48%) after a 12-week exercise training program in the absence of a significant BMI reduction (103). Among women with PCOS, exercise training also appears to reduce inflammation. Following a 3-month moderate-intensity exercise program, the average CRP level in the exercise group was significantly lower than that in the control group (104). In this study, though, the exercise group also showed improvements in BMI, waist-to-hip ratio, and insulin resistance, so one cannot be sure that exercise per se influenced CRP. One study providing evidence for a more direct, robust effect of exercise on inflammation revealed that IL-6 gene expression in mesenteric adipose tissue was down-regulated following 4 to 5 weeks of free access to a wheel among PCOS-induced rats compared to the PCOS-induced control rats, but it was not reported if other changes after exercise (i.e., reduced body weight, reduced fat depots) were related to this finding (101).
Regarding psychological parameters, regular exercise appears to improve body image independently of weight loss. In one study, previously non-exercising women with PCOS endorsed significantly fewer body dysmorphic symptoms after six months of self-reported regular (i.e., 3 days a week) moderate exercise compared to their baseline body dysmorphic symptomatology (105). This study provides evidence that exercise may contribute to improved psychological functioning directly, and not simply via established physiological improvements (i.e., weight loss, fertility).
In conclusion, though the effect of exercise on androgen levels and clinical manifestations of hyperandrogenism (i.e., hirsutism)is unclear at this time, several studies suggest that regular physical activity, irrespective of anthropometric changes, improves insulin sensitivity, body image, and the PCOS management primary goal endpoints of increased ovulation and fertility rates. Given these and other benefits of exercise, fostering an interest in exercise during childhood may be especially important and effective in reducing the likelihood of PCOS emergence. To this end, fortunately it appears that young girls who are predisposed to develop PCOS are more likely than other girls to participate in less traditionally-feminine behaviors, which may include physical activity. In fact, one study reported that women with PCOS retrospectively endorsed interest and engagement in significantly more masculine behaviors (i.e., preferring more masculine toys and games, having a higher activity level than other girls) during their childhood than women without PCOS (106). This study's finding should be interpreted as an encouraging sign that a young girl's demonstration of an early interest in masculine behaviors could be fostered, with hope that interest in these behaviors (especially physical activity) might be maintained throughout adulthood, thereby reducing the probability that she will develop PCOS.
Diet changes
Changes in diet may also affect physiological parameters and mood, even in the absence of weight loss or exercise. In one study (107), women with PCOS who were randomly assigned to adhere to a low-carbohydrate high-protein diet for 16 weeks showed significant improvements in depression and self-esteem, whereas there were no such changes in the group randomly assigned to consume a high-carbohydrate low-protein diet. The groups did not differ in weight loss or BMI reduction. A possible physiological explanation for these findings is that dietary protein enriched in tryptophan might increase brain serotonin, thereby enhancing coping skills and subsequent mood (108).
The results from the studies showing improvements in mood due to both weight loss and eating a high-protein low-carbohydrate diet are echoed in in vitro studies which suggest that pro-inflammatory marker secretion could be greater in PCOS versus non-PCOS women following a high-carbohydrate meal. For example, Gonzalez, Rote, Minium, and Kirwan showed that TNF-α release from mononuclear cells (MNC) under euglycemic conditions (5 mM) did not differ between PCOS women and controls (109). However, when MNC were exposed to a 10 mM glucose concentration (which is similar to a postprandial state), the change in TNF-α release in PCOS women increased significantly compared with that of controls. Further, these researchers showed that both lean and obese women with PCOS had a significantly greater percent change in reactive oxygen species (ROS) generation from MNC after induced hyperglycemia than did lean controls (110). Testosterone was positively correlated with the percent change in ROS generation from MNC for all groups, suggesting that testosterone and inflammation are related. These data also show that hyperglycemia stimulates ROS generation from MNC of women with PCOS independent of obesity, as lean women with PCOS exhibited greater ROS generation from MNC than lean controls. Results from the above studies suggest that hyperglycemia (perhaps from eating carbohydrates) is inherently disadvantageous with regard to inflammation among women with PCOS compared with controls and diet potentially may affect mood via inflammation.
In summary, lifestyle changes (i.e., weight loss, exercise, diet) can significantly improve several physiological indices of women with PCOS, including visceral fat, inflammation, insulin resistance and—most importantly—ovulation rate. Lifestyle changes and psychological health appear to have a bidirectional relationship in that individuals who are more psychologically healthy are more likely to maintain a healthy lifestyle and optimal self-care (111–114), and lifestyle changes may contribute to a positive mood.
Stress and Mood Management
Women with PCOS are likely to exhibit exaggerated SNS responses and HPA-axis abnormalities to negative stimuli, suggesting that teaching them techniques to better manage stressors may offer benefits. In addition to improving mood and decreasing stress, cognitive behavioral approaches, including stress management interventions, appear in other populations to be capable of normalizing HPA axis regulation (115–117) as well as lowering SNS activity (118). As such, techniques such as relaxation and cognitive behavioral therapy directed at better managing stress may be used to address the cortisol secretion abnormalities often present in PCOS women, especially since standard treatment with metformin does not appear to affect physiological responses to stress (75). If so, stress management approaches may also have the secondary effects of reducing hyperandrogenism, as has been demonstrated in women with other conditions (119). Very little research has employed cognitive behavioral therapy (CBT) or other psychological interventions among women with PCOS. The only such study published to date is a pilot study conducted among adolescents with PCOS (120). In this study, an 8-week CBT intervention resulted in significant decreases in weight and depressive symptoms and significant improvements in menstrual regularity and sleep-related breathing. Much more research investigating the effectiveness of CBT interventions among this population is needed, but this small study provides some evidence that these approaches are promising.
Among the general population, CBT interventions have helped individuals achieve changes such as reductions in BMI, percent fat, waist circumference, lipids, and caloric intake (121–123). Given the well-known challenge of achieving significant weight loss, especially among those who are insulin resistant, CBT interventions might be particularly salient to employ among women with PCOS.
Conclusions
In deciding between pursuing a pharmacologic versus a psychological intervention approach to managing PCOS a very reasonable question is: Why support a psychological intervention in lieu of a pharmacological intervention, such as prescribing metformin to women with PCOS? In fact, a study demonstrated that participants with PCOS who adhered to metformin for 6 months showed improvements in several psychiatric and physiological parameters (124). Thus, it could be argued that simply prescribing metformin or some other medication may alleviate many PCOS symptoms. The present review argues in favor of addressing psychological problems via behavioral and mental health interventions since they not only improve mood, but increase the probability that one will adopt a healthier lifestyle and reduce physiological abnormalities (i.e., inflammation, hyperandrogenism) that lead to various disorders (i.e., CVD, diabetes, infertility). Further, addressing depression and other mood disorders via psychotherapy versus pharmacology is potentially more cost-effective and longer-lasting (125–127). Treatment of PCOS, then, should include behavioral and psychological interventions as adjunctive to standard medical care. (See the Figure for an illustration of the interrelationships of several physiological and psychological characteristics described in detail in this review, along with suggested possible pharmaceutical, psychological, and behavioral interventions.) It is very likely that clinicians already employ behavioral and psychological interventions (e.g., smoking cessation advice, addressing barriers to adherence, brief assessment of mood) in the absence of established guidelines. Research protocols could help establish whether these interventions are indeed effective in managing this chronic disorder. Given the evidence supporting relationships among physiological and psychological characteristics common in PCOS, and the existence of behavioral and/or psychological intervention approaches that result in improved metabolic, anthropometric, and reproductive parameters, some aims for future research might include the following:
Investigate whether improvement in sleep quality—specifically, reduction in sleep apnea—among women with PCOS reduces not only physiological abnormalities such as visceral fat and inflammation but also improves other characteristics such as mood and ovulation rate.
Examine whether stress management and lifestyle change among PCOS women affects stress physiology (blood pressure, heart rate, HPA axis [i.e., cortisol] reaction to lab stressors), metabolic (weight, visceral fat, insulin resistance), immunologic (inflammatory markers), and clinical features of hyperandrogenism (i.e., acne, hirsutism).
Since muscle contractions stimulate glucose uptake in the absence of insulin and increased soleus muscle mass may reduce insulin resistance, future work should test whether strength training exercise increases muscle mass and reduces insulin resistance in young women with PCOS.
Elucidate the behavioral and interpersonal goals that might motivate an adolescent who is newly diagnosed with PCOS to improve her lifestyle to achieve weight loss (via exercise and/or diet).
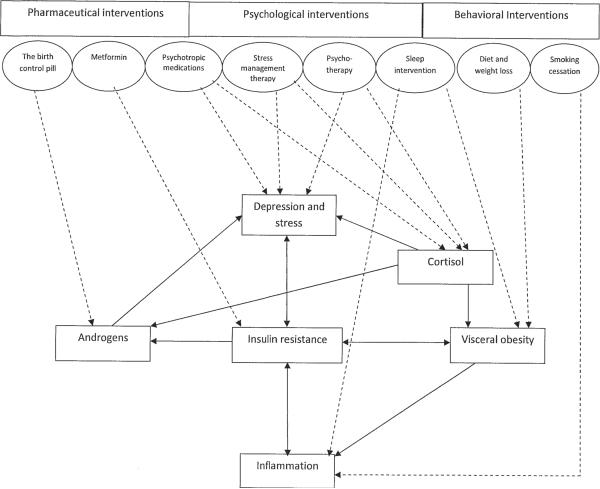
Figure.
Proposed model of interrelationships among physiological and psychological characteristics in women with PCOS and how various pharmaceutical, psychological, and behavioral interventions might impact physiological and psychological abnormalities. In the Figure, the physiological and psychological characteristics common among PCOS women are represented by rectangles, and their direct relations are represented by arrows. Suggested interventions are enclosed in ovals, their dashed arrows indicating which physiological and/or psychological abnormalities they might directly impact.
Summary
PCOS is a common and chronic endocrine disorder characterized by hyperandrogenism, menstrual cycle abnormalities, and polycystic ovaries. It is a common cause of infertility and pregnancy complications. Development of PCOS appears to be spurred by a chronic state of insulin resistance interacting with HPA axis and ovarian abnormalities that are likely `programmed' to be dysfunctional very early in life. Increased insulin resistance is associated with decreased SHBG, which then results in increased bioavailable testosterone. Increased testosterone causes infertility and increased acne, alopecia, and hirsutism, and it also appears to be associated with an increased inflammatory state, mood disorders, and obesity.
Women with PCOS also tend to exhibit central obesity and increased visceral fat compared with non-PCOS women matched for BMI. This phenomenon could be due to an HPA axis defect such that women with PCOS have increased or abnormal cortisol responses to physical and psychological stressors which cannot be explained by percent body fat, BMI, waist-to-hip ratio, fasting insulin, or androgen levels. Central obesity alone does not fully explain the presence of insulin resistance or inflammation, as even lean women with PCOS show evidence of insulin resistance and increased inflammatory markers, suggesting that these maladaptive physiological states are inherent among women with PCOS.
Embarrassing PCOS symptoms such as acne, hirsutism, and central obesity emerge during adolescence and significantly affect the well-being and mental health of the young woman during a phase in life in which she is expanding her social network and beginning to date. PCOS alone and possibly emergence of its symptoms set the stage for development of emotional disturbances such as depression and social phobia. These emotional difficulties may, in turn, exacerbate already-existing obesity and other maladaptive physiological characteristics (i.e., inflammation, HPA axis abnormalities, hyperandrogenism) via behavioral pathways (i.e., increased consumption of carbohydrates, smoking, reduced exercise) and physiological pathways, as previously discussed.
This review presented the evidence supporting the robust relationships among numerous physiological and psychological processes and physical and emotional symptoms in women with PCOS. It is argued herein that addressing and attempting to alleviate emotional disturbances might result in improved physiological processes with the result of improving insulin resistance, HPA axis functioning, obesity, and hyperandrogenism. Subsequently, via the psychological-physiological pathways described, hyperandrogenemic symptoms of PCOS might be alleviated, and the incidence and exacerbation of PCOS and its symptoms, especially among adolescents, could be reduced.
Capsule.
Physiological and psychological abnormalities common in women with PCOS are strongly inter-related. These relations suggest that medical management of PCOS would benefit greatly from inclusion of psychological and/or behavioral approaches.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azziz R. Controversy in clinical endocrinology: diagnosis of polycystic ovary syndrome: the Rotterdam criteria are premature. J Clin Endocrinol Metab. 2006;91:781–785. doi: 10.1210/jc.2005-2153. [DOI] [PubMed] [Google Scholar]
- 2.Chang WY, Knochenbauer ES, Bartolucci AA, Azziz R. Phenotypic spectrum of polycystic ovary syndrome: clinical and biochemical characterization of the three major clinical subgroups. Fertil Steril. 2005;83:1717–1723. doi: 10.1016/j.fertnstert.2005.01.096. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12:324–332. doi: 10.1016/j.molmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53–58. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 6.Norman RJ, Dewailly D, Legro R, Hickey T. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 7.Franks S, McCarthy M, Hardy K. Development of polycystic ovary syndrome: involvement of genetic and environmental factors. Int J Androl. 2006;29:278–285. doi: 10.1111/j.1365-2605.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharma ST, Nestler JE. Prevention of diabetes and cardiovascular disease in women with PCOS: Treatment with insulin sensitizers. Best Pract Res Clin Endocrinol Metab. 2006;20:245–260. doi: 10.1016/j.beem.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Goodarzi MO, Korenman SG. The importance of insulin resistance in polycystic ovary syndrome. Fertil Steril. 2002;77:255–258. doi: 10.1016/s0015-0282(03)00734-9. [DOI] [PubMed] [Google Scholar]
- 10.Ehrmann DA, Barnes RB, Rosenfield RI, Cavaghan MK, Imperial J. Prevalance of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care. 1999;22:141–146. doi: 10.2337/diacare.22.1.141. [DOI] [PubMed] [Google Scholar]
- 11.Norman RJ, Masters L, Milner CR, Wang JX, Davies MJ. Relative risk of conversion from normoglycemia to impaired glucose tolerance or non-insulin dependent diabetes mellitus in polycystic ovary syndrome. Hum Reprod. 2001;16:1995–1998. doi: 10.1093/humrep/16.9.1995. [DOI] [PubMed] [Google Scholar]
- 12.Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clin Endocrinol. 2004;60:1–17. doi: 10.1046/j.1365-2265.2003.01842.x. [DOI] [PubMed] [Google Scholar]
- 13.Diamanti-Kandarakis E, Mitrakou A, Hennes MM, Platanissiotis D, Kaklas N, Spina J, et al. Insulin sensitivity and antiandrogenic therapy in women with polycystic ovary syndrome. Metab Clin Exp. 1995;44:525–531. doi: 10.1016/0026-0495(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 14.Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000;85:139–146. doi: 10.1210/jcem.85.1.6293. [DOI] [PubMed] [Google Scholar]
- 15.Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 16.Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83:2001–2005. doi: 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 17.Cascella T, Palomba S, Di Sio I, Manguso F, Giallauria F, De Simone B, et al. Visceral fat is associated with cardiovascular risk in women with polycystic ovary syndrome. Hum Reprod. 2008;1:153–159. doi: 10.1093/humrep/dem356. [DOI] [PubMed] [Google Scholar]
- 18.Carmina E, Guastella E, Longo RA, Rini GB, Lobo RA. Correlates of increased muscle mass in women with polycystic ovary syndrome. Eur J Endocrinol. 2009;4:583–589. doi: 10.1530/EJE-09-0398. [DOI] [PubMed] [Google Scholar]
- 19.Georgopoulos NA, Saltamavros AD, Vervita V, Karkoulias K, Adonakis G, Decavalas G, et al. Basal metabolic rate is decreased in women with polycystic ovary syndrome and biochemical hyperandrogenemia and is associated with insulin resistance. Fertil Steril. 2009;92:250–255. doi: 10.1016/j.fertnstert.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 20.Kiddy DS, Sharp PS, White DM, Scanlon MF, Mason HD, Bray CS, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol. 1990;32:213–220. doi: 10.1111/j.1365-2265.1990.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 21.Polson DW, Adams J, Wadsworth J, Franks S. Polycystic ovaries—a common finding in normal women. Lancet. 1988;1:870–872. doi: 10.1016/s0140-6736(88)91612-1. [DOI] [PubMed] [Google Scholar]
- 22.Hirschberg AL, Naessen S, Stridesberg M, Brystrom B, Holtet J. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol Endocrinol. 2004;19:79–87. doi: 10.1080/09513590400002300. [DOI] [PubMed] [Google Scholar]
- 23.Stewart PM, Shackleton CH, Beastall GH, Edwards CR. 5-alpha reductase activity in polycystic ovary syndrome. Lancet. 1990;335:431–433. doi: 10.1016/0140-6736(90)90664-q. [DOI] [PubMed] [Google Scholar]
- 24.Chin D, Shackleton C, Prasad VK, Kohn B, David R, Imperato-McGinley J, et al. Increased 5 alpha-reductase and normal 11 beta-hydroxysteroid dehydrogenase metabolism of C19 and C21 steroids in a young population with polycystic ovary syndrome. J Pediatr Endocrinol Metab. 2000;13:253–259. doi: 10.1515/jpem.2000.13.3.253. [DOI] [PubMed] [Google Scholar]
- 25.Rodin A, Thakkar H, Taylor N, Clayton R. Hyperandrogenism in polycystic ovary syndrome. Evidence of dysregulation of 11 betahydroxysteroid dehydrogenase. N Engl J Med. 1994;330:460–465. doi: 10.1056/NEJM199402173300703. [DOI] [PubMed] [Google Scholar]
- 26.Lanzone A, Petraglia F, Fulghesu AM, Ciampelli M, Caruso A, Mancuso S. Corticotropin-releasing hormone induces an exaggerated response of adrenocorticotropic hormone and cortisol in polycystic ovary syndrome. Fertil Steril. 1995;63:1195–1199. doi: 10.1016/s0015-0282(16)57596-7. [DOI] [PubMed] [Google Scholar]
- 27.Carmina E, Levin JH, Malizia G, Lobo RA. Ovine corticotropin-releasing factor and dexamethasone responses in hyperandrogenic women. Fertil Steril. 1990;54:245–250. doi: 10.1016/s0015-0282(16)53697-8. [DOI] [PubMed] [Google Scholar]
- 28.Battaglia C, Mancini F, Cianciosi A, Busacchi P, Persico N, Paradisi R, et al. Cardiovascular risk in normal weight, eumenorrheic, nonhirsute daughters of patients with polycystic ovary syndrome: a pilot study. Fertil Steril. 2008;92:240–249. doi: 10.1016/j.fertnstert.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Birdsall MA, Farquar CM, White HD. Association between polycystic ovaries and the extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med. 1997;126:32–35. doi: 10.7326/0003-4819-126-1-199701010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Lakhani K, Seifalian AM, Hardiman P. Impaired carotid viscoelastic properties in women with polycystic ovaries. Circulation. 2002;106:81–85. doi: 10.1161/01.cir.0000020681.19400.8a. [DOI] [PubMed] [Google Scholar]
- 31.Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562–2568. doi: 10.1210/jc.2003-030334. [DOI] [PubMed] [Google Scholar]
- 32.Talbott EO, Guzick DS, Sutton-Tyrrell K, McHugh-Pemu KP, Zborowski JV, Remsberg KE, et al. Evidence for association between polycystic ovary syndrome and premature carotid atherosclerosis in middle-aged women. Arterioscler Thromb Vasc Biol. 2000;20:2414–2421. doi: 10.1161/01.atv.20.11.2414. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 34.Engin-Ustun Y, Ustun Y, Meydanli M, Kafkasli A, Yetkin G. Are polycystic ovaries associated with cardiovascular disease risk as polycystic ovary syndrome? Gynecol Endocrinol. 2006;22:324–328. doi: 10.1080/09513590600630447. [DOI] [PubMed] [Google Scholar]
- 35.Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millan JI. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: relationship to insulin resistance and to obesity. J Clin Endocrinol Metab. 2004;89:806–811. doi: 10.1210/jc.2003-031365. [DOI] [PubMed] [Google Scholar]
- 36.Kerchner A, Lester W, Stuart SP, Dokras A. Risk of depression and other mental health disorders in women with polycystic ovary syndrome: a longitudinal study. Fertil Steril. 2009;91:207–212. doi: 10.1016/j.fertnstert.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Benson S, Hahn S, Tan S, Mann K, Janssen OE, Schedlowski M, et al. Prevalence and implications of anxiety in polycystic ovary syndrome: results of an internet-based survey in Germany. Hum Reprod. 2009;24:1446–1451. doi: 10.1093/humrep/dep031. [DOI] [PubMed] [Google Scholar]
- 38.Tan S, Hahn S, Benson S, Janssen OE, Dietz T, Kimmig R, et al. Psychological implications of infertility in women with polycystic ovary syndrome. Hum Reprod. 2008;23:2064–2071. doi: 10.1093/humrep/den227. [DOI] [PubMed] [Google Scholar]
- 39.Adali E, Yildizhan R, Kurdoglu M, Kolusari A, Edirne T, Sahin HG, et al. The relationship between clinic-biochemical characteristics and psychiatric distress in young women with polycystic ovary syndrome. J Int Med Res. 2008;36:1188–1196. doi: 10.1177/147323000803600604. [DOI] [PubMed] [Google Scholar]
- 40.Mansson M, Holte J, Landin-Wilhelmsen K, Dahlgren E, Johansson A, Landen M. Women with polycystic ovary syndrome are often depressed or anxious—a case control study. Psychoneuroendocrinology. 2008;33:1132–1138. doi: 10.1016/j.psyneuen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Hollinrake E, Abreu A, Maifeld M, Van Voorhis B, Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril. 2007;87:1369–1376. doi: 10.1016/j.fertnstert.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 42.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003;54:330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 43.Bishop SC, Basch S, Futterweit W. Polycystic ovary syndrome, depression, and affective disorders. Endocr Pract. 2009;15:475–482. doi: 10.4158/EP09083.RAR. [DOI] [PubMed] [Google Scholar]
- 44.Trent ME, Rich M, Austin SB, Gordon CM. Fertility concerns and sexual behavior in adolescent girls with polycystic ovary syndrome: implications for quality of life. J Pediatr Adolesc Gynecol. 2003;16:33–37. doi: 10.1016/s1083-3188(02)00205-x. [DOI] [PubMed] [Google Scholar]
- 45.Elsenbruch S, Benson S, Hahn S, Tan S, Mann K, Pleger K, et al. Determinants of emotional distress in women with polycystic ovary syndrome. Hum Reprod. 2006;21:1092–1099. doi: 10.1093/humrep/dei409. [DOI] [PubMed] [Google Scholar]
- 46.Sonino N, Fava GA, Mani E, Belluardo P, Boscaro M. Quality of life of hirsute women. Postgrad Med J. 1993;69:186–189. doi: 10.1136/pgmj.69.809.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mallon E, Newton JN, Klassen A, Stewart-Brown SL, Ryan TJ, Finlay AY. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672–676. doi: 10.1046/j.1365-2133.1999.02768.x. [DOI] [PubMed] [Google Scholar]
- 48.Keegan A, Liao LM, Boyle M. `Hirsutim': A psychological analysis. J Health Psychol. 2003;8:327–345. doi: 10.1177/13591053030083004. [DOI] [PubMed] [Google Scholar]
- 49.Elsenbruch S, Hahn S, Kowalsky D, Offner AH, Schedlowski M, Mann K, et al. Quality of life, psychosocial well-being, and sexual satisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:5801–5807. doi: 10.1210/jc.2003-030562. [DOI] [PubMed] [Google Scholar]
- 50.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 52.Maes M, Scharpe S, Meltzer HY, Bosmans E, Suy E, Calabrese J, et al. Relationships between interleukin-6 activity, acute phase proteins, and function of the hypothalamic-pituitary-adrenal axis in severe depression. Psychiatry Res. 1993;49:11–27. doi: 10.1016/0165-1781(93)90027-e. [DOI] [PubMed] [Google Scholar]
- 53.Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- 54.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 55.Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 56.Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Immunol Allergy Clin N Am. 2009;29:309–320. doi: 10.1016/j.iac.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kubera M, Symbirtsev A, Basta-Kaim A, Borycz, Roman A, Papp M, et al. Effect of chronic treatment with imipramine on interleukin 1 and interleukin 2 production by splenocytes obtained from rats subjected to a chronic mild stress model of depression. Pol J Pharmacol. 1996;48:503–506. [PubMed] [Google Scholar]
- 58.Dantzer R. Cytokine-induced sickness behavior: Where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 59.Kent S, Bluthe RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 60.Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, et al. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 61.Chandler S, Miller KM, Clements JM, Lury J, Corkill D, Anthony DC, et al. Matrix metalloproteinases, tumor necrosis factor and multiple sclerosis: an overview. J Neuroimmunol. 1997;72:155–161. doi: 10.1016/s0165-5728(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 62.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and and cortico-releasing hormone. Proc Natl Acad Sci USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker Medical progress: Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 65.Link H. The cytokine storm in multiple sclerosis. Mult Scler. 1998;4:12–15. doi: 10.1177/135245859800400104. [DOI] [PubMed] [Google Scholar]
- 66.Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. 2005;76:469–475. doi: 10.1136/jnnp.2004.054635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pollak Y, Ovadia H, Goshen I, Gurevich R, Monsa K, Avitsur R, et al. Behavioral aspects of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;104:31–36. doi: 10.1016/s0165-5728(99)00257-x. [DOI] [PubMed] [Google Scholar]
- 68.Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: Role of insulin resistance. J Clin Endocrinol Metab. 2001;86:517–520. doi: 10.1210/jcem.86.2.7185. [DOI] [PubMed] [Google Scholar]
- 69.Forbes EE, Bertocci MA, Gregory AM, Ryan ND, Axelson DA, Birmaher B, et al. Objective sleep in pediatric anxiety disorders and major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:148–155. doi: 10.1097/chi.0b013e31815cd9bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem. 2008;114:211–223. doi: 10.1080/13813450802364627. [DOI] [PubMed] [Google Scholar]
- 71.Mehra R, Redline S. Sleep apnea: a proinflammatory disorder that coaggregates with obesity. J Allergy Clin Immunol. 2008;121:1096–1102. doi: 10.1016/j.jaci.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 73.Nissel H, Hjemdahl P, Linde B, Beskow C, Lunell NO. Sympathoadrenal and cardiovascular responses to mental stress in pregnancy-induced hypertension. Obstet Gynecol. 1986;68:531–536. [PubMed] [Google Scholar]
- 74.Gallinelli A, Matteo ML, Volpe A, Facchinetti F. Autonomic and neuroendocrine responses to stress in patients with functional hypothalamic secondary amenorrhea. Fertil Steril. 2000;73:812–816. doi: 10.1016/s0015-0282(99)00601-9. [DOI] [PubMed] [Google Scholar]
- 75.Benson S, Arck PC, Tan S, Hahn S, Mann K, Rifaie N, et al. Disturbed stress responses in women with polycystic ovary syndrome. Psychoneuroendocrinology. 2009;34:727–35. doi: 10.1016/j.psyneuen.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Drapeau V, Therrien F, Richard D, Tremblay A. Is visceral obesity a physiological adaptation to stress? Panminerva Med. 2003;45:189–195. [PubMed] [Google Scholar]
- 77.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissue of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 78.Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, et al. Stress and body shape: Stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 79.van Honk J, Tuiten A, Hermans E, Putman P, Koppeschaar H, Thijssen J, et al. A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Beh Neuro. 2001;115:238–242. doi: 10.1037/0735-7044.115.1.238. [DOI] [PubMed] [Google Scholar]
- 80.Black H. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 81.Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: comparison of women with polycystic ovary syndrome to healthy controls. Psychosom Med. 2004;66:356–362. doi: 10.1097/01.psy.0000127871.46309.fe. [DOI] [PubMed] [Google Scholar]
- 82.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 83.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci. 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- 84.Timonen M, Laakso M, Jokelainen J, Rajala U, Meyer-Rochow VB, Keinänen-Kiukaanniemi S. Insulin resistance and depression: cross sectional study. BMJ. 2005;330:17–18. doi: 10.1136/bmj.38313.513310.F71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan A, Ye X, Franco OH, Li H, Yu Z, Zou S, et al. Insulin resistance and depressive symptoms in middle-aged and elderly Chinese: findings from the Nutrition and Health of Aging Population in China Study. J Affect Disord. 2008;109:75–82. doi: 10.1016/j.jad.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 86.The Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23:462–477. doi: 10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- 87.Moll E, van der Veen F, van Wely M. The role of metformin in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2007;13:527–537. doi: 10.1093/humupd/dmm026. [DOI] [PubMed] [Google Scholar]
- 88.Nestler JE. Metformin for the treatment of polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 89.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13:251–257. doi: 10.1016/s1043-2760(02)00612-4. [DOI] [PubMed] [Google Scholar]
- 90.Giallauria F, Orio F, Palomba S, Lombardi G, Colao A, Vigorito C. Cardiovascular risk in women with polycystic ovary syndrome. J Cardiovasc Med. 2008;9:987–992. doi: 10.2459/JCM.0b013e32830b58d4. [DOI] [PubMed] [Google Scholar]
- 91. [Accessed February 20, 2009];Polycystic ovary syndrome. (n.d.) Available at: http://www.mayoclinic.com/health/polycystic-ovary-syndrome/DS00423.
- 92. [Accessed February 20, 2009];Polycystic ovary syndrome. (n.d.) Available at: http://www.patient.co.uk/health/Polycystic-Ovary-Syndrome.htm.
- 93.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moran LJ, Brinkworth G, Noakes M, Norman RJ. Effects of lifestyle modification in polycystic ovary syndrome. Reprod Biomed Online. 2006;12:569–578. doi: 10.1016/s1472-6483(10)61182-0. [DOI] [PubMed] [Google Scholar]
- 95.Despres JP, Lemieux I, Prud`homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. BMJ. 2001;322:716–720. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moran LJ, Noakes M, Clifton PM, Tomlinson L, Galletly C, Norman RJ. Dietary composition in restoring reproductive and metabolic physiology in overweight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:812–819. doi: 10.1210/jc.2002-020815. [DOI] [PubMed] [Google Scholar]
- 97.Huber-Buchholz MM, Carey DGP, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: Role of insulin sensitivity and luteinizing hormone. J Clin Endocrinol Metab. 1999;84:1470–1474. doi: 10.1210/jcem.84.4.5596. [DOI] [PubMed] [Google Scholar]
- 98.Palomba S, Giallauria F, Falbo A, Russo T, Oppedisano R, Tolino A, et al. Structured exercise training programme versus hypocaloric hyperproteic diet in obese polycystic ovary syndrome patients with anovulatory infertility: a 24-week pilot study. Hum Reprod. 2008;23:642–650. doi: 10.1093/humrep/dem391. [DOI] [PubMed] [Google Scholar]
- 99.Brown AJ, Setji TL, Sanders LL, Lowry KP, Otvos JD, Kraus WE, et al. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Med Sci Sports Exerc. 2009;41:497–504. doi: 10.1249/MSS.0b013e31818c6c0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ryder JW, Chibalin AV, Zierath JR. Intracellular mechanisms underlying increases in glucose uptake in response to insulin or exercise in skeletal muscle. Acta Physiol Scand. 2001;171:249–257. doi: 10.1046/j.1365-201x.2001.00827.x. [DOI] [PubMed] [Google Scholar]
- 101.Manneras L, Jonsdottir IH, Holmang A, Lonn M, Stener-Victorin E. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology. 2008;149:3559–3568. doi: 10.1210/en.2008-0053. [DOI] [PubMed] [Google Scholar]
- 102.Church TS, Barlow CD, Earnest CP, Kampert JB, Priest EL, Blaire SN. Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol. 2002;22:1869–1876. doi: 10.1161/01.atv.0000036611.77940.f8. [DOI] [PubMed] [Google Scholar]
- 103.Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein W, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100:93–99. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 104.Giallauria F, Palomba S, Maresca L, Vuolo L, Tafuri D, Lombardi G, et al. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS) Clin Endocrinol. 2008;69:792–798. doi: 10.1111/j.1365-2265.2008.03305.x. [DOI] [PubMed] [Google Scholar]
- 105.Liao LM, Nesic J, Chadwick PM, Brooke-Wavell K, Prelevic GM. Exercise and body image distress in overweight and obese women with polycystic ovary syndrome: A pilot investigation. Gynecol Endocrinol. 2008;24:555–561. doi: 10.1080/09513590802288226. [DOI] [PubMed] [Google Scholar]
- 106.Manlove HA, Guillermo C, Gray PB. Do women with polycystic ovary syndrome (PCOS) report differences in sex-typed behavior as children and adolescents? Results of a pilot study. Ann Human Biol. 2008;35:584–595. doi: 10.1080/03014460802337067. [DOI] [PubMed] [Google Scholar]
- 107.Galletly C, Moran L, Noakes M, Clifton P, Tomlinson L, Norman R. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome—A pilot study. Appetite. 2007;49:590–593. doi: 10.1016/j.appet.2007.03.222. [DOI] [PubMed] [Google Scholar]
- 108.Markus CR, Olivier B, Panhuysen GE, Van der Gugten J, Alles MS, Tuiten A, et al. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids and in vulnerable subjects raises brain serotonin activity reduces cortisol concentration and improves mood under stress. Am J Clin Nutr. 2000;71:1536–1544. doi: 10.1093/ajcn/71.6.1536. [DOI] [PubMed] [Google Scholar]
- 109.Gonzalez F, Rote N, Minium J, Kirwan J. In vitro evidence that hyperglycemia stimulates tumor necrosis factor-α release in obese women with polycystic ovary syndrome. J Endocrinol. 2006;188:521–529. doi: 10.1677/joe.1.06579. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez F, Rote N, Minium J, Kirwan J. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336–34. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- 111.Bonnet F, Irving K, Terra JL, Nony P, Berthezene F, Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178:339–344. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 112.Lysy Z, Da cost A, Dasgupta K. The association of physical activity and depression in Type 2 diabetes. Diabet Med. 2008;25:1133–1141. doi: 10.1111/j.1464-5491.2008.02545.x. [DOI] [PubMed] [Google Scholar]
- 113.Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32:741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- 114.Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–638. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 115.Antoni MH, Cruess S, Cruess D, Kumar M, Lutgendorf S, Ironson G, et al. Cognitive behavioral stress management reduces distress and 24-hour urinary free cortisol among symptomatic HIV-infected gay men. Ann Behav Med. 2000;22:29–37. doi: 10.1007/BF02895165. [DOI] [PubMed] [Google Scholar]
- 116.Cruess DG, Antoni MH, McGregor BA, Kilbourn KM, Boyers AE, Alferi SM, et al. Cognitive-behavioral stress management reduces serum cortisol by enhancing positive contributions among women being treated for early stage breast cancer. Psychosom Med. 2000;62:304–308. doi: 10.1097/00006842-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 117.Phillips K, Antoni MH, Lechner S, Blomberg BB, Llabre MM, Avisar E, et al. Stress Management Intervention Reduces Serum Cortisol and Increases Relaxation During Treatment for Non-Metastatic Breast Cancer. Psychosom Med. 2008;70:1044–1049. doi: 10.1097/PSY.0b013e318186fb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Antoni MH, Cruess D, Cruess S, Lutgendorf S, Kumar M, Ironson G, et al. Cognitive behavioral stress management effects on anxiety, 24-hour urinary catecholamine output, and T-Cytotoxic/suppressor cells over time among symptomatic HIV-infected gay men. J Consult Clin Psychol. 2000;68:31–45. doi: 10.1037//0022-006x.68.1.31. [DOI] [PubMed] [Google Scholar]
- 119.Cruess D, Antoni MH, Mahendra K, McGregor B, Alferi S, Boyers A, et al. Effects of stress management on testosterone levels in women with early-stage breast cancer. Int J Behav Med. 2001;8:194–207. [Google Scholar]
- 120.Rofey DL, Szigethy EM, Noll RB, Dahl RE, Lobst E, Arslanian SA. Cognitive-behavioral therapy for physical and emotional disturbances in adolescents with polycystic ovary syndrome: A pilot study. J Pediatr Psychol. 2009;34:156–163. doi: 10.1093/jpepsy/jsn057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mefferd K, Nichols J, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat. 2007;104:145–152. doi: 10.1007/s10549-006-9410-x. [DOI] [PubMed] [Google Scholar]
- 122.Vignolo M, Rossi F, Bardazza G, Pistorio A, Parodi A, Spigno S, et al. Five-year follow-up of a cognitive-behavioural lifestyle multidisciplinary programme for childhood obesity outpatient treatment. Eur J Clin Nutr. 2008;62:1047–1057. doi: 10.1038/sj.ejcn.1602819. [DOI] [PubMed] [Google Scholar]
- 123.Ash S, Reeves M, Bauer J, Dover T, Vivanti A, Leong C, et al. A randomized control trial comparing lifestyle groups, individual counseling and written information in the management of weight and health outcomes over 12 months. Int J Obes. 2006;30:1557–1564. doi: 10.1038/sj.ijo.0803263. [DOI] [PubMed] [Google Scholar]
- 124.Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, Mann K, et al. Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress, and sexuality. Hum Reprod. 2006;21:1925–1934. doi: 10.1093/humrep/del069. [DOI] [PubMed] [Google Scholar]
- 125.Blackburn IM, Moore RG. Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in out-patients with recurrent depression. Br J Psychiatry. 1997;171:328–334. doi: 10.1192/bjp.171.4.328. [DOI] [PubMed] [Google Scholar]
- 126.Fava GA, Rafanelli C, Grandi S, Conti S, Belluardo P. Prevention of recurrent depression with cognitive behavioral therapy: preliminary findings. Arch Gen Psychiatry. 1998;55:816–820. doi: 10.1001/archpsyc.55.9.816. [DOI] [PubMed] [Google Scholar]
- 127.Gould RA, Ott MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Clin Psychol Rev. 1995;15:819–844. [Google Scholar]