Abstract
Potential mechanisms of Passiflora incarnata extracts and the effect of extraction methods on ingredients and biological effects were explored. Using the same batch of plant material, total flavonoid yields as measured by high performance liquid chromatography coupled to diode array detection (HPLC-DAD) increased substantially with hot vs. cold extraction methods.
Whole Passiflora extract induced prominent, dose-dependent direct GABAA currents in hippocampal slices, but the expected modulation of synaptic GABAA currents was not seen. GABA was found to be a prominent ingredient of Passiflora extract, and GABA currents were absent when amino acids were removed from the extract.
Five different extracts, prepared from a single batch of Passiflora incarnata, were administered to CF-1 mice for one week in their drinking water prior to evaluation of their behavioral effects. Anticonvulsant effects against PTZ induced seizures were seen in mice that received two of the five Passiflora extracts. Instead of the anxiolytic effects described by others, anxiogenic effects in the elevated plus maze were seen in mice receiving any of the five Passiflora extracts.
Keywords: Flavonoid, GABAA receptor, Epileptic seizure, Elevated plus maze, Rotarod, Pentylenetetrazol
Introduction
Passiflora incarnata (Purple Passionflower) is an indigenous American vine with white and blue or purple flowers and an edible fruit (Dhawan et al. 2001a). Its medicinal use originated with native Americans (Spinella 2001), and its most popular uses are for insomnia and anxiety (Carlini 2003) as well as epilepsy (Spinella 2001). Passiflora incarnata is listed in the pharmacopoeias of Great Britain, United States, India, France, Germany, Switzerland and others (Dhawan et al. 2001b). The active ingredients have not been conclusively defined (Carlini 2003). Most available data suggests flavonoids as possible active ingredients (Speroni and Minghetti 1988; Dhawan et al. 2001b; Dhawan et al. 2003).
Studies in animal models show efficacy of Passiflora extracts and flavonoid fractions against pentylenetetrazol (PTZ) induced seizures (Speroni and Minghetti 1988; Speroni and Billi 1996; Nassiri-Asl et al. 2007; Nassiri-Asl et al. 2008). This effect of Passiflora can be inhibited by the benzodiazepine site antagonist Ro 15-1788, suggesting the involvement of GABAA receptors (Medina et al. 1990). Flavonoids bind with high affinity to the benzodiazepine site of the GABAA receptor (Medina et al. 1997; Marder and Paladini 2002), but appear to modulate GABAA and also GABAC receptor currents by a different mechanism than benzodiazepines (Goutman et al. 2003; Kavvadias et al. 2004).
In 3 clinical trials, Passiflora extracts showed anxiolytic efficacy. One of the trials compared Passiflora to placebo (Movafegh et al. 2008), and two others showed Passiflora to have anxiolytic efficacy similar to benzodiazepines (Mori et al. 1993; Akhondzadeh et al. 2001b). In addition, Passiflora extract showed sedative effects in 2 clinical trials (Akhondzadeh et al. 2001a; Movafegh et al. 2008).
In preparation for a clinical trial in epilepsy patients, potential mechanisms of Passiflora extracts and the effect of extraction method on ingredients and biological effects were explored. An initial extract was tested with full and with reduced amino acid content in a hippocampal slice preparation. Using several extraction methods, another 5 extracts were prepared from the same original plant material. All 5 extracts were analyzed for flavonoid and amino acid content, and tested for neurological effects in mice using the elevated plus maze, the rotarod, and the subcutaneous PTZ model of epileptic seizures after application in the drinking water for one week.
Methods
Passiflora extracts for in vitro testing in hippocampal slices
Whole extract
An extract of passionflower (Lot# PAS 02034C) was obtained from a local dietary supplement manufacturer, Oregon’s Wild Harvest (OWH), Sandy, OR. Fresh passionflower, collected from the wild, was steeped in 44% ethanol for 35 days. The extract was distilled to remove ethanol, and freeze dried to a dry powder (1 g equivalent to 25.78 g of fresh Passionflower herb or 5.6 g of dried plant material).
Amino acid-reduced extract
The freeze dried powder from above (3 g) was dissolved in a minimum amount of water and applied to a column containing EMD C-18 silica gel 60 (Sigma; 100 g), previously conditioned with methanol and water. Amino acids were eluted with water (1 l), and flavonoids by elution with 80% aqueous methanol containing 1% ammonia (400 ml), methanol:chloroform; water (48:30:12; 150 ml), chloroform (100 ml) and finally dichloromethane; methanol; ammonia (200:75:5; 175 ml). The water elution containing amino acids was freeze dried (extracted amino acids, 2.4 g). The combined organic elutions were dried on a centrifugal evaporator (amino-acid reduced extract, 0.8 g).
Thin layer chromatography (TLC) of amino acids in Passiflora extracts
Amino acids in the various Passiflora extracts (50 mg/ml; 10 μl) were compared by TLC on silica gel with n-butanol-acetone-glacial acetic acid-water 35:35:10:20 as mobile phase. Standards applied (1 mg/ml; 10 μl) were alanine, aspartic acid, cystine, GABA, glutamine, glycine, methionine, serine, tryptophan, and tyrosine (Sigma-Aldrich). Amino acids were visualized by spraying with 0.3% ninhydrin in n-butanol containing 3% acetic acid and heated for 10 minutes. Amino acids, including GABA were prominent in the total extract and amino acid extract, and virtually absent in the amino acid reduced extract. Using NIH ImageJ for simple densitometry, GABA content was semi-quantified as compared to a 1 mg/ml (10 μl) GABA standard.
Passiflora extracts for in vivo testing in CF1 mice
Passiflora herb source
Fresh Passiflora herb (flower, fruit, leaf, and stem) was collected from the wild in Salisbury, North Carolina, USA by Botanical Supply, Inc. and obtained from OWH (Lot# PAS035FWBO). A voucher specimen was deposited with the herbarium at Portland State University and confirmed to be Passiflora incarnata L.. A portion of the batch was air-dried in a drying room at OWH. Moisture content of the fresh and dried herb was determined as 75% and 5 % respectively, using an A&D MF-50 moisture analyzer.
Extraction methods
Five different extracts were prepared from fresh or dried Passiflora herb as described in Table 1. Extract PAS 1 was prepared at OWH, whereas extracts PAS 4, 5, 7 and 8 were prepared at OHSU. Ethanol was removed from extracts using a rotary evaporator, and residual water by freeze drying. The weight of the freezedried residue was calculated as percent of the weight of fresh or dried herb extracted, and corrected for moisture content to enable comparison of the extracts (Table 1).
Table 1. Extraction methods of the five extracts from P. incarnata.
Extraction methods of the five extracts from P. incarnata whole herb, and the relative yields of each extraction method are shown.
| Extract | Condition of herb | Extraction Temperature | Solvent | Duration of extraction | Extract weight as % w/w of plant material extracted | Extract weight as % w/w of dry plant material* |
|---|---|---|---|---|---|---|
| PAS 1 | Fresh | 25°C | 65% Ethanol | 14 days | 2.12 | 8.5 |
| PAS 4 | Fresh | 100°C then 4°C |
Water Water |
75 min 21 h |
6.00 | 24.0 |
| PAS 5 | Dried | 4°C | 65% Ethanol | 14 days | 7.30 | 7.7 |
| PAS 7 | Dried | 100°C then 4°C |
65% Ethanol 65% Ethanol |
65 min 19 h |
9.34 | 9.8 |
| PAS 8 | Dried | 100°C then 4°C |
Water Water |
60 min 20 h |
8.40 | 8.8 |
Corrected for moisture content i.e.75% of fresh herb and 5% of dry herb.
HPLC
A chemical fingerprint of the freeze-dried Passiflora extracts was obtained using an Agilent HPLC-DAD apparatus with an Econosil 5 micron C18 column. A stepwise, binary gradient of acetonitrile and water, each containing 0.1% acetic acid, was applied. The percentage of acetonitrile was 5, 25, 50 and 70 at 0, 20, 35 and 40 min respectively, with a return to starting conditions from 45 to 48 min. UV absorbance was monitored at 205 nm, 254 nm, 290 nm, 330 nm, and 350 nm. For LC-MS, full scan electrospray ionization mass spectra of the eluent were obtained using a ThermoElectron LCQ Advantage 3D Ion trap tandem mass spectrometer (San Jose, CA). The ionization source was operated in the negative mode with spray voltage 4500 V, sheath gas 35, auxiliary gas 15, capillary temperature 275°C and tube lens 40 V. Total and individual flavonoids were estimated against vitexin as a standard, using peak areas obtained at 330 nm.
Hippocampal slice physiology
Male Sprague Dawley albino rats were obtained from and housed in the OHSU NSI vivarium. Care and use of the animals was approved by the OHSU Animal Research Committee and performed according to its policies and guidelines.
Hippocampal slices were prepared from young (2–3 week old) rats and kept viable by perfusion with O2-saturated artificial CSF as described (Rossi et al. 2000). Electrical currents induced in the cell membrane by Passiflora total extract and amino acid reduced extract were measured under whole-cell voltage clamp conditions (Rossi et al. 2000). All current traces and the mean values are from CA1 pyramidal cells voltage-clamped at −30 mV with ECl- set to −60 mV, so GABAA mediated chloride currents were outward currents. Blocking a GABAA mediated current resulted in an inward current. All experiments were performed in the presence of the glutamate receptor antagonist kynurenic acid (1mM).
In vivo studies in CF-1 mice
Male CF-1 mice were obtained from and housed in the OHSU vivarium. Care and use of the animals was approved by the OHSU Animal Research Committee and performed according to its policies and guidelines. Each of the 5 different passionflower extracts was tested on a group of CF-1 mice after continuous administration in the drinking water (1000 mg freeze dried extract/kg/day; equivalent to between 4.2 and 13 g of dry herb/kg/day depending on preparation method) for one week and compared to a group of control mice which received normal drinking water.
PTZ-induced seizure model
Effects of passionflower on seizure activity induced by subcutaneous injection of 85 mg/kg pentylenetetrazol (PTZ) (Swinyard et al. 1989) were determined in groups of 8 CF-1 mice for each of the 5 passionflower extracts and compared to a group of 28 control mice. At this dose of the proconvulsant, 97% of unprotected mice typically experience clonic seizures for at least 5 minutes (Swinyard et al. 1989). Over an observation time of one hour after PTZ injection, the number of stage 2 seizures (face and forelimb clonus), and of stage 4 seizures (tonic hindlimb extension) was counted for each mouse simultaneously by 2 or 3 trained observers blinded to the animals’ treatment status.
Measures of Anxiety in the Elevated Plus maze and Sensorimotor Function on the Rotarod
Groups of 8 CF-1 mice for each of the 5 passionflower extracts and 8 control mice were tested for sensorimotor function using a rotarod (Kinder Scientific, Poway, CA), as described (Acevedo et al. 2006). In the same mice, measures of anxiety were assessed using the elevated plus maze (Kinder Scientific, Poway, CA), as described (Acevedo et al. 2006).
Results
Chemical analysis of Passiflora extract ingredients
1. Passionflower extract analysis by high-performance liquid chromatography (HPLC)
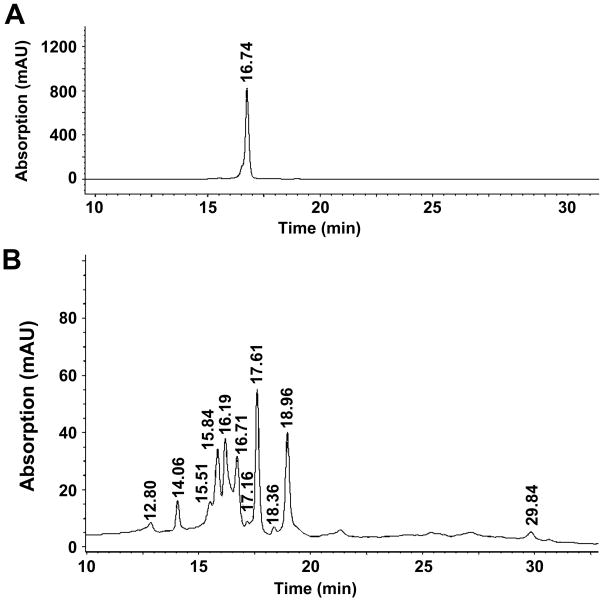
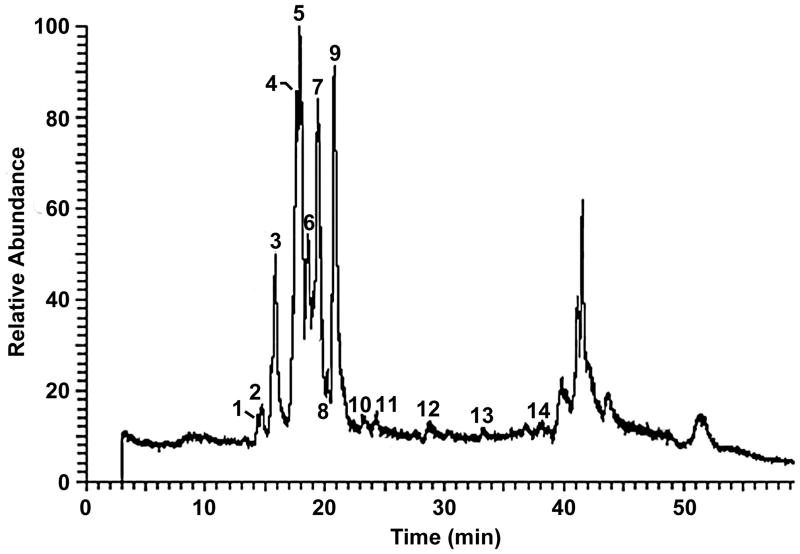
To determine the effect of different extraction methods on flavonoid content, chemical fingerprints of the five Passiflora extracts prepared by different methods (Table 1) were obtained using HPLC-DAD and LC-MS. A typical Passiflora flavonoid profile with 11 peaks was detected; Fig. 1 shows the profile of PAS1 as an example. The identity of the major peaks (Table 2) was derived by obtaining molecular weights from LC-MS (Fig. 2) and comparison of their elution order to published reports (Raffaelli et al. 1997; Bilia et al. 2002). Peaks seen by LC-MS beyond 39 minutes elution time (Fig. 2) do not appear when using UV detection at wavelengths above 250nm, suggesting that these relatively lipophilic components are not flavonoids, lack aromatic rings and may represent terpenoids.
Fig. 1.
HPLC analysis of (A) the flavonoid standard vitexin, and (B) Passiflora incarnata extract (PAS1) using UV detection at 330 nm.
Table 2. Likely identity of flavonoid peaks in Passiflora extract (PAS1).
Likely identity of flavonoid peaks in Passiflora extract (PAS1) based on elution order and molecular weight derived from negative ion electrospray LC-MS. Peak# corresponds to peak numbers in Fig. 2.
| Peak # | Retention Time (min.) | m/z from mass spectrum (relative abundance) | Likely identity of peak (MW) |
|---|---|---|---|
| 1 | 14.40 | 609.33(100) | Isoorientin-2″-O-β-glucopyranoside (610) |
| 2 | 14.77 | 609.27(100) | Isoorientin-2″-O-β-glucopyranoside (610) |
| 3 | 15.87 | 593.27(100) & 661.07(13) | Vicenin-2 (594) |
| 4 | 17.65 | 563.27(100) | Isoschaftoside (564) |
| 5 | 17.93 | 563.27(100) | Schaftoside (564) |
| 6 | 18.59 | 447.20(100) | Isoorientin (448) |
| 7 | 19.69 | 593.20(100) & 431.20(17) | Isovitexin-2″-O-β-glucopyranoside (594) & Vitexin (432) |
| 8 | 20.18 | 623.20(100) & 447.20(75) | Unknown compound (624) & Orientin (448) |
| 9 | 20.78 | 431.20(100) | Isovitexin (432) |
| 10 | 23.24 | 520.80(100) & 608.60(97) | Unknown |
| 11 | 24.36 | 520.80(100) & 515.27(83) | Unknown |
| 12 | 28.69 | 301.13(100) & 520.87(94) | Unknown compound (521) & Quercetin (302) |
| N/A | 31.72 | 520.87(100) & 269.33(26) | Unknown compound (521) & Apigenin (270) |
| 13 | 33.27 | 520.87(100) & 248.93(56) | Unknown |
| 14 | 36.83 | No data | Unknown |
Fig. 2.
LC-MS analysis of Passiflora incarnata extract (PAS1). Numbers above the peaks indicate the designated peak number. The likely identification of the numbered peaks is given in Table 2.
A comparison of flavonoid content obtained with each of the 5 different extraction methods (Table 3) showed that there were large differences in total flavonoid yields with different extraction methods. Highest flavonoid yields were obtained using a hot (100°C) solvent (PAS 4, PAS 7, PAS 8, Table 1). Using only cold (4°C) extraction (PAS 1, PAS 5) resulted in about 10-fold lower total flavonoid yield. This was independent of solvent composition (ethanol or water), and independent of the use of fresh or dry herb. Similar results were also seen with additional, different extraction methods (data not shown). In contrast, the general flavonoid profile was not substantially affected by different extraction methods. All extracts appear to follow a similar pattern, with isoschaftoside, schaftoside, isovitexin and isovitexin glucoside forming the major components. The relative proportions of these components varied somewhat.
Table 3. LC-MS flavonoid analysis of the 5 different Passiflora extracts.
Summary LC-MS flavonoid analysis of the 5 different Passiflora extracts using UV detection at 330 nm. The total flavonoid content, obtained as the sum of peak areas relative to vitexin, of each of the 5 different extracts is shown, along with the relative percent distribution of those flavonoids that could be identified with some certainty by comparison to the mass spectrometric analysis (Fig. 2, Table 2).
| Extract | Total Flavonoid content (% w/w of freeze-dried extract) | Relative content of individual flavonoids (% of total flavonoid) | ||||||
|---|---|---|---|---|---|---|---|---|
| Isoorientin-2″-O-β-glucopyran-oside | Vicenin-2 | Isoschafto-side | Schaftoside | Isoorientin | Isovitexin-2″-O-β-glucopyrano-side | Isovitexin | ||
| PAS 1 | 3.0% | 1.8% | 3.6% | 14.5% | 19.1% | 13.2% | 20.1% | 18.9% |
| PAS 4 | 30.6% | 0.5% | 2.8% | 13.3% | 18.3% | 7.6% | 24.2% | 14.5% |
| PAS 5 | 2.8% | 1.6% | 3.9% | 15.4% | 19.7% | 16.1% | 15.9% | 20.0% |
| PAS 7 | 46.4% | 0.9% | 5.3% | 20.7% | 13.9% | 10.3% | 17.4% | 19.3% |
| PAS 8 | 20.3% | 1.1% | 7.1% | 23.4% | 14.9% | 8.0% | 17.6% | 12.2% |
2. Passionflower extract amino acids by thin-layer chromatography (TLC)
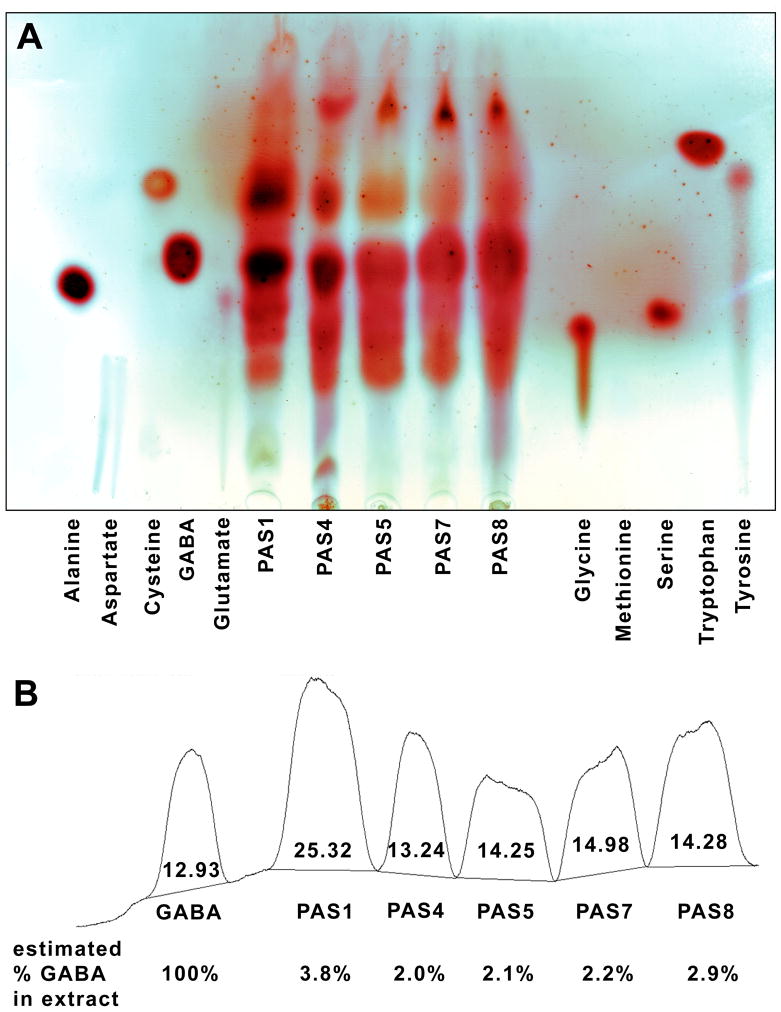
To determine the effect of different extraction methods on amino acid content, a simple analysis was done using TLC-densitometry. Fig. 3 shows a TLC chemical fingerprint analysis for amino acids in the five different Passiflora extracts.
Fig. 3.
TLC chromatogram (A) and semiquantification by densitometry (B) of amino acids in the five different Passiflora extracts.
GABA was the dominant amino acid in all extracts (Fig. 3A). The highest amino acid and GABA content was seen in extract PAS 1 (Fig. 3B), prepared from fresh herb by cold extraction (Table 1). This suggests that using either dried herb or hot extraction may reduce the proportion of amino acids in the extract.
In vivo and in vitro biological assays of extract properties
3. Direct GABA agonist effect of Passiflora extract on hippocampal pyramidal cells
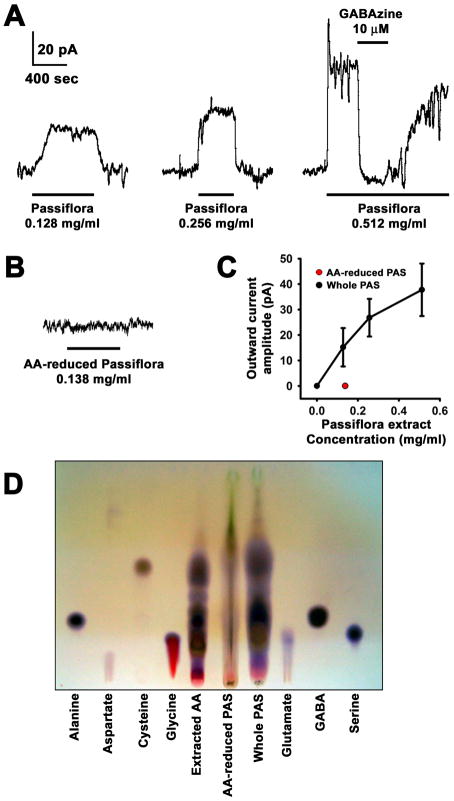
Experiments were conducted to test potential effects of whole Passiflora extract in CA1 pyramidal cells in hippocampal slices. Bath application of Passiflora extract to voltage-clamped pyramidal cells did not affect the rise time, amplitude or decay time of electrically-evoked synaptic currents mediated by pharmacologically identified GABAA receptors (data not shown). However, the extract did evoke a direct current with a dose-dependent amplitude (Figs. 4A and C). This was mediated by GABAA receptors, as evidenced by its complete but reversible block by the GABAA receptor antagonist GABAzine (10 μM, Fig. 4A). The GABAA receptor current was likely elicited by GABA present in the extract, because no current was induced by extracts from which GABA and other amino-acids had been largely removed (Figs. 4B, C and D).
Fig. 4.
Hippocampal pyramidal cell responses to Passiflora extract. Each specimen trace is from a different cell. The scale bar is the same for all traces. A, Traces of inhibitory currents elicited by Passiflora extract. Inhibition by GABAzine was tested in 2 cells at 0.128 mg/ml, 3 cells at 0.256 mg/ml and 2 cells at 0.512 mg/ml Passiflora extract. In all 7 cases, the block by GABAzine (10 μM) was 100%. B, No currents were seen with amino acid reduced Passiflora extract. C, Dose response curve with points representing 0 mg/ml, 0.128 mg/ml (n=6), 0.256 mg/ml (n=5) and 0.512 mg/ml (n=4). The amino acid reduced extract (red dot) was tested at 0.138 mg/ml (n=8). D, TLC comparison of whole Passiflora extract (Whole PAS), amino acid-reduced PAS extract (AA-reduced PAS), and amino acids removed from PAS extract (Extracted AA) with standard amino acids including GABA.
4. Effects of Passiflora incarnata extracts on PTZ-induced seizures, anxiety and sensorimotor function
To determine potential effects of extraction method on biological effects, the five different Passiflora extracts were tested in established animal models of epilepsy (PTZ-induced seizures, Fig. 5), anxiety (elevated plus maze, Fig. 6) and sensorimotor function (rotarod, Fig. 7) in mice.
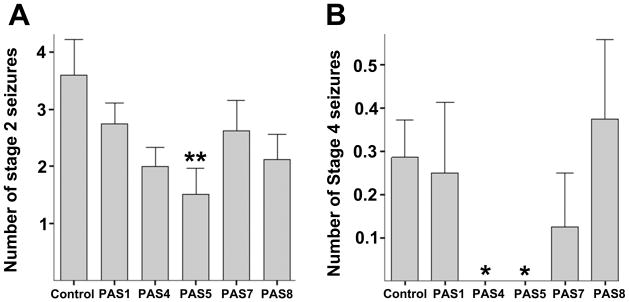
Fig. 5.
Effects of Passiflora extracts on pentylenetetrazol (PTZ) induced seizures in CF-1 mice. A, the average number of stage 2 seizures (face and forelimb clonus). B, the average number of stage 4 seizures (fatal tonic hindlimb extension). Means and standard errors are shown, and statistically significant differences versus control (**) as well as trends toward significance (*) are noted (**p < 0.05, *p < 0.08, one-way ANOVA followed by Tukey’s LSD, two-tailed).
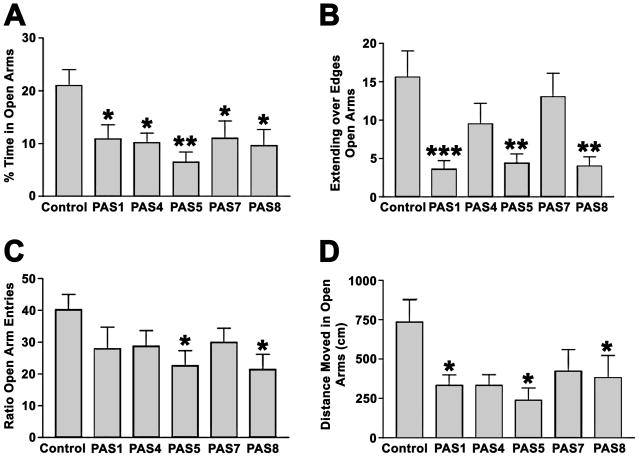
Fig. 6.
Effect of Passiflora extracts on CF-1 mice in the elevated plus maze. A, percent time spent in the open arms (time spent in open arms/(time spent in open + closed arms). B, Extending over edges of the open arms. C, ratio of entries into open arms over entries into open + closed arms. D, distance moved in the open arms. Means and standard errors are shown, *p < 0.05 versus control, **p < 0.01 versus control, ***p < 0.001 versus control, one-way ANOVA followed by Dunnet’s posthoc test, two-tailed. All extracts show anxiogenic effects in one or more measures of anxiety.
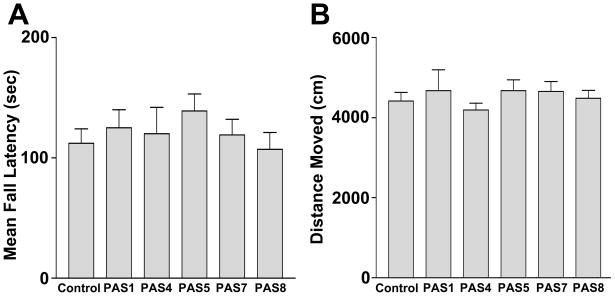
Fig. 7.
Effect of 5 extracts on sensorimotor activity in the rotarod (A, average of 3 trials) and total distance moved in the plus maze (B). None of the extracts resulted in a reduction of locomotor activity.
Two of the extracts, PAS4 and PAS5, reduced the frequency and severity of PTZ-induced seizures (Fig. 5). Surprisingly, all of the Passiflora extracts increased anxiety levels when compared to control, as evidenced by significantly reduced time in the open arms of the elevated plus maze and other measures of anxiety (Fig. 6). These anxiogenic effects were most prominent in mice treated with extracts PAS 5 and PAS 8. None of the extracts had an effect on sensorimotor function, assessed as fall latency on the rotarod (Fig. 7A) or the total distance moved in the elevated plus maze (Fig. 7B). These data confirm that potential sedative effects of Passiflora extracts did not contribute to their observed behavioral effects in the plus maze.
5. Comparison of extract properties following 5 different extraction methods
A summary of all extract properties is shown in Table 4. Comparing ingredient and biological activity from all extracts, there was no correlation between total flavonoid or GABA content with anxiogenic or anticonvulsant effects. The two extracts with the highest GABA content (PAS 1 and PAS 8) did not show anticonvulsant effects, and the two extracts with anticonvulsant effects differed widely in their total flavonoid content (PAS 4 and PAS 5). Similarly, there was no correlation between GABA content and anxiogenic effects.
Table 4.
Content and biological effects of the five Passiflora extracts
| Extract | Extract yield (% w/w of dry plant material) | Flavonoid content (% w/w of the extract) | GABA content (% w/w of the extract) | Dose of dry passiflora herb equivalent to 1000 mg/kg/day of extract (g/kg/day) | Anxiogenic effects | Anti-convulsant effects |
|---|---|---|---|---|---|---|
| PAS 1 | 8.5 | 3.0% | 3.8% | 11.8 | Medium | Absent |
| PAS 4 | 24.0 | 30.6% | 2.0% | 4.2 | Low | Present |
| PAS 5 | 7.7 | 2.8% | 2.1% | 13.0 | High | Present |
| PAS 7 | 9.8 | 46.4% | 2.2% | 10.2 | Low | Absent |
| PAS 8 | 8.8 | 20.3% | 2.9% | 11.4 | High | Absent |
Discussion
In the present study of 5 extracts prepared from a single batch of Passiflora incarnata, we found that different extraction methods influenced the extract yield and total flavonoid and GABA content. Extraction method also affected the anticonvulsant potential. In contrast, the relative proportions of specific flavonoids, and the surprising anxiogenic effects seen in all tested extracts were not much affected by the extraction method. There was no correlation between measured flavonoid and GABA content with anticonvulsant effects of the extracts.
GABA and anticonvulsant effects
While many plant extracts contain amino acids, Passiflora extracts were found to have the highest GABA content of 21 examined plants (Carratù et al. 2008). Our studies show that Passiflora extracts not only contain a high amount of GABA, but are also able to induce direct GABAA currents in CA1 hippocampal pyramidal neurons. Since the extract with reduced amino acid levels induced no current, it is likely that the GABA content of the extract is sufficient to explain the observed GABA currents in vitro. As the main endogenous inhibitory neurotransmitter, GABA might be expected to act as a natural anticonvulsant. However, we did not find a correlation between an extract’s GABA content and its anticonvulsant activity in vivo. Since orally administered GABA is subject to active transport across membranes both in the intestinal tract as well as across the blood-brain barrier (Takanaga et al. 2001), the pharmacological significance of GABA-content in Passiflora extract is still unclear.
Anxiogenic effects
Our study is the first to administer Passiflora incarnata extracts in the drinking water for 1 week prior to behavioral testing in CF1 mice, thus closely mimicking regular intake using the oral route in humans. Strikingly, our data showed an increase in anxiety measures. Importantly, the activity of the mice was not affected, excluding the possibility that potential alterations in activity contributed to the increase in anxiety measures. Consistent with this, we also found that intraperitoneal (i.p.) injections of C57Bl6/J wild-type mice or mice deficient in apolipoprotein E once 1 hour prior to behavioral testing increased measures of anxiety (data not shown). In contrast to our findings, other studies have reported anxiety-reducing effects of Passiflora extracts following single i.p. injections or oral (p.o.) administration (Table 5). Differences between Passiflora botanical species tested, geographical source of plants, extraction methods, dose, method and duration of administration, vehicle used, test animal species and strain, baseline anxiety levels and potential effects of the extracts on activity levels might all have contributed to the divergent findings. In our studies, the baseline anxiety levels in the vehicle-treated animals were such that we could easily detect both increases and decreases in measures of anxiety. However, for all previous animal studies on effects of P. incarnata on anxiety (Table 5), baseline anxiety levels were very high, with animals spending 5% or less time spent in open arms of the plus maze. Clearly, it is difficult if not impossible to detect a potential anxiogenic effect under these experimental conditions. In addition, potential effects on activity levels could contribute to group differences in performance in anxiety mazes. There, it is key to assess activity levels to exclude these potential confounds but not in all studies is this actually done. This is particularly important for anxiety-modulating compounds that often affect activity levels at higher doses. Clearly, future studies are warranted to determine the mechanisms underlying these differential effects. However, together these data support the concept that the effects of Passiflora incarnata extracts on measures of anxiety probably depend on the baseline anxiety state of the animals. In turn, this suggests that the extracts might function as modulators of one or several neurotransmitter systems, similar to positive and negative allosteric modulators whose action critically depends on the presence or absence of endogenous neurotransmitters whose levels would vary with baseline anxiety states. The anxiogenic results observed here, in vivo, also contradict reported clinical anxiolytic effects of Passiflora incarnata. However, the clinical results are specific to the Passiflora product tested. Plant extracts containing so many different constituents can exhibit a variety of effects, sometimes contradictory, depending on the chemical composition, route of administration and dosage of the individual extracts used. Furthermore in vitro and in vivo effects of pharmacological agents do not always correlate directly to clinical studies, due to issues of bioavailability, metabolism, and species differences. Additionally, the clinical data (Table 5) is not without limitations. Two of the published clinical studies of Passiflora extracts did not have a placebo control (Mori et al. 1993; Akhondzadeh et al. 2001b), and the third (Movafegh et al. 2008) measured anxiety by an unusual, possibly not standardized scale. Large, well-designed clinical studies, possibly comparing a variety of Passiflora extracts, are needed for a more complete understanding of the range of clinical actions of Passionflower.
Table 5.
Previous reports on anxiolytic effects of Passiflora extracts
| Species tested and strain |
Plant species |
Source of plant material |
Effective extraction method |
Type of effect |
Vehicle: % time in open arms |
Motility change? |
Effective dose |
Vehicle used |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| 10 Swiss albino mice/group | P. incarnata | Collected in Bari, Italy | Hydroethanolic | Anxiolytic (staircase test) | N/A | increased | 100–800 mg/kg i.p. | 0.9% saline | (Soulimani al. 1997) |
| 5 Swiss mice/group | P. incarnata | Cultivated in Saharanpur, India | Methanol, petroleum ether, chloroform, or water | Anxiolytic (plus maze) | 0 % | not measured | 75–300 mg/kg p.o. | 1% carboxymethyl cellulose, 66% sucrose | (Dhawan et al. 2001b) |
| 5 Swiss albino mice/group | P. incarnata | Cultivated in Saharanpur, India | Fractions from methanolic extract | Anxiolytic (plus maze) | 0 % | not measured | 20–100 mg/kg p.o. | 1% carboxymethyl cellulose, 66% sucrose | (Dhawan et al. 2001c) |
| 5 Swiss albino mice/group | P. incarnata | German and Indian Suppliers | Multiple methods | Anxiolytic (plus maze) | 0 % | not measured | 100–400 mg/kg p.o. | 1% carboxymethyl cellulose, 66% sucrose | (Dhawan et al. 2002) |
| 9–10 Wistar rats/group | P. alat P. edulis |
Collected in Rio Grande, Brazil | Hydroethanolic | Anxiolytic (plus maze) | 35% | no change/increased | 50–150 mg/kg i.p. | Propylene glycol and saline | (Petry et al. 2001) |
| 12 Wistar rats/group | P. alata P. edulis |
Collected in Rio Grande, Brazil | Hot water extraction | Anxiolytic (plus maze) | 35% | no change/increased | 400–800 mg/kg p.o. | Propylene glycol and saline | (Reginatto al. 2006) |
| 10 BL6/C57 mice/group | P. incarnata | Pascoe Pharmaceutic al Co. Germany | Hydroethanolic | Anxiolytic (plus maze) | 5 % | No change | 150–600 mg/kg p.o. | 0.5% propylene glycol | (Grundmann et al. 2008) |
| 7–10 Swiss mice/group | P. edulis | Collected in Antonio Carlos, Brazil | Hot water extraction of pericarp | Anxiolytic (light/dark box) | N/A | No change | 100–300 mg/kg p.o. | 0.9% saline | (Sena et al. 2009) |
| RCT in 79–83 patients/group | P. incarnata | Maruho Co. Japan | Hydroethanolic | Anxiolytic (clinical scoring) | 90–180 mg/day p.o. | (Mori et al. 1993) | |||
| RCT in 18 patients/group | P. incarnata | Iran Darouk Co. | Hydroethanolic | Anxiolytic (HAM-A) | 45 drops/day p.o. | (Akhondzadeh et al. 2001b) | |||
| RCT in 30 patients/group | P. incarnata | Iran Darouk Co. | Hydroethanolic | Anxiolytic (NRS) | 500 mg p.o. x1 | (Movafegh al. 2008) |
Role of flavonoids
Are flavonoids the active ingredient of Passiflora? In the more recent literature, flavonoids are considered the most likely active ingredient (Speroni and Minghetti 1988; Dhawan et al. 2001b; Dhawan et al. 2004), because some flavonoids have anxiolytic properties in mice similar to benzodiazepines (Zanoli et al. 2000), and modulate (Kavvadias et al. 2004) or inhibit (Goutman et al. 2003) GABAA and GABAC receptor currents. However, while benzodiazepines typically show anticonvulsant, anxiolytic and sedative effects, Passiflora extracts in our study showed only anticonvulsant effects, no sedation and anxiogenic instead of the expected anxiolytic effects. Recent studies have shown that specific benzodiazepine-induced pharmacological effects are mediated by specific GABAA receptor subtypes; agonists acting on the α1 subunit show predominantly sedative effects and partially anticonvulsant effects, while agonists acting on the α2 subunit show predominantly anxiolytic effects (Rudolph and Möhler 2006; Fradley et al. 2007). Since the natural flavonoids tested to date (hispidulin, apigenin, and quercetin) all seem to modulate α1GABA receptors, they may be more effective as anticonvulsants than as anxiolytics. It is also conceivable that the unexpected anxiogenic activity of Passiflora extracts under some conditions, such as dosage changes, may result from flavonoids which change from weak agonists to weak antagonists at specific GABAA receptor subtypes (Rudolph and Möhler 2006). The reported narrow dose range of anxiolytic effects of Passiflora (Dhawan et al. 2001b; Grundmann et al. 2008) may also suggest the presence of both anxiolytic and anxiogenic components with different dose/response profiles, possibly with increased anxiogenic effect at higher doses.
Our studies in CA1 pyramidal cells in hippocampal slices are the first to examine whole Passiflora extract rather than isolated flavonoids, and, contrary to expectation, did not show benzodiazepine-like modulation of synaptic GABAA currents. One reason for this may be the low concentration of individual flavonoids in whole Passiflora extract, as pointed out by other authors (Speroni et al. 1996). At the concentrations of whole extract used in our experiments (0.128–0.512 mg/ml), according to our HPLC analysis individual flavonoids are likely to have ranged up to 2 μg/ml; e.g. quercetin can be estimated at about 0.34 pM, which is well below its reported ED50 of 30 μM to inhibit α1β1γ2s GABAA and ρ1 GABAC receptors.
Another reason for the absence of GABAA receptor modulation by whole Passiflora extract could be that the receptor characteristics of the hippocampal CA1 pyramidal cells tested were different from the receptor types shown to be modulated by flavonoids (inhibition by apigenin and quercetin, and modulation by hispidulin in α1β1γ2sGABAA, α1γ2γ2sGABAA and ρ 1GABAC receptors, but no modulation in α1β2GABAA receptors (Kavvadias et al. 2004)).
It is also possible that we missed a phenomenon described as “second order positive modulation” of GABAA receptors (Campbell et al. 2004). This has been described in flavonoids such as apigenin (present in Passiflora incarnata), and means that GABA responses are only enhanced in the presence of a first order positive modulator such as diazepam. Endogenous first order GABAA modulators such as steroids and other hormones would not have been present in our slice experiments.
There are several reports suggesting that a poorly defined, tri-substituted benzoflavone compound termed BZF is the active ingredient of Passiflora incarnata (Dhawan et al. 2003; Dhawan et al. 2004). No matching compound was detected in our studies after careful examination by TLC or LC-MS following the published descriptions (Dhawan et al. 2003; Dhawan et al. 2004). Since our studies showed little variation in relative abundance of specific flavonoids with extraction methods, if present but not detected, the benzoflavone compound would have been expected to co-vary with total flavonoid content, which was not correlated with anticonvulsant or anxiogenic effects in our study.
In summary, the true active ingredients of Passiflora incarnata extract remain elusive. They might comprise one or more specific flavonoids that we were unable to individually detect and characterize, or they might be compounds of altogether different chemical characteristics such as the unidentified lipophilic compounds seen in our LC-MS study. Passiflora bioactivity may also result from synergistic actions of several compounds, such as a combination of GABA with additional compounds which may facilitate its membrane permeation, and possibly along with second order positive modulation of GABAA receptors by flavonoids (Campbell et al. 2004). The recent literature, e.g. on interactions between multiple sedative ingredients of Valeriana officinalis (Marder et al. 2003; Fernández et al. 2004; Granger et al. 2005), suggests that such synergistic interactions between several active constituents within the same extract may be a common finding in natural products (Johnston et al. 2006). Future efforts are clearly warranted to determine the active ingredients of Passiflora and the mechanisms underlying their behavioral effects.
Acknowledgments
This study was supported by NCCAM K23 grant AT01993-01 to S.M.E., NINDS R01 NS051561 to D.J.R, NIMH R01 MH77647 to J.R., NCCAM grant AT002656 (W.G.) and a career development award from NIH P50 AT00066 to A.S. The authors wish to thank Randy Buresh, Nate Couture, Joanne Roberts and Matthew Cresswell of Oregon’s Wild Harvest for providing Passiflora incarnata fresh herb and preparing some of the extracts for these studies. HPLC-DAD and LC-MS were performed at the Bioanalytical Shared Resource Pharmacokinetic Core laboratories at OHSU.
Footnotes
Disclosure of Conflict of Interest
A. S. is a part-time employee of Oregon’s Wild Harvest, a company which manufactures botanical extracts and has provided Passiflora incarnata fresh herb and some of the extracts for these studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo SF, I, de Esch JP, Raber J. Sex- and Histamine-Dependent Long-Term Cognitive Effects of Methamphetamine Exposure. Neuropsychopharmacology. 2006;32(3):665–672. doi: 10.1038/sj.npp.1301091. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Kashani L, Mobaseri M, Hosseini S, Nikzad S, Khani M. Passionflower in the treatment of opiates withdrawal: a double-blind randomized controlled trial. Journal of Clinical Pharmacy and Therapeutics. 2001a;26:369–373. doi: 10.1046/j.1365-2710.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Nhaghavi H, Vazirian M, Shayeganpour A, Rashidi H, Khani M. Passionflower in the treatment of generalized anxiety: a pilot double-blind randomized controlled trial with oxazepam. Journal of Clinical Pharmacy and Therapeutics. 2001b;26:363–367. doi: 10.1046/j.1365-2710.2001.00367.x. [DOI] [PubMed] [Google Scholar]
- Bilia AR, Bergonzi MC, Gallori S, et al. Stability of the constituents of Calendula, Milk-thistle and Passionflower tinctures by LC-DAD and LC-MS. Journal of Pharmaceutical and Biomedical Analysis. 2002;30:613–624. doi: 10.1016/s0731-7085(02)00352-7. [DOI] [PubMed] [Google Scholar]
- Campbell EL, Chebib M, Johnston GAR. The dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABAA receptors. Biochemical Pharmacology. 2004;68(8):1631. doi: 10.1016/j.bcp.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Carlini EA. Plants and the central nervous system. Pharmacology, Biochemistry and Behavior. 2003;75:501–512. doi: 10.1016/s0091-3057(03)00112-6. [DOI] [PubMed] [Google Scholar]
- Carratù B, Boniglia C, Giammarioli S, Mosca M, Sanzini E. Free Amino Acids in Botanicals and Botanical Preparations. Journal of Food Science. 2008;73(5):C323–C328. doi: 10.1111/j.1750-3841.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- Dhawan K, Dhawan S, Chhabra S. Attenuation of benzodiazepine dependence in mice by a tri-substituted benzoflavone moiety of Passiflora incarnata Linneaus: A non-habit forming anxiolytic. J Pharm Pharmaceut Sci. 2003;6(2):215–222. [PubMed] [Google Scholar]
- Dhawan K, Dhawan S, Sharma A. Passiflora: a review update. Journal of Ethnopharmacology. 2004;94:1–23. doi: 10.1016/j.jep.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Dhawan K, Kumar R, Kumar S, Sharma A. Correct identification of Passiflora incarnata L., a promising herbal anxiolytic and sedative. J Med Food. 2001a;4(3):137–144. doi: 10.1089/109662001753165710. [DOI] [PubMed] [Google Scholar]
- Dhawan K, Kumar S, Sharma A. Anti-anxiety-studies on extracts of Passiflora incarnata L. Journal of Ethnopharmacology. 2001b;78:165–170. doi: 10.1016/s0378-8741(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Dhawan K, Kumar S, Sharma A. Anxiolytic activity of aerial and underground parts of Passiflora incarnata. Fitoterapia. 2001c;72(8):922–926. doi: 10.1016/s0367-326x(01)00322-7. [DOI] [PubMed] [Google Scholar]
- Dhawan K, Kumar S, Sharma A. Comparative anxiolytic activity profile of various preparations of Passiflora incarnata L.: a comment on medicinal plants’ standardization. The Journal of Alternative and Complementary Medicine. 2002;8(3):283–291. doi: 10.1089/10755530260127970. [DOI] [PubMed] [Google Scholar]
- Fernández S, Wasowski C, Paladini AC, Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacology Biochemistry and Behavior. 2004;77(2):399. doi: 10.1016/j.pbb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Fradley RL, Guscott MR, Bull S, Hallett DJ, Goodacre SC, Wafford KA, Garrett EM, Newman RJ, O’Meara GF, Whiting PJ, Rosahl TW, Dawson GR, Reynolds DS, Atack JR. Differential contribution of GABAA receptor subtypes to the anticonvulsant efficacy of benzodiazepine site ligands. J Psychopharmacol. 2007;21(4):384–391. doi: 10.1177/0269881106067255. [DOI] [PubMed] [Google Scholar]
- Goutman JD, Waxemberg MD, Donate-Oliver F, et al. Flavonoid modulation of ionic currents mediated by GABA A and GABA C receptors. European Journal of Pharmacology. 2003;461:79–87. doi: 10.1016/s0014-2999(03)01309-8. [DOI] [PubMed] [Google Scholar]
- Granger RE, Campbell EL, Johnston GAR. (+)- And (−)-borneol: efficacious positive modulators of GABA action at human recombinant [alpha]1[beta]2[gamma]2L GABAA receptors. Biochemical Pharmacology. 2005;69(7):1101. doi: 10.1016/j.bcp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Grundmann O, Wang J, McGregor G, Butterweck V. Anxiolytic activity of a phytochemically characterized Passiflora incarnata extract is mediated via the GABAergic system. Planta Medica. 2008;74:1769–1773. doi: 10.1055/s-0028-1088322. [DOI] [PubMed] [Google Scholar]
- Johnston GAR, Hanrahan JR, Chebib M, Duke RK, Mewett KN, Enna SJ. Advances in Pharmacology. Vol. 54. Academic Press; 2006. Modulation of Ionotropic GABA Receptors by Natural Products of Plant Origin; p. 285. [DOI] [PubMed] [Google Scholar]
- Kavvadias D, Sand P, Youdim K, Qaiser AMZ, Rice-Evans C, Baur R, Sigel E, Rausch W-D, Riederer P, Schreier P. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood– brain barrier and exhibits anticonvulsive effects. British Journal of Pharmacology. 2004;142(5):811–820. doi: 10.1038/sj.bjp.0705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder M, Paladini AC. GABA A receptor ligands of flavonoid structure. Curr Top Med Chem. 2002;2(8):853–867. doi: 10.2174/1568026023393462. [DOI] [PubMed] [Google Scholar]
- Marder M, Viola H, Wasowski C, Fernández S, Medina JH, Paladini AC. 6-Methylapigenin and hesperidin: new valeriana flavonoids with activity on the CNS. Pharmacology Biochemistry and Behavior. 2003;75(3):537. doi: 10.1016/s0091-3057(03)00121-7. [DOI] [PubMed] [Google Scholar]
- Medina JH, Paladini AC, Wolfman C, et al. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochemical Pharmacology. 1990;40(10):2227–2231. doi: 10.1016/0006-2952(90)90716-x. [DOI] [PubMed] [Google Scholar]
- Medina JH, Viola H, Wolfman C, et al. Flavonoids: a new family of benzodiazepine receptor ligands. Neurochem Res. 1997;22:419–425. doi: 10.1023/a:1027303609517. [DOI] [PubMed] [Google Scholar]
- Mori A, Hasegawa K, Murasaki M, Yamauchi T, Ito S, Asai M, Kudo Y, Nakajima T, Nishimura T, Saito M, Hanada M, Ikawa G, Higashi Y, Kawakita Y. Clinical evaluation of Passiflamin (Passiflora extract) on neurosis - multicenter, double-blind study in comparison with mexazolam. Rinsho Hyoka [Clinical evaluation for drugs] 1993;21(3):383–440. [Google Scholar]
- Movafegh A, Alizadeh R, Hajimohamadi F, Esfehani F, Nejatfar M. Preoperative oral Passiflora Incarnata reduces anxiety in ambulatory surgery patients: a double-blind, placebo-controlled study. Anesthesia and Analgesia. 2008;106(6):1728–1732. doi: 10.1213/ane.0b013e318172c3f9. [DOI] [PubMed] [Google Scholar]
- Nassiri-Asl M, Shariati-Rad S, Zamansoltani F. Anticonvulsant effects of aerial parts of Passiflora incarnata extract in mice: involvement of benzodiazepine and opioid receptors. BMC Complementary and Alternative Medicine. 2007;7:26. doi: 10.1186/1472-6882-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassiri-Asl M, Shariati-Rad S, Zamansoltani F. Anticonvulsive effects of intracerebroventricular administration of rutin in rats. Progess in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:989–993. doi: 10.1016/j.pnpbp.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Petry RD, Reginatto F, de-Paris HF, Gosmann G, Salgueiro JB, Quevedo J, Kapczinski F, Ortega GG, Schenkel EP. Comparative pharmacological study of hydroethanol extracts of Passiflora alata and Passiflora edulis leaves. Phytotherapy Research. 2001;15(2):162–164. doi: 10.1002/ptr.694. [DOI] [PubMed] [Google Scholar]
- Raffaelli A, Moneti G, Mercati V, Toja E. Mass spectrometric characterization of flavonoids in extracts from Passiflora incarnata. Journal of Chromatography A. 1997;777:223–231. [Google Scholar]
- Reginatto F, De-Paris HF, Petry RD, Quevedo J, Ortega GG, Gosmann G, Schenkel EP. Evaluation of anxiolytic activity of spray dried powders of two South Brazilian Passiflora species. Phytotherapy Research. 2006;20(5):348–351. doi: 10.1002/ptr.1853. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Reversed uptake is the major mechanism of glutamate release in severe brain ischaemia. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Current Opinion in Pharmacology. 2006;6(1):18. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sena L, Zucolotto S, Reginatto F, Sxhenkel E, Monteiro de Lima T. Neuropharmacological activity of the pericarp of Passiflora edulis flavicarpa Degener: putative involvement of C-glycosylflavonoids. Exp Biol Med. 2009;234:967–975. doi: 10.3181/0902-RM-84. [DOI] [PubMed] [Google Scholar]
- Soulimani R, Younos C, Jarmouni S, et al. Behavioral effects of Passiflora incarnata L. and its indole alkaloid and flavonoid derivatives and maltol in the mouse. Journal of Ethnopharmacology. 1997;57:11–20. doi: 10.1016/s0378-8741(97)00042-1. [DOI] [PubMed] [Google Scholar]
- Speroni E, Billi R. Sedative effects of crude extract of Passiflora incarnata after oral administration. Phytotherapy Research. 1996;10:S92–S94. [Google Scholar]
- Speroni E, Billi R, Crespi Perellino N, Minghetti A. Role of chrysin in the sedative effects of Passiflora incarnata L. Phytotherapy research. 1996;10:S98–S100. [Google Scholar]
- Speroni E, Minghetti A. Neuropharmacological activity of extracts from Passiflora incarnata. Planta Med. 1988;54:488–491. doi: 10.1055/s-2006-962525. [DOI] [PubMed] [Google Scholar]
- Spinella M. Herbal medicines and epilepsy: the potential for benefit and adverse effects. Epilepsy and Behavior. 2001;2:524–532. doi: 10.1006/ebeh.2001.0281. [DOI] [PubMed] [Google Scholar]
- Swinyard EA, Woodhead JH, White HS, Franklin MR. General principles: experimental selection, quantification, and evaluation of anticonvulsants. In: Levy RH, Mattson RH, Melrum B, Penry JK, Dreifuss FE, editors. Antiepileptic drugs. New York: Raven Press; 1989. pp. 85–102. [Google Scholar]
- Takanaga H, Ohtsuki S, Hosoya K-i, Terasaki T. GAT2/BGT-1 as a System Responsible for the Transport of Gamma-Aminobutyric Acid at the Mouse Blood-Brain Barrier. J Cereb Blood Flow Metab. 2001;21(10):1232. doi: 10.1097/00004647-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Zanoli P, Avallone R, Baraldi M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia. 2000;71(S1):S117–S123. doi: 10.1016/s0367-326x(00)00186-6. [DOI] [PubMed] [Google Scholar]