Abstract
Women treated with chest radiation for a pediatric cancer have low mammography screening rates despite their high risk for breast cancer. This study characterized the relationship between perceptions of mammography and screening practices. A cross-sectional survey was administered to 523 women in North America who were treated with chest radiation before 21 years of age. Women with inconsistent mammography perceptions and practices were identified using the Pros and Cons of Mammography for perceptions and Transtheoretical Model stages of adoption for prior and intended screening practices. Classification and regression tree (CART) analysis was used to identify barriers to and facilitators of screening among women with positive and negative perceptions. Nearly one-third of the cohort had inconsistent perceptions and practices: 37.4% had positive perceptions and were not having mammograms; 27.6% had negative/neutral perceptions and were having mammograms. Regardless of perceptions, a recent physician’s recommendation for mammography, age ≥ 40, and interest in routine health care were universally associated with mammography practices. For women with positive perceptions and a physician’s recommendation, barriers to screening included high acceptance coping, low active-planning coping, and high internal health locus of control. For women with negative perceptions, acknowledging the importance of asymptomatic screening was associated with mammography.
Keywords: Cancer survivorship, Late effects, Screening, Transtheoretical model, Stages of adoption
Introduction
Female survivors of pediatric cancer have an elevated risk of breast cancer following moderate- to high-dose chest radiation during childhood or adolescence [1–7]. An estimated 12–20% develop breast cancer by 45 years of age [1, 4, 6]. For these women, the Children’s Oncology Group recommends annual screening mammography and adjunct breast MRI, beginning at age 25 or 8 after radiation exposure, whichever occurs last [8, 9]. Still, many high-risk survivors are not being screened appropriately, with only 36.5% of women ages 25–39 and 76.5% of women ages 40–50 reporting a screening mammogram in the past 2 years [10]. Interventions to improve screening rates are essential.
The literature on mammography interventions in the general and hereditary risk populations is extensive; however, little is known about the optimal approach for cancer survivors at risk of breast cancer following chest radiation at a young age. Among the most common theoretical frameworks for mammography interventions is the transtheoretical model (TTM) [11, 12]. Building from the observation that perceptions of mammography, as measured by the pros and cons of mammography, tend to be more positive among those who are screening, TTM-based interventions counsel women to increase positive perceptions (pros) and decrease negative perceptions (cons), so that they will be more likely to advance through the stages of adoption, moving from precontemplation and contemplation (not considering mammography or considering but not yet screening) to action and maintenance (screening and planning to continue) [13–15]. Though intuitive, this approach may not be effective for some women, as perceptions of mammography vary even within the same stage of adoption [16, 17]. Some women have inconsistent perceptions and practices, meaning that they either report positive perceptions but are not having mammograms (precontemplation or contemplation) or report negative perceptions and are having mammograms (action or maintenance). For women in these groups, additional facilitators or barriers are likely to influence their mammography practices. It follows that incorporating elements from other health behavior theories may improve the effectiveness of TTM-based interventions.
Mammography screening studies have traditionally used multivariate regression procedures to identify factors that are independently associated with screening. Since these models measure average effects while holding all else constant, interventions that are based on them may be most appropriate for the average member of a population [18]. Recently, there has been a greater emphasis on investigating interactions between factors to distinguish subgroups of participants that may benefit from different interventions. One promising approach is classification and regression tree (CART) analysis, a nonparametric method that identifies interactions by splitting a sample into subgroups with shared characteristics [19]. The resulting models tend to be clinically intuitive and hypothesis-generating, making them particularly helpful during the early stages of intervention development [18, 20, 21].
Among women at risk of breast cancer following chest radiation for pediatric cancer, we have previously reported an association between positive perceptions of mammography and increased likelihood of screening [10]. Although this suggests that an intervention guided by the TTM may be appropriate, more information is needed to describe interactions and identify subgroups that may respond to different intervention approaches. Seeking to optimize the effectiveness of future theory-based interventions in this high-risk population, this study aimed to determine whether and to what extent women in this cohort had inconsistent perceptions of mammography and screening practices. After identifying inconsistent subgroups, we used CART analysis to explore (1) barriers that may interfere with screening among women with positive perceptions of mammography and (2) facilitators that may prompt screening among women with negative perceptions of mammography.
Methods
Study population
The study population included 523 women in the Childhood Cancer Survivor Study (CCSS) who were randomly selected for a cross-sectional study of breast cancer surveillance practices. The CCSS is a longitudinal cohort study of over 14,000 survivors of pediatric cancer who were diagnosed with Hodgkin lymphoma, non-Hodgkin lymphoma, leukemia, neuroblastoma, kidney tumor, brain tumor, soft tissue sarcoma, or bone tumor before age 21 at one of 26 collaborating institutions in the United States and Canada (listed in “Appendix A”). All participants were diagnosed between 1970 and 1986 and survived at least 5 years from their diagnosis.
Female CCSS participants who were treated with moderate- to high-dose radiation (≥20 Gy) to the chest area and had not been diagnosed with breast cancer were eligible for this study. From a random sample of 625 women who were successfully contacted, 551 (88.2%) participated. Nonparticipants were similar to participants with respect to age at study, age at cancer diagnosis, and elapsed time between diagnosis and study, but were more likely to be racial or ethnic minorities (17.8 vs. 7.7%). The present study of 523 women excludes 28 (5.1%) with insufficient information about mammography perceptions (n = 10) or practices (n = 18).
Details of the CCSS recruitment methodology for the overall cohort and for this cohort of women have been published previously [10, 22–24]. The Institutional Review Board at each collaborating site approved the study protocol (survey available at www.stjude.org/ccss).
Primary outcome: inconsistent perceptions and practices
Inconsistent mammography perceptions and practices were defined by the pros and cons of mammography and TTM stage of adoption. Women had inconsistent perceptions and practices if they either (1) had positive perceptions (pros > cons) and were in precontemplation or contemplation or (2) had negative or neutral perceptions (cons ≥ pros) and were in action.
Perceptions of mammography
Perceptions were assessed using the pros and cons of mammography, a validated instrument measuring six positive perceptions (pros) and seven negative perceptions (cons) of screening mammography [25], provided in “Appendix B”. All items were rated on a five-point Likert scale, where 1 = strongly disagree and 5 = strongly agree. The internal reliability was high for pros (Cronbach coefficient alpha [α] = 0.73) and cons (α = 0.78). As in the published literature [13–15, 25, 26], the pros index and cons index were each standardized to t-scores. Mammography perceptions were indicated by decisional balance (pros t-score - cons t-score), with a more positive decisional balance representing more favorable perceptions.
A continuous measure of decisional balance is difficult to interpret in a clinical setting, as a one-unit change may not signify a meaningful difference in perceptions of mammography. For this reason, decisional balance was categorized into two groups. Women who reported more cons than pros or had an approximately equal balance were classified as having negative or neutral perceptions. Women who reported more pros than cons (pros outweighed cons by a margin of at least 0.25*standard deviation for the sample) were classified as having positive perceptions.
Mammography practices
According to standard accepted criteria, participants were assigned to one of six TTM stages of adoption based upon self-reported mammography practices within the past 2 years and intended screening within the next year [14, 15, 26]. Although annual mammography is recommended, a 2-year interval was considered acceptable for prior mammograms in order to minimize misclassification of women who generally follow recommendations, but may not have their exams in the same month each year.
Women who did not intend to have a mammogram within the next year were in precontemplation if they had never had a mammogram, relapse if they had one more than 2 years ago, or risk of relapse if they had one within the past 2 years. Those who intended to have a mammogram within the next year were in contemplation if they had never had a mammogram or had one more than 2 years ago, action if they had one within the past 2 years, or maintenance if they had two within the past 4 years (summarized in “Appendix C”). In this study, as in previous investigations [14], precontemplation and relapse were combined (“precontemplation”), as were contemplation and risk of relapse (“contemplation”) and action and maintenance (“action”).
Independent variables
Independent variables included: demographics (age, race/ethnicity, living area, weight, height, age at diagnosis) and health care factors (primary care physician or usual source of care, written summary of cancer treatment, physician recommendation for a mammogram within the past year, prior breast problems including an abnormal mammogram or benign breast lump, regular monthly breast self exams, clinical breast exam and Papanicolaou (Pap) smear within the past 2 years). General and cancer-specific health concerns (two items each) and cancer health worries (six items) were measured by five-point Likert scales, where 1 = not at all and 5 = extremely. Risk knowledge was assessed by a true/false statement of the association between chest radiation and breast cancer risk, and risk perception was measured by each participant’s estimate of her breast cancer risk compared to average women, where 1 = much less and 5 = much more. Coping was measured by five scales from the COPE inventory, completed with reference to how participants generally cope with stressful experiences: behavioral disengagement, denial, acceptance, active coping, and planning coping [27, 28]. Active-planning coping was included as a composite (average) of the active and planning subscales because they were strongly correlated (r = 0.68). Perceived health locus of control was evaluated using Wallston et al. Multidimensional Health Locus of Control (MHLC) scales (Form A). Three independent domains in the MHLC describe an individual’s perception of the extent to which she controls her health: internal (“I am in control”), chance (“what will be, will be”) and powerful others (“health professionals control my health”) [29, 30].
Statistical analysis
CART analysis was conducted to identify factors that were associated with inconsistent mammography practices among two groups of women with differing perceptions of mammography (positive, n = 230; negative/neutral, n = 293). The CART algorithm and statistical theory have been explained in detail [19, 31, 32]. All independent variables were loaded into the CART model. Age was categorized (25–39; 40–50) to reflect mammography recommendations in the general population. The Gini impurity criterion [33] and tenfold cross-validation [34] were used to build the models, specifying minimum node sizes of 20 observations for parent nodes and 10 observations for terminal nodes. CART models were constructed with the automated CART software version 6.0 (Salford Systems, San Diego, CA).
Results
Study participants
The mean age of participants was 38.7 (SD, 6.3) and just over half were under age 40 at the time of study (Table 1). Racial and ethnic diversity was limited, but participants were approximately evenly distributed among rural, urban, and suburban living areas. Since Hodgkin lymphoma (HL) is one of the most common pediatric cancers and is generally treated with chest radiation, patients with HL represented the largest group in the study (58.1%). About two-thirds of the participants were diagnosed between the ages of 10–20 (mean, 12.1 years; SD, 5.7). Nearly 45% of the women had not had a screening mammogram within the previous 2 years. More than half demonstrated a low commitment to mammography, with 57.0% in precontemplation (n = 125), relapse (n = 47), contemplation (n = 55), or risk of relapse (n = 71). In contrast, 43.0% of the women were in action (n = 53) or maintenance (n = 172).
Table 1.
Characteristics of the study population (n = 523)
| Characteristics | N | % |
|---|---|---|
| Age at time of study | ||
| 25–39 years | 279 | 53.3 |
| 40–50 years | 244 | 46.7 |
| Race and ethnicity | ||
| White, non-Hispanic | 482 | 92.3 |
| Minority | 40 | 7.7 |
| Living area | ||
| Rural | 163 | 32.2 |
| Urban | 126 | 24.9 |
| Suburban | 217 | 42.9 |
| BMI | ||
| <25 | 303 | 58.4 |
| 25–29 | 123 | 23.7 |
| ≥30 | 93 | 17.9 |
| Cancer diagnosis | ||
| Hodgkin lymphoma | 304 | 58.1 |
| Kidney tumor | 72 | 13.8 |
| Non-Hodgkin lymphoma | 42 | 8.0 |
| Neuroblastoma | 33 | 6.3 |
| Bone tumor | 28 | 5.3 |
| Soft tissue sarcoma | 26 | 5.0 |
| Leukemia | 13 | 2.5 |
| Brain tumor | 5 | 1.0 |
| Age at cancer diagnosis | ||
| 0–9 years | 172 | 32.9 |
| 10–20 years | 351 | 67.1 |
| Screening mammogram | ||
| Within the past 2 years | 291 | 55.6 |
| More than 2 years ago or nevera | 232 | 44.4 |
| Stage of adoption | ||
| Precontemplation | 125 | 23.9 |
| Relapse | 47 | 9.0 |
| Contemplation | 55 | 10.5 |
| Risk of relapse | 71 | 13.6 |
| Action | 53 | 10.1 |
| Maintenance | 172 | 32.9 |
Includes five women in risk of relapse who had a mammogram within the past 2 years for a specific breast problem
Correspondence between mammography perceptions and practices
Consistent with the TTM and pros and cons of mammography, women in action tended to have more positive perceptions of mammography than women in precontemplation or contemplation (mean decisional balance: action = 9.1; SD, 13.8; contemplation = 1.8; SD, 16.0; precontemplation = −8.8; SD, 16.4; p < 0.001). Still large standard deviations indicated variability, and nearly one-third of women (n = 176, 31.9%) had inconsistent mammography practices given their perceptions. Among women with positive perceptions, 37.4% were in precontemplation or contemplation, and among those with negative or neutral perceptions, 27.6% were in action (Table 2).
Table 2.
Subgroups of women with inconsistent mammography perceptions and practices
| TTM stage of adoption | Positive perceptions (n = 230) |
Negative/neutral perceptions (n = 293) |
||||||
|---|---|---|---|---|---|---|---|---|
| n | % | Mean | SD | n | % | Mean | SD | |
| Precon + contmp | 86 | 37.4 | 14.8 | 8.0 | 212 | 72.3 | −12.1 | 13.1 |
| Action | 144 | 62.6 | 17.5 | 8.1 | 81 | 27.6 | −6.0 | 7.4 |
Shading denotes subgroups with inconsistent perceptions and practices
TTM transtheoretical model, Precon + Contmp precontemplation and contemplation
Why women with positive perceptions of mammography are not having mammograms
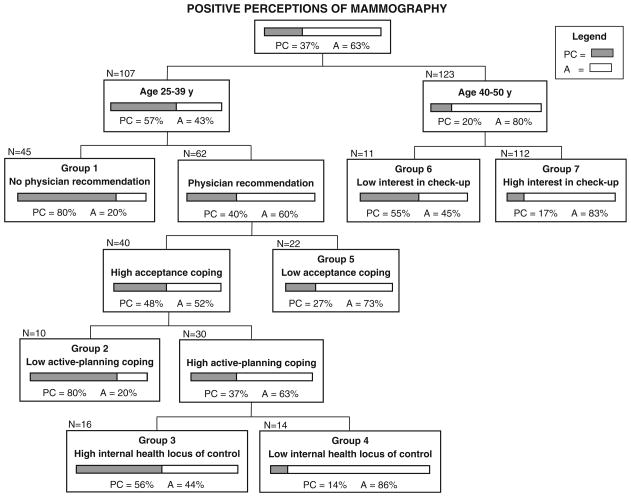
The CART model in Fig. 1 presents potential barriers that may have discouraged women with positive perceptions from having mammograms. Notably, 80% of women who were under age 40 and did not report a physician’s recommendation for mammography within the past year were in precontemplation and contemplation (Group 1 in Fig. 1). Those who reported a physician’s recommendation were similarly likely to be in precontemplation and contemplation (80%) if they had high acceptance coping and low active-planning coping (Group 2). Of the women who ranked high in both acceptance and active-planning coping, the proportion not having mammograms was greater among those who reported high internal control over their health than among those who did not (Group 3 vs. Group 4). Women aged 40 and older were more likely to be in precontemplation and contemplation if they were not interested in routine medical checkups (Group 6 vs. Group 7).
Fig. 1.
CART model predicting inconsistent mammography practices (precontemplation + contemplation) for 230 women with positive perceptions (why women who have positive perceptions of mammography are not having mammograms). Notes: PC precontemplation + contemplation (shaded); A = action (white); Interest in checkup: low = not at all or a little bit; high = moderately, quite a bit, or extremely; Acceptance coping: low = not at all or a little bit; high = a medium amount or a lot; Active-planning coping: low = -not at all, a little bit, or a medium amount; high = a lot; Internal health locus: low = disagree strongly, moderately, or slightly, or agree slightly; high = agree moderately or strongly; Misclassification percentage = 36.1% (proportion whose true mammography practices differed from what the model would predict)
Why women with negative perceptions of mammography are having mammograms
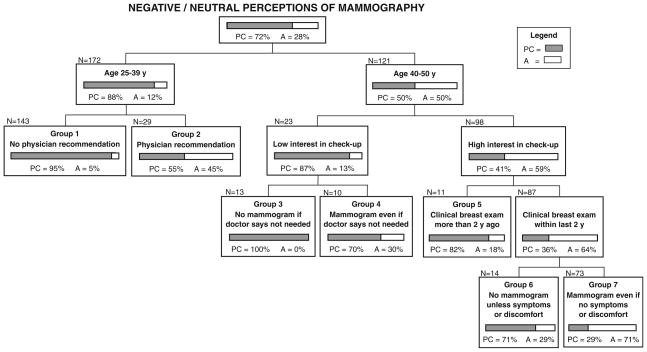
A second CART model presents facilitators that may have prompted mammography for women with negative or neutral perceptions (Fig. 2). Forty-five percent of women under age 40 who reported a recent physician’s recommendation for mammography were in action, compared to only 5% who reported no such recommendation (Group 2 vs. Group 1). Women aged 40 and older were more likely to be in action than younger women, particularly if they were interested in routine medical checkups, had a recent clinical breast exam, and believed mammograms are necessary even in the absence of symptoms or discomfort (Group 7 vs. Groups 3–6).
Fig. 2.
CART model predicting inconsistent mammography practices (action) for 293 women with negative or neutral perceptions (why women who have negative perceptions of mammography are having mammograms). Notes: PC = precontemplation + contemplation (shaded); A = action (white); Interest in checkup: low = not at all or a little bit; high = moderately, quite a bit, or extremely; No mammogram if doctor says not needed: strongly agree or agree (Group 3) versus strongly disagree, disagree, don’t agree or disagree (Group 4); No mammogram unless symptoms or discomfort: strongly agree, agree, don’t agree or disagree (Group 6) versus strongly disagree or disagree (Group 7); Misclassification percentage = 28.3% (proportion whose true mammography practices differed from what the model would predict)
Discussion
In this cohort of women at risk of breast cancer following chest radiation for pediatric cancer, we show that although perceptions of mammography tended to be more positive among those who were having mammograms, about one-third of the women had inconsistent perceptions and practices. These women may not respond to standard pros and cons focused counseling, as positive perceptions were not sufficient to ensure screening and negative or neutral perceptions were noted among those who were regularly screened. We used CART analysis to explore additional factors to consider in future studies. A recent physician’s recommendation, aged ≥ 40, and interest in routine medical checkups were associated with mammography regardless of women’s perceptions. Other barriers and facilitators differed according to perceptions, indicating the value of considering additional factors when designing interventions.
Although we have previously reported the role of a physician’s recommendation and older age in encouraging screening [10], we now draw attention to the consistency of these associations in two groups of women with different perceptions of mammography. A high proportion of women who were under age 40 or did not receive a physician’s recommendation were not having mammograms despite having positive perceptions. Likewise, even among women with negative or neutral perceptions, the proportion having mammograms was much greater among those who were over age 40 or received a physician’s recommendation. This underscores the importance of targeting young high-risk women for screening interventions, as their mammography practices reflect recommendations for the general population irrespective of their perceptions. Furthermore, these findings indicate that the influence of a physician’s recommendation may be strong enough to overcome or offset women’s perceptions of mammography, making this a valuable component of interventions for this high-risk cohort. Indeed, while almost three-quarters (72%) of women under 40 reported a clinical breast examination within the past year and 92% had a Papanicolaou smear within the past 2 years, only 33% reported a physician recommendation for a mammogram. Women who received a recommendation from a physician were three times as likely to have a mammogram.
Regardless of women’s perceptions, interest in health care was associated with mammography, reinforcing the importance of discussing breast cancer surveillance in the context of other routine health care practices. For women with negative or neutral perceptions, the proportion in action was greater among those who understood that mammograms were needed in the absence of symptoms or discomfort. Although a similar association has been documented in the general population [35, 36], we expand our knowledge by showing that modifying this particular perception may encourage screening even among those who do not recognize many benefits of mammography.
While several health behavior theories include coping and health locus of control, associations with breast cancer surveillance have been inconsistent [37–40]. In this study, both constructs were linked with mammography in women who had positive perceptions, were under age 40, and received a recent physician’s recommendation, indicating interactions for further study. Specifically, it is plausible that the physician’s recommendation could be particularly stressful for these women, given their young age and increased risk of breast cancer. Once stress activates the coping response, effects on screening practices may manifest. Despite their positive perceptions, 8 of 10 women with high acceptance coping and low active-planning coping were not having mammograms. This association is plausible, as acceptance is common among those who believe that a stressful situation (e.g., breast cancer risk) cannot be changed, and low active-planning coping signifies difficulty preparing and executing an appropriate response [27]. Among women with high active-planning coping, those who perceived themselves to be in control of their health were less likely to have mammograms. Although internal health locus of control has been linked with greater health care utilization [30] and active coping [41] in the general population, it is important to consider interactions with other factors [30, 42, 43]. For example, associations may differ for adult survivors of pediatric cancer as a result of their health care experiences. Of the few small studies to describe health locus of control in childhood cancer survivors [44–48], one linked greater internality with increased psychological distress [46], a factor that has been associated with low mammography rates among women with a family history of breast cancer [49, 50]. While additional research is needed in this population, interventions may wish to draw from a study showing that written messages which were matched to women’s health locus of control orientation increased mammography more effectively than unmatched messages [51].
In considering these findings, several limitations warrant discussion. By consolidating TTM stages of adoption and categorizing decisional balance into positive and negative/neutral perceptions, clinical interpretability improved, but residual heterogeneity within groups limited our ability to assess subtle differences among individuals. The CART models were exploratory and were not tested with an independent sample, so the misclassification percentages may be overly optimistic. Although the cross-sectional study design precluded inferences of temporality and causality, associations may inform future studies. Questions regarding social support factors that may influence mammography, such as marital status, were not included in the survey, thus limiting our ability to assess the association of these factors on screening practices. In the general population, it has been observed that mammography screening rates are often lower among racial and ethnic minority women in comparison with white non-Hispanic women. In contrast, we did not find a difference between minority and non-Hispanic white survivors. While generalization of this observation is limited by the small sample of minority participants in this study, we have previously reported from the entire CCSS cohort that general screening rates tend to be as high or higher (and unhealthy lifestyle behaviors lower) among African American and Hispanic women in comparison with white non-Hispanic women [52]. Lastly, as participants in the CCSS, the women in this study may be more aware of late effects and involved with health care than survivors who have not been followed in this longitudinal cohort study. However, it is unlikely that the correspondence between mammography perceptions and practices would differ systematically.
In this study of long-term pediatric cancer survivors at risk of breast cancer following chest radiation, we have identified and characterized a subgroup of women with inconsistent mammography perceptions and practices. Separate analyses for women with different perceptions of mammography expanded previous research by demonstrating the universality of some correlates of screening and specificity of others. We recommend that future interventions for this high-risk population consider a constellation of factors in addition to standard pros and cons focused counseling.
Acknowledgments
This work was supported by Grant R21-CA-106972 (K. C. Oeffinger, Principal Investigator) from the National Cancer Institute/Department of Health and Human Services and the Centers for Disease Control and Prevention, Grant U24-CA-55727 (L. L. Robison, Principal Investigator) from the Department of Health and Human Services, funding to the University of Minnesota from the Children’s Cancer Research Fund and funding to St. Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities (ALSAC).
Appendix A. Childhood Cancer Survivor Study (CCSS) institutions and investigators
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived 5 or more years after diagnosis of childhood cancer. CCSS is a retrospectively ascertained cohort of 20,346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4,000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (NCI grant # U24 CA55727) awarded to St. Jude Children’s Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss. See Table 3.
Table 3.
CCSS institutions and investigators
| St. Jude Children’s Research Hospital, Memphis, TN | Leslie L. Robison, PhD#‡, Melissa Hudson, MD*‡ |
| Greg Armstrong, MD, MSCE‡, Daniel M. Green, MD‡ | |
| Children’s Healthcare of Atlanta/Emory University, Atlanta, GA | Lillian Meacham, MD*, Ann Mertens, PhD‡ |
| Children’s Hospitals and Clinics of Minnesota Minneapolis, St. Paul, MN | Joanna Perkins, MD, MS* |
| Children’s Hospital and Medical Center, Seattle, WA | Douglas Hawkins, MD*, Eric Chow, MD, MPH‡ |
| Children’s Hospital, Denver, CO | Brian Greffe, MD* |
| Children’s Hospital Los Angeles, CA | Kathy Ruccione, RN, MPH* |
| Children’s Hospital, Oklahoma City, OK | John Mulvihill, MD*‡ |
| Children’s Hospital of Orange County, Orange, CA | Leonard Sender, MD* |
| Children’s Hospital of Philadelphia, Philadelphia, PA | Jill Ginsberg, MD*, Anna Meadows, MD‡ |
| Children’s Hospital of Pittsburgh, Pittsburgh, PA | Jean Tersak, MD* |
| Children’s National Medical Center, Washington, DC | Gregory Reaman, MD*, Roger Packer, MD‡ |
| Cincinnati Children’s Hospital Medical Center, Cincinnati, OH | Stella Davies, MD, PhD*‡ |
| City of Hope Medical Center, Los Angeles, CA | Smita Bhatia, MD*‡ |
| Cook Children’s Medical Center, Ft. Worth, TX | Paul Bowman, MD, MPH* |
| Dana-Farber Cancer Institute/Children’s Hospital, Boston, MA | Lisa Diller, MD*‡ |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, ScD*‡ |
| Hospital for Sick Children, Toronto, ON | Mark Greenberg, MBChB*, Paul C. Nathan, MD*‡ |
| International Epidemiology Institute, Rockville, MD | John Boice, ScD*‡ |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, MD* |
| Memorial Sloan-Kettering Cancer Center, New York, NY | Charles Sklar, MD*‡, Kevin Oeffinger, MD‡ |
| Miller Children’s Hospital, Long Beach, CA | Jerry Finklestein, MD* |
| National Cancer Institute, Bethesda, MD | Roy Wu, PhD‡, Nita Seibel, MD‡, Preetha Rajaraman, PhD‡ |
| Nationwide Children’s Hospital, Columbus, Ohio | Amanda Termuhlen, MD*, Sue Hammond, MD‡ |
| Northwestern University, Chicago, IL | Kimberley Dilley, MD, MPH* |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, MD* |
| Roswell Park Cancer Institute, Buffalo, NY | Martin Brecher, MD* |
| St. Louis Children’s Hospital, St. Louis, MO | Robert Hayashi, MD* |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, MD*, Sarah S. Donaldson, MD‡ |
| Texas Children’s Hospital, Houston, TX | Zoann Dreyer, MD* |
| University of Alabama, Birmingham, AL | Kimberly Whelan, MD, MSPH* |
| University of Alberta, Edmonton, AB | Yutaka Yasui, PhD*‡ |
| University of California-Los Angeles, CA | Jacqueline Casillas, MD, MSHS*, Lonnie Zeltzer, MD‡ |
| University of California-San Francisco, CA | Robert Goldsby, MD* |
| University of Chicago, Chicago, IL | Tara Henderson, MD, MPH* |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, MD* |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, MD, MPH*‡ |
| University of Southern California, Los Angeles, CA | |
| Dennis Deapen, DrPH*‡ | |
| UT-Southwestern Medical Center, Dallas, TX | Daniel Bowers, MD* |
| U.T.M.D. Anderson Cancer Center, Houston, TX | Louise Strong, MD*‡, Marilyn Stovall, MPH, PhD‡ |
Institutional principal investigator
Member CCSS steering committee
Project principal investigator (U24 CA55727)
Appendix B
See Table 4.
Table 4.
Pros and cons of mammography
| Pros items |
| Those people who are close to me will benefit if I have a mammogram |
| I would be more likely to have a mammogram if my doctor told me how important it was |
| Having a mammogram every year or two will give me a feeling of control over my health |
| Regular mammograms give you peace of mind about your health |
| Mammograms are necessary even when there is no history of breast problems in a family |
| Mammograms are most helpful when you have one every year or two |
| Cons items |
| If I have a breast exam from a doctor or nurse, I don’t need to have a mammogram |
| Mammograms have a high chance of leading to breast surgery that is not needed |
| Once you have a couple of mammograms that are normal, you don’t need to have any more for a few years |
| If a mammogram finds something, then whatever is there will be too far along to do anything about it anyway |
| I would probably not have a mammogram if my doctor seemed to doubt that I really needed one |
| If I eat a healthy diet, I will lower my risk of cancer far enough that I probably do not need to have a mammogram |
| I would probably not have a mammogram unless I had some breast symptoms or discomfort |
Appendix C
See Table 5.
Table 5.
Transtheoretical model stages of adoption
| Stage of adoption | Prior mammogram within the last 2 years | Plan mammogram within the next year |
|---|---|---|
| Precontemplation | No—never had a mammogram | No |
| Relapse | No—more than 2 years ago | No |
| Contemplation | No—never or more than 2 years ago | Yes |
| Risk of relapse | Yes—at least 1 in the last 2 years | No |
| Action | Yes—at least 1 in the last 2 years | Yes |
| Maintenance | Yes—at least 2 in the last 4 years | Yes |
Footnotes
A portion of this research was presented at the 10th International Conference on Long-Term Complications of Treatment of Children & Adolescents for Cancer in Niagara-on-the-Lake, Ontario, Canada (June 2008).
Contributor Information
Stephanie M. Smith, Department of Pediatrics, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA. Stanford University School of Medicine, 300 Pasteur Drive, Stanford, CA 94305, USA
Jennifer S. Ford, Departments of Psychiatry & Behavioral Sciences and Pediatrics, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA
William Rakowski, Department of Community Health, Brown University, Box G-S121, Providence, RI 02912, USA.
Chaya S. Moskowitz, Department of Epidemiology and Biostatistics, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065, USA
Lisa Diller, Dana-Farber Cancer Institute/Childrens Hospital Boston, 44 Binney Street, Boston, MA 02115, USA.
Melissa M. Hudson, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, USA
Ann C. Mertens, Emory University, 201 Dowman Drive, Atlanta, GA 30322, USA
Annette L. Stanton, University of California, 405 Hilgard Ave, Los Angeles, CA 90095, USA
Tara O. Henderson, University of Chicago, 5801 South Ellis Avenue, Chicago, IL 60637, USA
Wendy M. Leisenring, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., PO Box 19024, Seattle, WA 98109, USA
Leslie L. Robison, St. Jude Children’s Research Hospital, 262 Danny Thomas Place, Memphis, TN 38105, USA
Kevin C. Oeffinger, Email: oeffingk@mskcc.org, Departments of Pediatrics and Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10021, USA
References
- 1.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the late effects study group. J Clin Oncol. 2003;21(23):4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Constine LS, Tarbell N, Hudson MM, et al. Subsequent malignancies in children treated for Hodgkin’s disease: associations with gender and radiation dose. Int J Radiat Oncol Biol Phys. 2008;72(1):24–33. doi: 10.1016/j.ijrobp.2008.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guibout C, Adjadj E, Rubino C, et al. Malignant breast tumors after radiotherapy for a first cancer during childhood. J Clin Oncol. 2005;23(1):197–204. doi: 10.1200/JCO.2005.06.225. [DOI] [PubMed] [Google Scholar]
- 4.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: a report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141(8):590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 5.Metayer C, Lynch CF, Clarke EA, et al. Second cancers among long-term survivors of Hodgkin’s disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18(12):2435–2443. doi: 10.1200/JCO.2000.18.12.2435. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AJ, Winter DL, Stiller CA, Murphy M, Hawkins MM. Risk of breast cancer in female survivors of childhood Hodgkin’s disease in Britain: a population-based study. Inter J Cancer. 2007;120(2):384–391. doi: 10.1002/ijc.22261. [DOI] [PubMed] [Google Scholar]
- 7.Wolden SL, Lamborn KR, Cleary SF, Tate DJ, Donaldson SS. Second cancers following pediatric Hodgkin’s disease. J Clin Oncol. 1998;16(2):536–544. doi: 10.1200/JCO.1998.16.2.536. [DOI] [PubMed] [Google Scholar]
- 8.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group late effects committee and nursing discipline. J Clin Oncol. 2004;22(24):4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Children’s Oncology Group; 2007. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. [Online]. Updated October 2008 [cited 2009 April 17]; Available from: www.survivorshipguidelines.org. [Google Scholar]
- 10.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA. 2009;301(4):404–414. doi: 10.1001/jama.2008.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohl SJ, Moyer A. Tailored interventions to promote mammography screening: a meta-analytic review. Prev Med. 2007;45(4):252–261. doi: 10.1016/j.ypmed.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer L, Pagell F, Adams T. Applying the transtheoretical model to cancer screening behavior. Am J Health Behav. 2005;29(1):36–56. doi: 10.5993/ajhb.29.1.4. [DOI] [PubMed] [Google Scholar]
- 13.Rakowski W, Andersen MR, Stoddard AM, et al. Confirmatory analysis of opinions regarding the pros and cons of mammography. Health Psychol. 1997;16(5):433–441. doi: 10.1037//0278-6133.16.5.433. [DOI] [PubMed] [Google Scholar]
- 14.Rakowski W, Dube CA, Goldstein MG. Considerations for extending the transtheoretical model of behavior change to screening mammography. Health Educ Res. 1996;11(1):77–96. [Google Scholar]
- 15.Rakowski W, Fulton JP, Feldman JP. Women’s decision making about mammography: a replication of the relationship between stages of adoption and decisional balance. Health Psychol. 1993;12(3):209–214. doi: 10.1037//0278-6133.12.3.209. [DOI] [PubMed] [Google Scholar]
- 16.Clark MA, Rakowski W, Ehrich B, et al. Stages of adopting regular screening mammography: do women differ in decisional balance within stages? J Health Psychol. 1998;3(4):491–506. doi: 10.1177/135910539800300404. [DOI] [PubMed] [Google Scholar]
- 17.Velicer WF, Hughes SL, Fava JL, Prochaska JO, DiClemente CC. An empirical typology of subjects within stage of change. Addict Behav. 1995;20(3):299–320. doi: 10.1016/0306-4603(94)00069-b. [DOI] [PubMed] [Google Scholar]
- 18.Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172–181. doi: 10.1207/S15324796ABM2603_02. [DOI] [PubMed] [Google Scholar]
- 19.Breiman L, Friedman J, Olshen R, Stone C. Classification and regression trees. Wadsworth; Pacific Grove: 1984. [Google Scholar]
- 20.Cook EF, Goldman L. Empiric comparison of multivariate analytic techniques: advantages and disadvantages of recursive partitioning analysis. J Chron Dis. 1984;37(9/10):721–731. doi: 10.1016/0021-9681(84)90041-9. [DOI] [PubMed] [Google Scholar]
- 21.Calvocoressi L, Kasl SV, Lee CH, Stolar M, Claus EB, Jones BA. A prospective study of perceived susceptibility to breast cancer and nonadherence to mammography screening guidelines in African American and White women ages 40–79 years. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2096–2105. [PubMed] [Google Scholar]
- 22.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 23.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakowski W, Clark MA, Pearlman DN, et al. Integrating pros and cons for mammography and Pap testing: extending the construct of decisional balance to two behaviors. Prev Med. 1997;26(5 Pt 1):664–673. doi: 10.1006/pmed.1997.0188. [DOI] [PubMed] [Google Scholar]
- 26.Rakowski W, Dube CA, Marcus BH, Prochaska JO, Velicer WF, Abrams DB. Assessing elements of women’s decisions about mammography. Health Psychol. 1992;11(2):111–118. doi: 10.1037//0278-6133.11.2.111. [DOI] [PubMed] [Google Scholar]
- 27.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56(2):267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 28.Pui CH, Pei D, Sandlund JT, et al. Risk of adverse events after completion of therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(31):7936–7941. doi: 10.1200/JCO.2004.01.0033. [DOI] [PubMed] [Google Scholar]
- 29.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallston KA. The validity of the multidimensional health locus of control scales. J Health Psychol. 2005;10(5):623–631. doi: 10.1177/1359105305055304. [DOI] [PubMed] [Google Scholar]
- 31.Steinberg D, Colla P. CART—classification and regression trees. Salford Systems; San Diego: 1997. [Google Scholar]
- 32.Steinberg D, Golovnya M. CART 6.0 user’s manual. Salford Systems; San Diego: 2006. [Google Scholar]
- 33.Breiman L. Technical note: some properties of splitting criteria. Machine Learning. 1996;24:41–47. [Google Scholar]
- 34.Harrell FE., Jr . Regression modeling strategies. Springer; New York: 2001. [Google Scholar]
- 35.Rimer BK, Keintz MK, Kessler HB, Engstrom PF, Rosan JR. Why women resist screening mammography: patient-related barriers. Radiology. 1989;172(1):243–246. doi: 10.1148/radiology.172.1.2740510. [DOI] [PubMed] [Google Scholar]
- 36.Yabroff KR, Mandelblatt JS. Interventions targeted toward patients to increase mammography use. Cancer Epidemiol Biomarkers Prev. 1999;8(9):749–757. [PubMed] [Google Scholar]
- 37.Glenn BL, Moore LA. Relationship of self-concept, health locus of control, and perceived cancer treatment options to the practice of breast self-examination. Cancer Nurs. 1990;13(6):361–365. [PubMed] [Google Scholar]
- 38.Holm CJ, Frank DI, Curtin J. Health beliefs, health locus of control, and women’s mammography behavior. Cancer Nurs. 1999;22(2):149–156. doi: 10.1097/00002820-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Magai C, Consedine N, Neugut AI, Hershman DL. Common psychosocial factors underlying breast cancer screening and breast cancer treatment adherence: a conceptual review and synthesis. J Womens Health. 2007;16(1):11–23. doi: 10.1089/jwh.2006.0024. [DOI] [PubMed] [Google Scholar]
- 40.Murray M, McMillan C. Health beliefs, locus of control, emotional control and women’s cancer screening behaviour. Br J Clin Psychol. 1993;32(Pt 1):87–100. doi: 10.1111/j.2044-8260.1993.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 41.Masters K, Wallston K. Canonical correlation reveals important relations between health locus of control, coping, affect and values. J Health Psychol. 2005;10(5):719–731. doi: 10.1177/1359105305055332. [DOI] [PubMed] [Google Scholar]
- 42.Wallston K. The importance of placing measures of health locus of control beliefs in a theoretical context. Health Educ Res. 1991;6(2):251–252. [Google Scholar]
- 43.Wallston K. Hocus-pocus, the focus isn’t strictly on locus: Rotter’s Social Learning Theory modified for health. Cognit Ther Res. 1992;16(2):183–199. [Google Scholar]
- 44.Di Gallo A, Amsler F, Gwerder C, Burgin D. The years after: a concept of the psychological integration of childhood cancer. Support Care Cancer. 2003;11(10):666–673. doi: 10.1007/s00520-003-0494-0. [DOI] [PubMed] [Google Scholar]
- 45.Greenberg HS, Kazak AE, Meadows AT. Psychologic functioning in 8- to 16-year-old cancer survivors and their parents. J Pediatr. 1989;114(3):488–493. doi: 10.1016/s0022-3476(89)80581-5. [DOI] [PubMed] [Google Scholar]
- 46.Hill JM, Kornblith AB, Jones D, et al. A comparative study of the long term psychosocial functioning of childhood acute lymphoblastic leukemia survivors treated by intrathecal methotrexate with or without cranial radiation. Cancer. 1998;82(1):208–218. [PubMed] [Google Scholar]
- 47.Olson AL, Boyle WE, Evans MW, Zug LA. Overall function in rural childhood cancer survivors. The role of social competence and emotional health. Clin Pediatr (Phila) 1993;32(6):334–342. doi: 10.1177/000992289303200603. [DOI] [PubMed] [Google Scholar]
- 48.Jamison R, Lewis S, Burish T. Psychological impact of cancer on adolescents: self-image, locus of control, perception of illness and knowledge of cancer. J Chron Dis. 1986;39(8):609–617. doi: 10.1016/0021-9681(86)90186-4. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz MD, Taylor KL, Willard KS, Siegel JE, Lamdan RM, Moran K. Distress, personality, and mammography utilization among women with a family history of breast cancer. Health Psychol. 1999;18(4):327–332. doi: 10.1037//0278-6133.18.4.327. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz MD, Taylor KL, Willard KS. Prospective association between distress and mammography utilization among women with a family history of breast cancer. J Behav Med. 2003;26(2):105–117. doi: 10.1023/a:1023078521319. [DOI] [PubMed] [Google Scholar]
- 51.Williams-Piehota P, Schneider TR, Pizarro J, Mowad L, Salovey P. Matching health messages to health locus of control beliefs for promoting mammography utilization. Psychol Health. 2004;19(4):407–423. [Google Scholar]
- 52.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: a comparison of long-term outcomes, health care utilization and health related behaviors. J Clin Oncol. 2005;23(27):6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]