Abstract
Homozygous complement C4B deficiency is described in a Southern European young female patient with Membranoproliferative Glomerulonephritis (MPGN) type III characterized by renal biopsies with strong complement C4 and IgG deposits. Low C4 levels were independent of clinical evolution or type of immunosuppression and were found in three other family members without renal disease or infections. HLA typing revealed that the patient has homozygous A*02, Cw*06, B*50 at the class I region, and DRB1*08 and DQB1*03 at the class II region. Genotypic and phenotypic studies demonstrated that the patient has homozygous monomodular RCCX in the HLA class III region, with single long C4A genes coding for C4A3 and complete C4B deficiency. Her father, mother, son and niece have heterozygous C4B deficiency. The patient’s deceased brother had a history of Henoch-Schönlein Purpura (HSP), an immune complex-mediated proliferative glomerulonephritis. These findings challenge the putative pathophysiological roles of C4A and C4B and underscore the need to perform functional assays, C4 allotyping and genotyping on patients with persistently low serum levels of a classical pathway complement component and glomerulopathy associated with immune deposits.
Introduction
The complement system is an essential element of the innate immune system [1]. The lack of any complement component may severely disturb host defenses. Complement component C4 plays a central role in classical and lectin complement activation pathways. C4 has two isotypic forms, C4A and C4B, encoded by loci in the MHC class III region on chromosome 6. The number of C4 genes varies between two and eight in a diploid genome among different individuals [2]. A C4 gene duplication is always concurrent with its neighboring genes RP at the 5' region and CYP21 and TNX at the 3' region [3]. This unit is known as an RCCX module (Fig. 1). Low C4A and C4B copy numbers (i.e., 0 or 1 copy) have a combined frequency of 31.6% and are associated with a variety of autoimmune or infectious diseases [4]. A complete deficiency of both C4A and C4B is nevertheless rare but strongly associated with impaired immune complex clearance and systemic lupus erythematous (SLE) [5]. On the other hand, homozygous complement C4B deficiency has a frequency of 3% in the American Caucasian population [6]. There have been a few case reports regarding homozygous C4B deficiency and non-SLE glomerulonephritis [7–11].
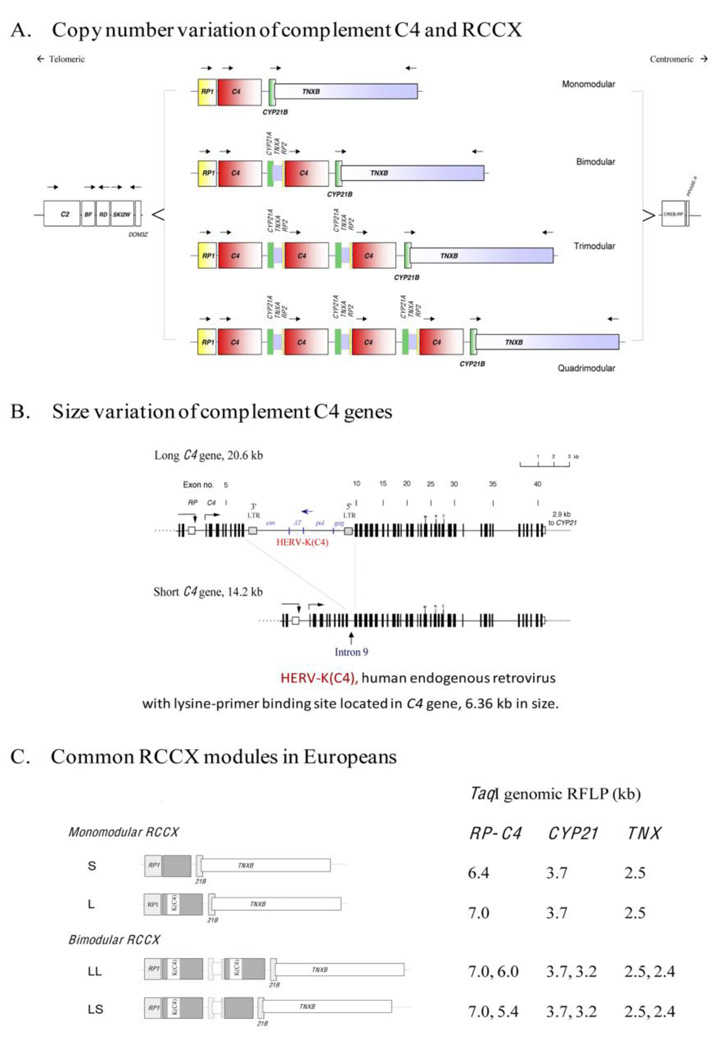
Figure 1.
The gene size and polygenic variations of human complement C4 and RP-C4-CYP21-TNX (RCCX modules) in the MHC class III region. A. Copy number variation of RCCX modules; B. Exon-intron structure and size variation of C4 gene; HERV-K(C4) is an endogenous virus inserted into intron 9 of long C4 genes; C. Common RCCX haplotypes and diagnostic TaqI restriction fragment size (in kb) for each haplotype (modified from ref. [50]).
Idiopathic MPGN represents only 6.4–7.3% of all primary glomerulopathies [12–15] and only 0.4% of all patients receive renal replacement therapy (USRDS). The renal outcome is poor, with a 10-year survival of 32–40%, and a remission rate of 5.0–7.6% [13,16]. MPGN type III is a rare renal disease of unknown cause, usually sporadic, representing less than 1% of glomerulonephritis in URSD registry data [17]. There are a few cases with MPGN and C4 deficiency reported in the literature [18–21] and even fewer cases associated with C4B*Q0 [19,20].
Here we report a family with two siblings who were inflicted with proliferative glomerulonephritis, one of whom had confirmed complete C4B-deficiency. Four family members had heterozygous C4B deficiency. The dosage of the C4A and C4B genes, three other constituent genes of the RCCX modules, and C4 protein phenotypes were investigated. The clinical presentation of MPGN type III for this C4B deficient patient is described.
Materials and Methods
Blood donors
Informed consents were obtained from blood donors according to IRB-approved protocol. Peripheral blood in EDTA-tubes was used for isolation of genomic DNA and plasma samples, as described [2].
Real-time PCR assays of C4 gene copy numbers
A TaqMan-based quantitative realtime PCR strategy was applied to determine the gene copy-numbers of total C4, C4A and C4B in DNA samples rapidly, as described [22].
Southern blot analysis
Genomic DNA samples were digested with TaqI restriction enzyme, resolved by agarose gel electrophoresis, Southern blotted, hybridized with probes that annealed to genomic regions for RP and C4, CYP21, and 3’ region of TNX. B. PshAI-PvuII RFLP for C4A and C4B. Genomic DNA samples were digested with PshAI that differentiates between the C4A and C4B isotypic sequences, and with PvuII to improve separation of genomic DNA fragments specific for C4A and C4B. After agarose gel electrophoresis and Southern blotting to nylon membrane, the membrane was hybridized to a C4d specific probe labeled by 32P-dCTP [2].
Allotyping of C4 proteins
EDTA-plasma samples were digested with neuraminidase and carboxylpeptidase B, resolved with high-voltage agarose gel electrophoresis, immunofixed with goat anti-human C4 antisera, blotted to remove diffusible proteins and stained [2,23].
Results
Index case
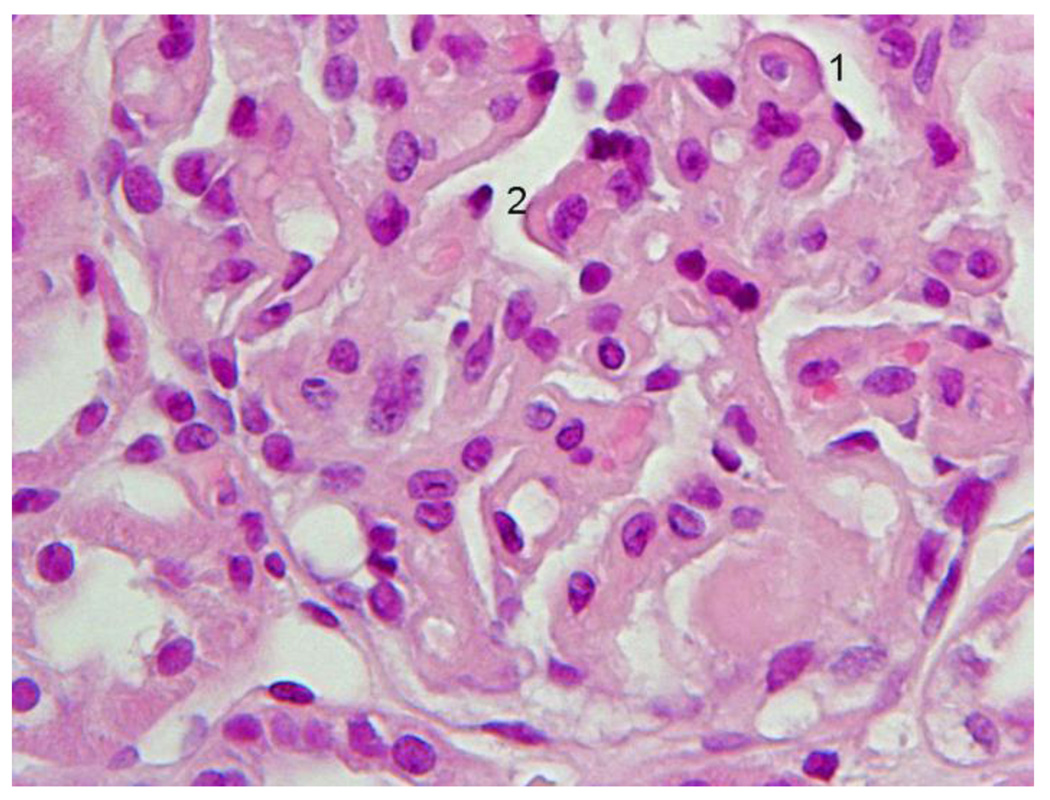
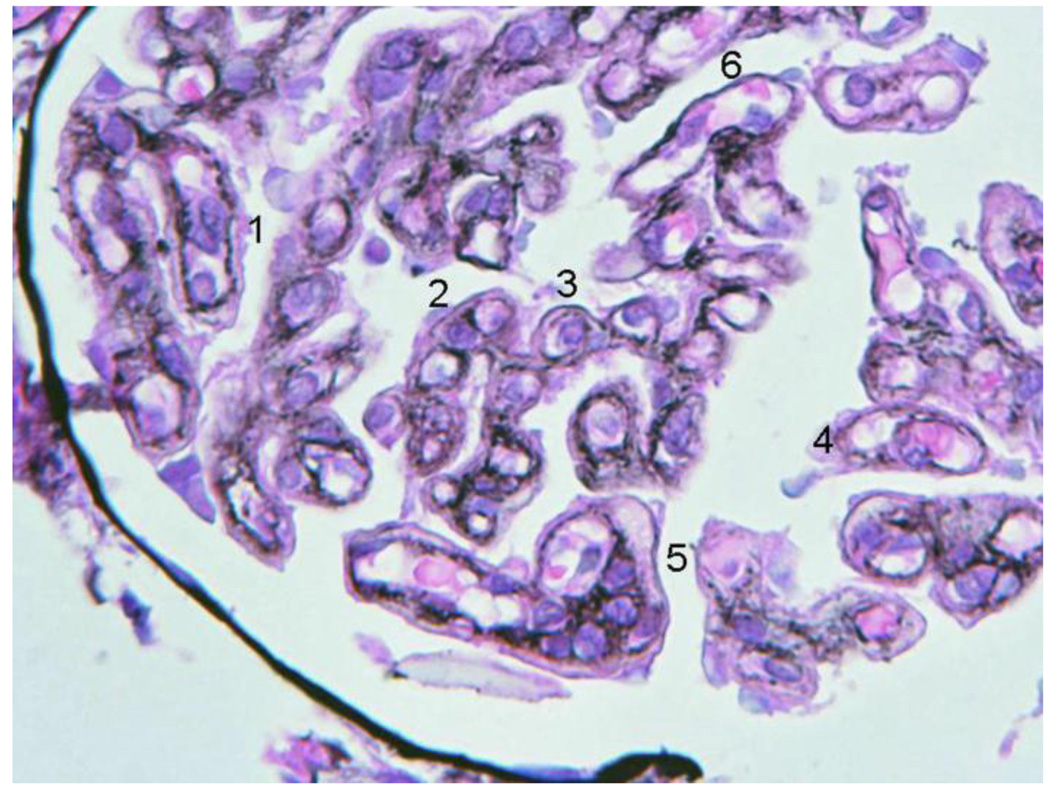
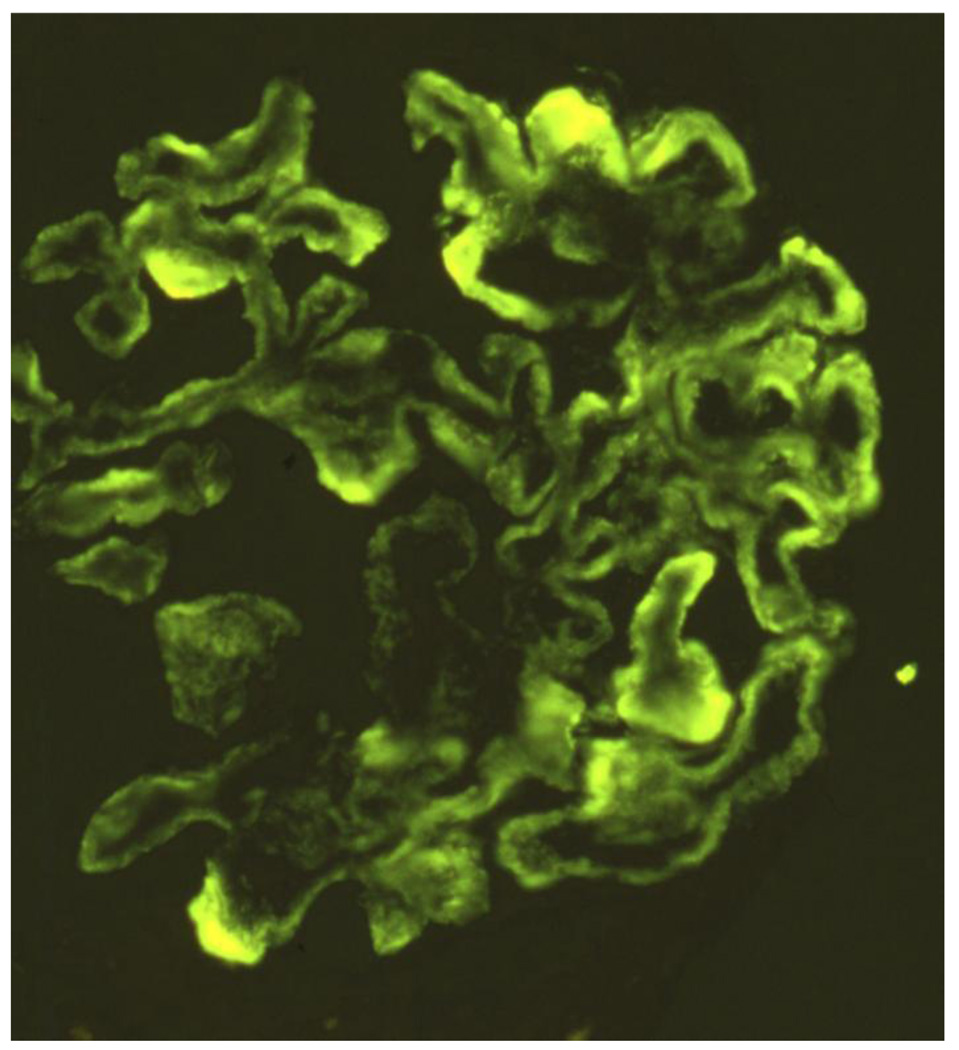
A 28-year old white female first presented with anasarca because of nephrotic syndrome. She was hypertensive; urine protein excretion was 7g/d and serum albumin 1.7g/dL, associated with microscopic hematuria, red cell casts and normal renal function. Serum C3 protein concentration was normal, C4 level was persistently low (5–7 mg/dL; normal range 17.4–52.2 mg/dL), IgG was low (247 mg/dL; normal range 690–1400 mg/dL). Autoantibodies (antinuclear, anti-DNA, anti-Sm, anti-RNP, anti-Ro/SSA, anticardiolipin), MPO-ANCA and cryoglobulins were absent. Bacterial cultures, and viral (HIV, HCV, HBV) serology were negative. She did not meet diagnostic criteria for SLE. Light microscopy of kidney biopsy showed a probable type III MPGN with extensive deposits (Fig. 2A). The most prominent anomaly was a general thickening of the capillary walls which sometimes assumed a broad glassy eosinophilic appearance and reduced capillary lumens. Hyperlobularity was not apparent. Inflammatory cells were not conspicuous and there were neither tuft necrosis nor crescents. Interstitial fibrosis or tubular atrophy were mild. The presence of frequent double contours in most loops but a lack of spikes was demonstrated with Jones’s silver stain. Any existing deposits within the loops were not stained with silver (Fig. 2B). Immunofluorescence (IF) showed intense (3+) C4 and IgG, moderate (2+) C1 and IgM, and weak (1+) IgA and C3, globally distributed large confluent granular deposits, forming a pseudolinear pattern within capillary walls (Fig. 2C). The more intense and larger deposits had globular or semilunar profiles. There was some granular mesangial staining with weaker intensity. These IF results suggested immune complex deposition. Electron microscopy was consistent with a Strife and Anders type of type III MPGN [24,25]. In most capillaries the profile of the normal lamina densa was not visible. In its place were numerous moderately dense deposits, usually uniformly textured aggregates of finely granular material, sometimes less well defined irregular aggregates. These deposits predominated on the epithelial side, but many extended from epithelium to endothelium. Mesangial deposits were noted more in the paramesangial region. A thin reactive type continuous lamina densa closely hugged and separated the dense deposits from the extensively thinned and effaced podocytes and, discontinuously, from endothelial cells. These inner and outer dense laminas corresponded to the double contours. Mesangial interposition into the capillary was confirmed. Spikes were not identified. In the glomerulus with a few less affected loops the lamina densa was single and normal; scattered fairly large and uniform subendothelial dense deposits were occasionally observed on these loops. No tubuloreticular structures were noted. (Fig. 2D). Lupus Nephritis was considered to be the most likely differential diagnosis.
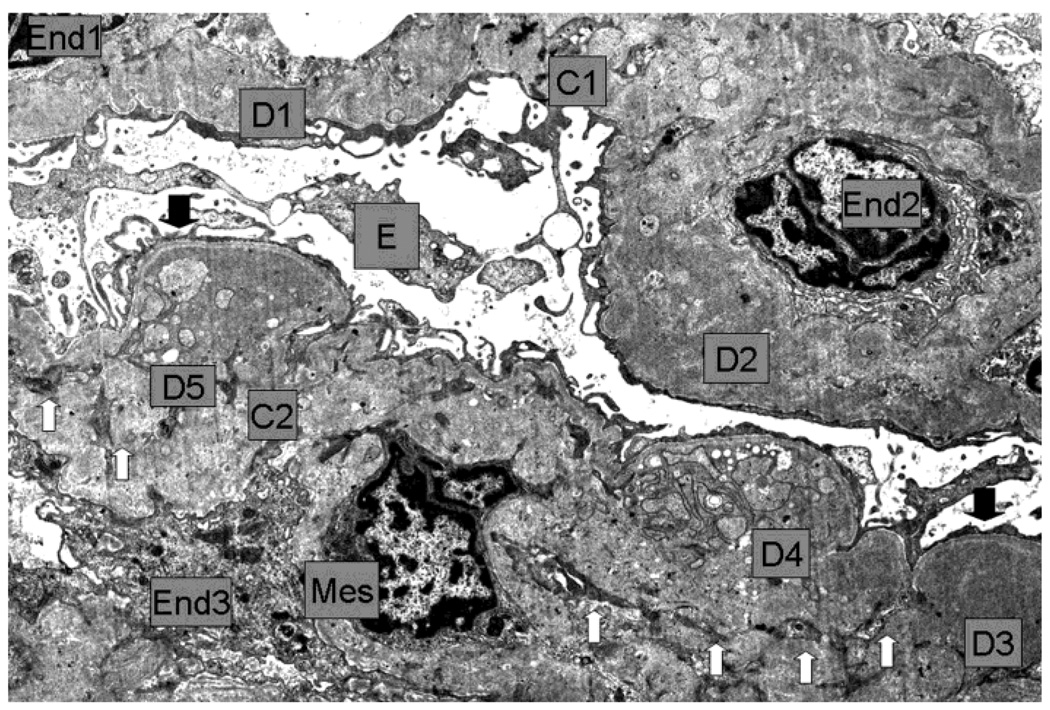
Figure 2.
First renal biopsy of the patient: Light microscopy (A) Silver stain (B), immunofluorescence (C), electromicroscopy (D)
2A Detail of the diffuse glomerulonephritis showing prominent generalized thickening of the capillary walls, sometimes with a broad glassy eosinophilic appearance (1, 2). There was slight to moderate increase in mesangial cells and expansion of the mesangial regions. Capillary lumina appear generally reduced. H+E.
2B. Within the thickened capillary walls the basement membrane appears irregular, interrupted or vacuolated (1, 3, 4). There are also several double contours some of which with a fairly thin outer contour (2, 3, 5). Any existing deposits within the wall appeared unstained with silver (5). One capillary wall has relatively normal wall (6). Mesangial matrix was mildly increased (5). Jones´s silver.
2C Intense confluent granular deposits of C4 with pseudolinear pattern globally distributed within the capillary walls and less intense mesangial staining. Large intense globular or semilunar collections in the wall were also seen.
2D Low magnification E.M: Capillaries (C1, C2) with endothelium (End1 to End3), Bowman´s space and visceral epithelium (E). In the thickened and deformed wall there are large, numerous moderately dense deposits (D1 to D5) more concentrated towards the subepithelial zone, but also extending through the whole wall (D2). Generally the dense deposits have uniform texture (D1 to D3) but some show complex irregular texture which includes less dense vesicular profiles, small clear spaces and groups of convoluted fine plasma membrane like structures (D4, D5). Generally there was no definition of the normal lamina densa of the basement membrane. Reactive type continuous thin lamina densa was seen along the epithelial side effectively separating the dense deposits from the podocytes (between black arrows). Clear cut formation of spikes was not seen. Reactive thin lamina densa on the endothelial side was ill defined and discontinuous. Mesangial cell (Mes) interposition was represented by multiple small cytoplasmic processes (white arrows) within the thickened capillary wall. There was severe thinning of podocytes and extensive effacement of foot processes. Negative magnification 2500×.
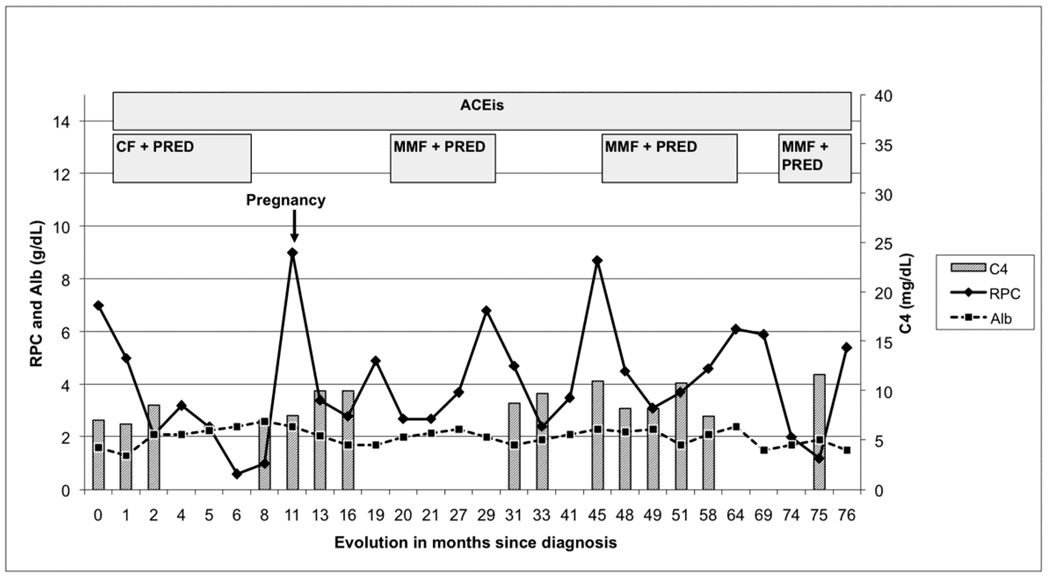
Therapy was started with methylprednisolone pulses, followed by oral prednisolone (1 mg/Kg) and six 1g Cyclophosphamide monthly pulses. After 16 weeks of therapy the protein/creatinine urinary ratio (PCUR g/g) decreased below 0.6 and prednisone was tapered over eight months. The patient was admitted at the eleven month of follow-up. She had a marked increase of PCUR to 9, and was eight weeks pregnant. Because of the risks for both patient and fetus, a therapeutic abortion was performed and the urine protein excretion declined. For the next 9 months the patient remained without immunosuppressants. The PCUR again increased to almost 5 and therapy with Mycophenolate Mophetil (MMF) 1g twice-a-day plus prednisolone (PRED) 20 mg every other day, was begun at month 18. There was a quick response, but after six months the PCUR rose progressively to 7, probably related to noncompliance, as admitted by the patient. In the following year and without immunosuppression the PCUR decreased to below 4 and increased again to 8.5 (Fig. 3). A second renal biopsy was performed at the 41st month of follow-up, which confirmed MPGN type III diagnosis, but with fewer immune deposits and without signs of chronicity (Fig. 4 A–C). The glomerular capsule appeared marginally thicker than that observed in the first biopsy. Immunofluorescence showed, in general, weaker reactions for all immune proteins. However there were still extensive moderate (2+) pseudolinear IgG and C4 and slight (1+) C1 and IgM deposits, all predominating within the capillary walls. Large globular or semilunar collections similar to those of the first biopsy remained present. IgA and C3 appeared much reduced, but there were strong segmental capsular and hilar deposits of C3. It was assumed that the evolution was related to the immunosuppressant therapy. MMF/PRED was re-started. During the six years of follow-up the patient received three cycles of MMF/PRED, serum C4 levels remained depressed persistently and the serum albumin and PCUR were never normalized. Renal function remained normal. The patient was under ACE inhibitor treatment from the start.
Figure 3.
Time course of proteinuria in response to different therapeutic approaches
CF: Cyclophosphamide, 6 1 g monthly pulses
PRED: prednisolone initially 1 mg/kg four month and then tapering
MMF: Mycophenolate Mophetil 0,5-1g twice-a-day
ACEI: angiotensin converting enzyme inhibitors
RPC: ratio of urinary protein/creatinine (g/g)
Alb: serum albumin (g/dL)
C4 - Complement component C4 mg/dL (normal range 17.4–52.2)
Figure 4.
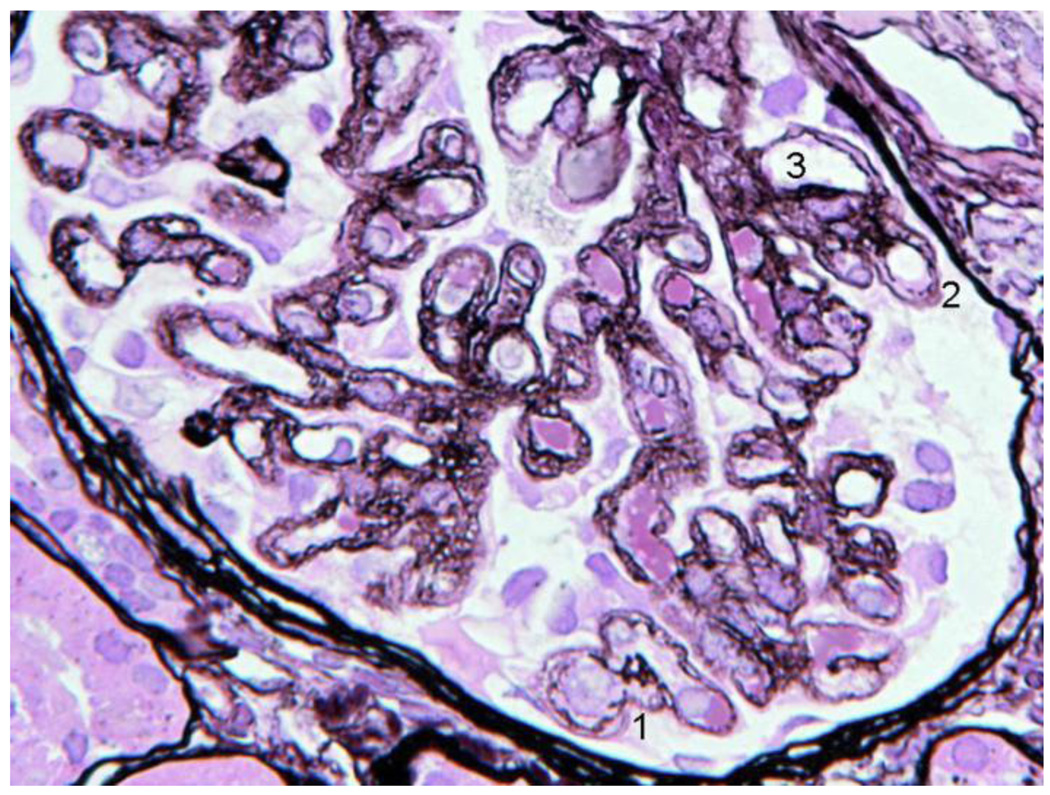
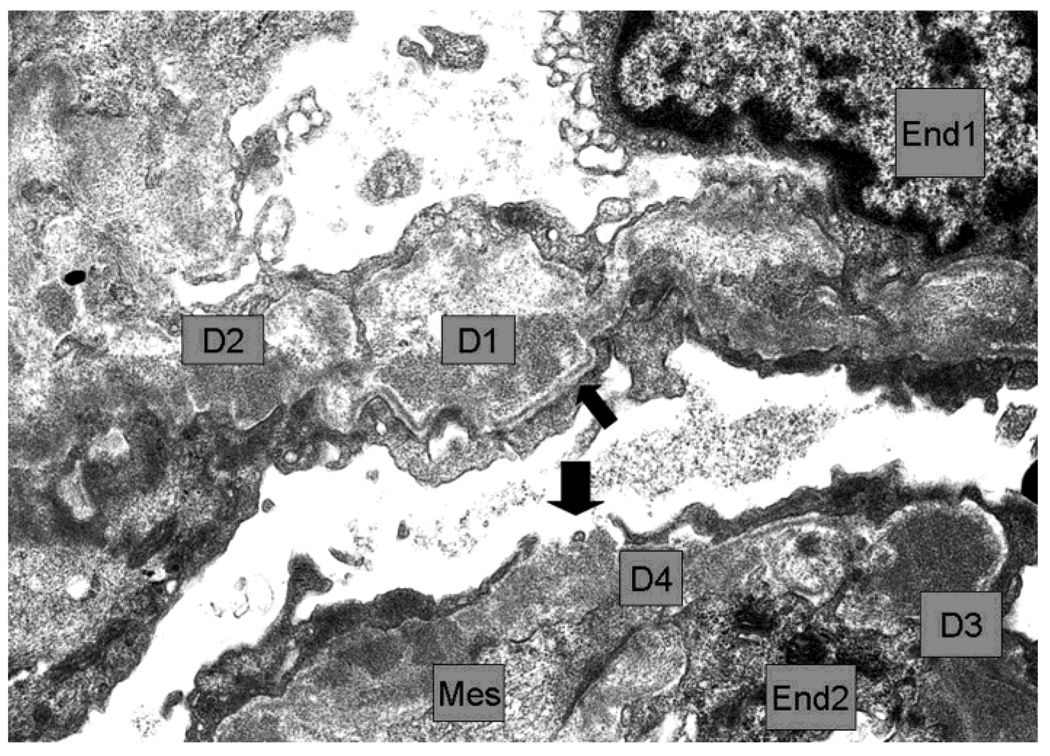
Second Biopsy: silver stain LM (A), EM (B)
4A. The basement membrane was generally irregular, sometimes interrupted (1,2) and double contours were present (3). The vacuolated or moth eaten profile of the walls, seen in the first biopsy, was widespread and also present in the paramesangium. Capillaries with normal single layer basement membrane were not seen. The glomerular capsule appeared marginally thicker than in the first biopsy.
4B. The walls of 2 capillaries with endothelial cells (End1 and End2) contained several dense deposits (examples D1 to D4). D1 appeared rarified and surrounded by thin reactive basement membrane which appeared well-defined and continuous in the subepithelium (thin arrow) and discontinuous in the subendothelium. D2 made direct contact with the podocyte. D3 had fairly uniform high density and was also partly surrounded by subepithelial basement membrane. There was a gap between podocyte processes leaving D4 in direct contact with Bowman´s space. Mesangial cell cytoplasm (Mes) interposition was seen in the wall near D4 and other deposits. Negative 6000×
Family study
The patient’s brother died on dialysis at age 28. A diagnosis of Henoch-Schonlein purpura (HSP) with proliferative glomerulonephritis was made at age 14. Neither the biopsy nor C4 protein levels were available for review.
Clinical and laboratory testing of four direct relatives of the patient revealed a normal urinalysis and renal function. Her father, son and niece (sister's daughter) had low C4 levels without disease. Therefore, we proceeded to a genetic analysis and protein allotyping of complement C4.
Genotypic and phenotypic studies of complement C4
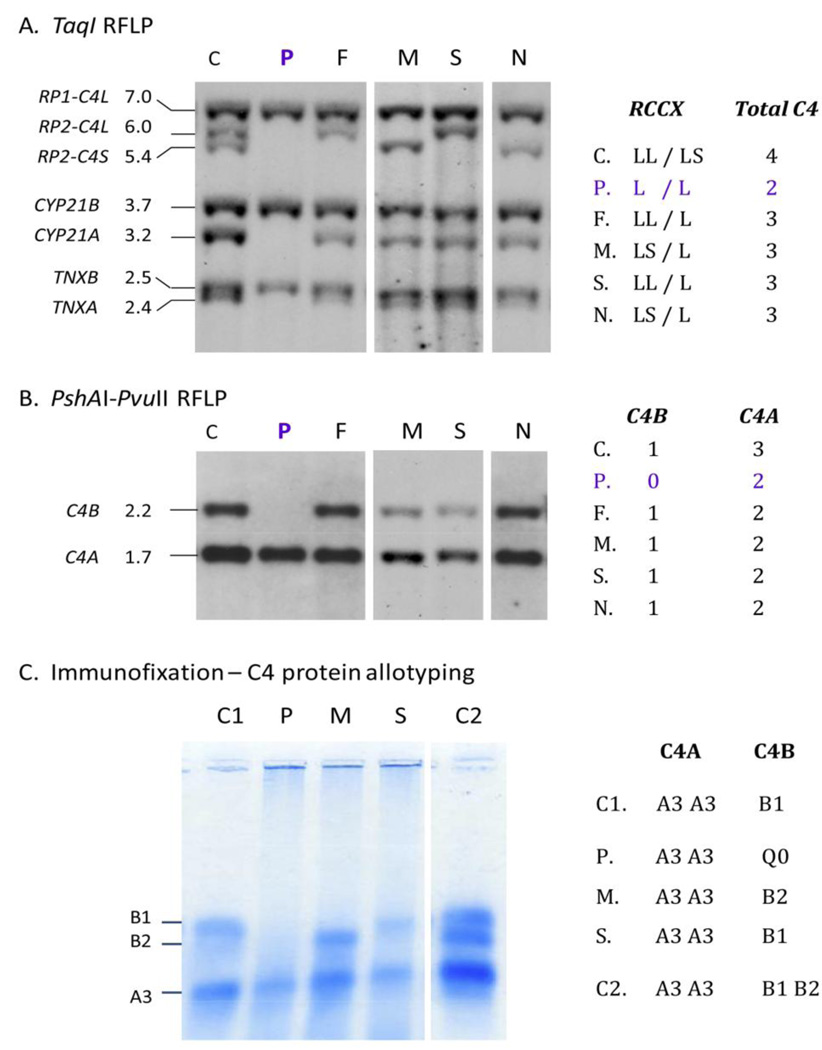
The results of the family study for complement C4 haplotypes are shown in Figure 5. Genomic DNA samples were isolated from peripheral blood mononuclear cells. A TaqMan-based realtime PCR strategy [22] was initially applied to determine the copy-number of C4A and C4B in each subject. The index patient has a total of two C4 genes in her diploid genome: two copies of C4A but no C4B. The father and the niece each has three copies of C4 genes: two copies of C4A and one copy of C4B.
Figure 5.
Genotyping and phenotyping of complement C4. A. TaqI restriction fragment length polymorphism (RFLP) and Southern blot analyses of RCCX modules. B. PshAI-PvuII RFLP for C4A and C4B. C. Immunofixation of C4A and C4B proteins.
Abbreviations: P, index patient; F, father; M, mother, S, son; N, niece; C, C1 and C2, three different healthy control subjects.
We further investigated the RP-C4-CYP21-TNX (RCCX) modular variation by genomic Southern blot analyses of TaqI and PshAI-PvuII restriction fragment length polymorphisms (RFLP) and probed for genes in the RCCX module [2]. TaqI RFLP identified numbers of long and short C4 genes and RCCX haplotypes. The index patient has homozygous, monomodular RCCX (L/L) with single copies of long C4 genes, and single copies of neighboring genes for steroid CYP21B and tenascin TNXB (Fig. 5 A). PshAI-PvuII RFLP showed that the C4 genes present in the index patient were C4A genes, while C4B genes were absent (Fig. 5B). Immunofixation using EDTA-plasma revealed the presence of C4A3 allotype and complete absence of C4B protein in the index case (Fig. 5C). The father has bimodular-LL and monomodular-L RCCX (LL/L) haplotypes with three copies of C4 genes: two C4A and one C4B. The mother has a total of three C4 genes with bimodular-LS and monomodular-L; two C4A and one C4B. The patient inherited the monomodular-L haplotypes with C4A (but no C4B) from each parent. Immunofixation of EDTA-plasma C4 showed that the mother has C4A3, C4A3 and C4B2 protein allotypes, while the son has C4A3, C4A3 and C4B1 allotypes, which are consistent with the genotypic data.
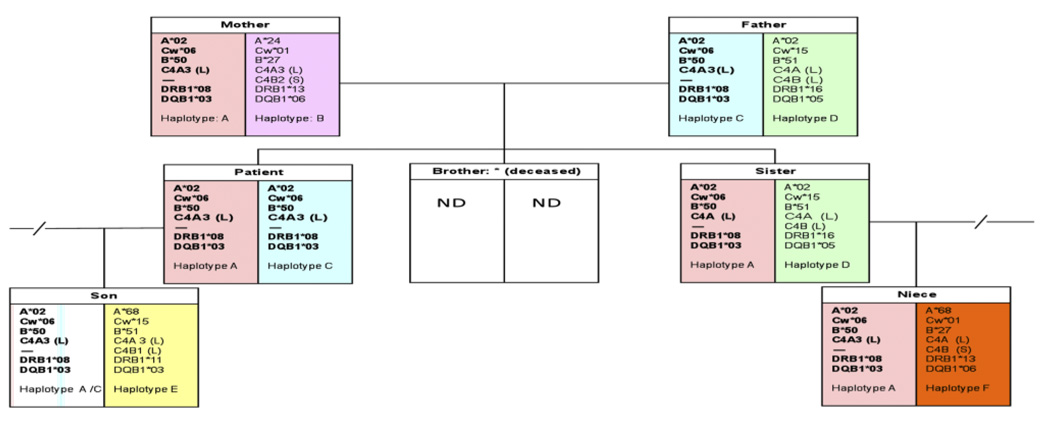
Complement C4 and the RCCX modules are located in the class III region of the human major histocompatibility complex, or the HLA. Thus, HLA typing of class I and class II genes was performed for the patient and her five direct family members to identify the haplotype associated with C4B deficiency. The patient has homozygous HLA A*02, Cw*06, and B*50 at the telomeric class I region, and DRB1*08 and DQB1*03 at the centromeric class II region. All other family members were heterozygous with this haplotype (Figure 6).
Figure 6.
Family tree with HLA and complement C4 genotypes. One of the patient’s siblings was deceased and no genetic data was available.
Discussion
MPGN type III is a rare, sporadic and progressive renal disease of unknown etiology. The finding of immune complexes with cellular inflammatory infiltrate suggests an immune mediated process. In some cases, a genetic basis of the diseases is evident, particularly in families [26] with inherited defects of the complement system [27–29]. Only a few cases have been reported with C4 deficiency [18–21,30] and one with isolated C4B deficiency [19].
Early in her disease presentation, the index patient was treated as if she had lupus nephritis, although she did not fulfill the diagnosis criteria for SLE clinically or serologically [31]. The patient received three alternated cycles of immunosuppression for about six years and spent almost two years without medication. There was a significant reduction of proteinuria during the treatment period but complete remission was never achieved and C4 levels remained depressed throughout the follow-up period.
The brother had reached an end-stage renal disease because of a proliferative immune complex-mediated glomerulonephritis with IgA deposits, and was diagnosed of Henoch-Schonlein purpura (HSP). The histology of IgA nephropathy and HSP is characterized by glomerular IgA, IgG and C3 deposits mainly in the mesangium but also in the capillary wall. Unfortunately, the brother’s renal biopsy was not available for review. It is unusual for two siblings to be inflicted with immune complex mediated glomerular injuries. We speculate that this could be related to the presence of C4B deficiency in the family. In this regard, the coexistence of IgA nephropathy and MPGN in several members of the same family had been reported previously, and it was associated with specific C4 polymorphisms [32].
Complement abnormalities in patients with HSP nephritis have previously been reported [7,11,33–35] and C4 deficiency was suggested as a risk factor for HSP nephritis and other immune complex glomerulonephritis, possibly due to a defective clearance mechanism [36,37]. C4 null alleles are common in HSP patients and the C4B*Q0 allele is thought to increase the risk of developing HSP [33]. Other authors have reported HSP patients with familiar C4 deficiency [30]. Low C4 levels in three family members, genotyping and phenotyping of C4 revealed that our patient was homozygous for C4B deficiency with HLA haplotype A*02-Cw*06-B*50-C4A3(L)-DRB1*08-DQB1*03. Her parents each was heterozygous with this haplotype, and had only one copy of C4B gene, similar to the patient’s son and niece. Phenotypically, heterozygous deficiency of either C4A or C4B is a common immune protein defect in all human populations as a consequence of the high frequency of the MHC haplotypes with C4 gene copy-number variations, although homozygous deficiency is rarer [38]. In Caucasians, more than two-thirds of the MHC haplotypes have two copies of C4 genes; the other haplotypes mainly possess either one C4 or three copies of C4 genes. Each of these C4 genes may be long (L) or short (S). The number of long or short C4A and C4B genes present in an individual plays a role in determining the basal level of C4 proteins in the peripheral blood plasma. The index patient has only single copy of long C4 gene coding for the C4A protein on each of her chromosome 6, which is one of the causes for persistently low levels of plasma C4. The varying efficiencies of complement activation that result from differential expression levels and protein polymorphisms of C4A and C4B may have a profound effect on an individual’s strengths in innate and adaptive immune systems, and susceptibility to systemic autoimmune diseases. C4 null alleles represent relevant disease markers and additive genetic risk factors for autoimmune phenomena in multiple races [36]. Cumulative results from more than 35 different studies revealed that heterozygous and homozygous deficiencies of C4A were present in 40–60% of SLE patients among northern and central Europeans, Anglo-Saxons, Caucasians in the US, Afro-Americans, Asian Chinese, Koreans and Japanese [5]. Interestingly, Spanish, Mexican and Australian Aborigine SLE patients had increased frequencies of C4B deficiency instead of C4A deficiency [39,40]. It is possible that different racial and genetic backgrounds could change the thresholds for the requirement of C4A or C4B protein levels in immune tolerance and immune regulation [4]. There is no information on the frequency of C4B deficiency in SLE and lupus-like patients in the Portuguese population. We speculate that the frequency might be similar to that in the Spanish population [40].
While the coexistence of C4B deficiency and type III MPGN could be coincidental, there are pathophysiological pathways that can explain the association. C4 deficiency is frequently associated with immune complex disease [36]. C4B is important in the formation of the classical pathway C3 covertase, leading to the assembly of the membrane-attack complex against microbes [37]. A C4B deficiency would adversely affect the efficiency and progression of the complement activation, and possibly diminish the capacity of phagocytosis and clearance of pathogenic immune complexes, apoptotic and necrotic cells, predisposing an individual to developing immune complex-mediated diseases such as HSP or MPGN. Human subjects with a genetic predisposition to infection or an impaired protection against autoimmunity would be vulnerable to immune complex glomerulonephritis. In this regard, infection may be complicated by MPGN or HSP/IgA nephropathy. Different reports have showed an association between glomerulonephritis and C4B deficiency in patients with SLE or Lupus-like disease [10,20,37,41]. Tsuda et al [19] also reported a MPGN-III patient from Japan with null C4B and persistently low C4 levels. Interestingly, the patient’s renal biopsy revealed strong positive staining for C3 and weak staining for C4.
We further investigated the status of two other immune effectors genes that exhibit gene copy-number variations and play a role in renal diseases, which were complement factor H-related genes CFHR3-R1 located at chromosome 1q32 [42,43], and immunoglobulin Fcγ-receptor FCGR3B located at chromosome 1q23 [44], in our patient and direct family members. Through genomic Southern blot analyses of TaqI digested genomic DNA with gene-specific probes for CFHR1, CFHR2, CFH3 and CFHR4, and for FCGR3B and FCGR3A, we did not observe a deletion of CFHR3-CFHR1, or low copy-number FCGR3B, in all six members of the family (data not shown).
The clinical course of MPGN type III is not clear, long-term kidney survival is poor, and there is little evidence for a specific treatment to improve outcome. Reviews of immunosuppression trials in adults did not reveal any significant benefit [45–48]. The onset with nephrotic syndrome worsens the prognosis. Lhotta [41] described a C4-deficient patient with membranous nephritis who responded well to MMF therapy. We therefore decided to use this option, bearing in mind that MMF inhibits the proliferation of activated B and T lymphocytes and antibody synthesis, and limits immune complex deposition in the glomeruli [49]. We observed that MMF reduced urinary protein excretion by almost 70%. The treatment was well tolerated, without infections. Renal function in our patient has remained normal during more than 6 years of follow-up.
Immunofluorescence staining for C4 is not a routine part of renal biopsy evaluation in the USA. However, in this case, it was the unusual pattern of very strong and widespread C4 capillary wall deposits observed on immunofluorescence that suggested from the outset that we were faced with a rare condition. This finding together with the persistent low C4 in the serum, absence of lupus or infection parameters initiated the search for a possible familial defect. We suggest that routine use of C4 staining could result in identification of further similar cases.
C4B deficiencies have not been well-documented in (and rarely associated with) MPGN III. Measurement of C4 protein levels may not detect C4A or C4B deficiencies; more elaborate diagnostic tests, such as C4 protein allotyping and genotyping, are required to identify complement C4 deficiencies in familial glomerulopathy. Our understanding of the exact mechanisms by which complement abnormalities lead to specific disease phenotypes is still poor. More in depth analyses are required for a better understanding of the pathogenesis and, thus, the development of specific therapies.
Acknowledgments
We thank the patient and her family for their participation. YLW and CYY were supported by NIH grant 1R01 AR054459; AO was supported by ISCII grants RETIC/REDINREN 06/0016 and PS09/00447.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement. None
Reference List
- 1.Walport MJ. Complement-part I. New Engl. J. Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Chung EK, Wu YL, Yang Y, Zhou B, Yu CY. Human complement components C4A and C4B genetic diversities: complex genotypes and phenotypes. Curr. Protoc. Immunol. 2005:13.8.1–13.8.36. doi: 10.1002/0471142735.im1308s68. [DOI] [PubMed] [Google Scholar]
- 3.Blanchong CA, Zhou B, Rupert KL, Chung EK, Jones KN, Sotos JF, Rennebohm RM, Yu CY. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in Caucasians: the load of RCCX genetic diversity on MHC-associated disease. J. Exp. Med. 2000;191:2183–2196. doi: 10.1084/jem.191.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Chung EK, Wu YL, Savelli SL, Nagaraja HN, Zhou B, Hebert M, Jones KN, Shu Y, Kitzmiller K, Blanchong CA, McBride K, Higgins GC, Rennebohm RM, Rice RR, Hackshaw KV, Roubey RA, Grossman JM, Tsao BP, Birmingham DJ, Rovin BH, Hebert LA, Yu CY. Gene copy number variation and associated polymorphisms of complement component C4 in human systemic erythematosus (SLE): low copy number is a risk factor for and high copy number is a protective factor against European American SLE disease susceptibility. Am. J. Hum. Genet. 2007;80:1037–1054. doi: 10.1086/518257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Chung EK, Zhou B, Lhotta K, Hebert LA, Birmingham DJ, Rovin BH, Yu CY. The intricate role of complement C4 in human systemic lupus erythematosus. Curr. Direct. Autoimmun. 2004;7:98–132. doi: 10.1159/000075689. [DOI] [PubMed] [Google Scholar]
- 6.Winkelstein J, Gewurz AT, Lint TF, Gewurz H. The complement system. In: Adkinson NF, Yungunger JW, Busse WW, editors. Middleton's Allergy Priniciples and Practice. Philadelphia: Mosby Inc; 2003. pp. 66–85. [Google Scholar]
- 7.Ault BH, Stapleton FB, Rivas ML, Waldo FB, Roy S, McLean RH, Bin JA, Wyatt RJ. Association of Henoch-Schonlein purpura glomerulonephritis with C4B deficiency. J Pediatr. 1990;117:753–755. doi: 10.1016/s0022-3476(05)83336-0. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt RJ, Schneider PD, Alpers CE, Hudson EC, Julian BA. C4B deficiency in two siblings with IgA nephropathy. Am J Kidney Dis. 1990;15:66–71. doi: 10.1016/s0272-6386(12)80594-4. [DOI] [PubMed] [Google Scholar]
- 9.Welch TR, Berry A, Beischel LS. C4 isotype deficiency in IgA nephropathy. Pediatr Nephrol. 1987;1:136–139. doi: 10.1007/BF00849283. [DOI] [PubMed] [Google Scholar]
- 10.Welch TR, McAdams AJ, Beischel LS. Glomerulonephritis associated with complete deficiency of the fourth component of complement. Response to intravenous immunoglobulin. Arthritis Rheum. 1995;38:1333–1337. doi: 10.1002/art.1780380923. [DOI] [PubMed] [Google Scholar]
- 11.Thors VS, Kristinsson JR, Laxdal T, Jonmundsson G, Eethvarethsson VO, Sigfusson A, Briem H, Haraldsson A. Henoch-Schonlein purpura, patients admitted to Landspitali-University Hospital 1984–2000. Laeknabladid. 2002;88:807–811. [PubMed] [Google Scholar]
- 12.West CD. Childhood membranoproliferative glomerulonephritis: an approach to management. Kidney Int. 1986;29:1077–1093. doi: 10.1038/ki.1986.110. [DOI] [PubMed] [Google Scholar]
- 13.Cameron JS, Turner DR, Heaton J, Williams DG, Ogg CS, Chantler C, Haycock GB, Hicks J. Idiopathic mesangiocapillary glomerulonephritis. Comparison of types I and II in children and adults and long-term prognosis. Am. J. Med. 1983;74:175–192. doi: 10.1016/0002-9343(83)90606-x. [DOI] [PubMed] [Google Scholar]
- 14.Stratta P, Segoloni GP, Canavese C, Sandri L, Mazzucco G, Roccatello D, Manganaro M, Vercellone A. Incidence of biopsy-proven primary glomerulonephritis in an Italian province. Am. J. Kidney Dis. 1996;27:631–639. doi: 10.1016/s0272-6386(96)90096-7. [DOI] [PubMed] [Google Scholar]
- 15.Simon P, Ramee MP, Autuly V, Laruelle E, Charasse C, Cam G, Ang KS. Epidemiology of primary glomerular diseases in a French region. Variations according to period and age. Kidney Int. 1994;46:1192–1198. doi: 10.1038/ki.1994.384. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt H, Bohle A, Reineke T, Mayer-Eichberger D, Vogl W. Long-term prognosis of membranoproliferative glomerulonephritis type I. Significance of clinical and morphological parameters: an investigation of 220 cases. Nephron. 1990;55:242–250. doi: 10.1159/000185969. [DOI] [PubMed] [Google Scholar]
- 17.Mathieson PW. Mesangiocapillary glomerulonephritis. In: Pusey CD, editor. The treatment of glomerulonephritis. Boston: Kluwer Acade,oc Publishers; 1999. pp. 81–92. [Google Scholar]
- 18.Lhotta K, Neunhauserer M, Solder B, Uring-Lambert B, Wurzner R, Rumpelt HJ, Konig P. Recurrent hematuria: a novel clinical presentation of hereditary complete complement C4 deficiency. Am. J. Kidney Dis. 1996;27:424–427. doi: 10.1016/s0272-6386(96)90367-4. [DOI] [PubMed] [Google Scholar]
- 19.Tsuda T, Moriguchi M, Asanuma Y, Imamura S, Toyoda A, Yamada S, Terai C, Suzuki K, Tabei K. C4B deficiency associated with membranoproliferative glomerulonephritis. Intern. Med. 2007;46:765–770. doi: 10.2169/internalmedicine.46.6162. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki J, Suzuki S, Nozawa R, Kawasaki Y, Suzuki H. Membranoproliferative glomerulonephritis associated with hereditary deficiency of the 4th component of complement. Clin. Nephrol. 2003;60:279–283. doi: 10.5414/cnp60279. [DOI] [PubMed] [Google Scholar]
- 21.Merkle M, Weiss M, Wornle M. Chronic urticaria and mesangial proliferative glomerulonephritis: a case report. Nephrol. Dial. Transplant. 2007;22:3327–3329. doi: 10.1093/ndt/gfm548. [DOI] [PubMed] [Google Scholar]
- 22.Wu YL, Savelli SL, Yang Y, Zhou B, Rovin BH, Birmingham DJ, Nagaraja HN, Hebert LA, Yu CY. Sensitive and specific real-time PCR Assays to accurately determine copy-number variations (CNVs) of human complement C4A, C4B, C4-Long, C4-Short and RCCX modules: Elucidation of C4 CNVs in 50 consanguineous subjects with defined HLA genotypes. J. Immunol. 2007;179:3012–3025. doi: 10.4049/jimmunol.179.5.3012. [DOI] [PubMed] [Google Scholar]
- 23.Sim E, Cross S. Phenotyping of human complement component C4, a class III HLA antigen. Biochem J. 1986;239:763–767. doi: 10.1042/bj2390763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders D, Agricola B, Sippel M, Thoenes W. Basement membrane changes in membranoproliferative glomerulonephritis. II. Characterization of a third type by silver impregnation of ultra thin sections. Virchows Arch. A Pathol. Anat. Histol. 1977;376:1–19. doi: 10.1007/BF00433081. [DOI] [PubMed] [Google Scholar]
- 25.Strife CF, McEnery PT, McAdams AJ, West CD. Membranoproliferative glomerulonephritis with disruption of the glomerular basement membrane. Clin. Nephrol. 1977;7:65–72. [PubMed] [Google Scholar]
- 26.Neary JJ, Conlon PJ, Croke D, Dorman A, Keogan M, Zhang FY, Vance JM, Pericak-Vance MA, Scott WK, Winn MP. Linkage of a gene causing familial membranoproliferative glomerulonephritis type III to chromosome 1. J. Am. Soc. Nephrol. 2002;13:2052–2057. doi: 10.1097/01.asn.0000022006.49966.f8. [DOI] [PubMed] [Google Scholar]
- 27.Alchi B, Jayne D. Membranoproliferative glomerulonephritis. Pediatr. Nephrol. 2009 doi: 10.1007/s00467-009-1322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varade WS, Forristal J, West CD. Patterns of complement activation in idiopathic membranoproliferative glomerulonephritis, types I, II, and III. Am. J. Kidney Dis. 1990;16:196–206. doi: 10.1016/s0272-6386(12)81018-3. [DOI] [PubMed] [Google Scholar]
- 29.Braun MC, Strife CF. Membranoproliferative glomerulonephritis. In: Kaplan BS, Meyers KEC, editors. Pediatric nephrology and urology: the requisites in pediatrics. Philadelphia: Elsevier; 2004. pp. 147–155. [Google Scholar]
- 30.Yang Y, Lhotta K, Chung EK, Eder P, Neumair F, Yu CY. Complete complement components C4A and C4B deficiencies in human kidney diseases and systemic lupus erythematosus. J. Immunol. 2004;173:2803–2814. doi: 10.4049/jimmunol.173.4.2803. [DOI] [PubMed] [Google Scholar]
- 31.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 32.Scolari F, Amoroso A, Savoldi S, Prati E, Scaini P, Manganoni A, Borelli I, Mazzola G, Canale L, Sacchi G. Familial occurrence of primary glomerulonephritis: evidence for a role of genetic factors. Nephrol Dial Transplant. 1992;7:587–596. doi: 10.1093/ndt/7.7.587. [DOI] [PubMed] [Google Scholar]
- 33.Stefansson T, Kolka VR, Sigurdardottir SL, Edvardsson VO, Arason G, Haraldsson A. Increased frequency of C4B*Q0 alleles in patients with Henoch-Schonlein purpura. Scand. J. Immunol. 2005;61:274–278. doi: 10.1111/j.1365-3083.2005.01533.x. [DOI] [PubMed] [Google Scholar]
- 34.Jin DK, Kohsaka T, Koo JW, Ha IS, Cheong HI, Choi Y. Complement 4 locus II gene deletion and DQA1*0301 gene: genetic risk factors for IgA nephropathy and Henoch-Schonlein nephritis. Nephron. 1996;73:390–395. doi: 10.1159/000189098. [DOI] [PubMed] [Google Scholar]
- 35.McLean RH, Wyatt RJ, Julian BA. Complement phenotypes in glomerulonephritis: increased frequency of homozygous null C4 phenotypes in IgA nephropathy and Henoch-Schonlein purpura. Kidney Int. 1984;26:855–860. doi: 10.1038/ki.1984.228. [DOI] [PubMed] [Google Scholar]
- 36.Hauptmann G, Tappeiner G, Schifferli J. Inherited deficiency of the fourth component of human complement. Immunodefic. Rev. 1988;1:3–22. [PubMed] [Google Scholar]
- 37.Rupert KL, Moulds JM, Yang Y, Arnett FC, Warren RW, Reveille JD, Myones BL, Blanchong CA, Yu CY. The molecular basis of complete C4A and C4B deficiencies in a systemic lupus erythematosus (SLE) patient with homozygous C4A and C4B mutant genes. J. Immunol. 2002;169:1570–1578. doi: 10.4049/jimmunol.169.3.1570. [DOI] [PubMed] [Google Scholar]
- 38.Chung EK, Yang Y, Rupert KL, Jones KN, Rennebohm RM, Blanchong CA, Yu CY. Determining the one, two, three or four long and short loci of human complement C4 in a major histocompatibility complex haplotype encoding for C4A or C4B proteins. Am. J. Hum. Genet. 2002;71:810–822. doi: 10.1086/342778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reveille JD, Moulds JM, Arnett FC. Major histocompatibility complex class II and C4 alleles in Mexican Americans with systemic lupus erythematosus. Tissue Antigens. 1995;45:91–97. doi: 10.1111/j.1399-0039.1995.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 40.Naves M, Hajeer AH, Teh LS, vies EJ, di-Ros J, Perez-Pemen P, Vilardell-Tarres M, Thomson W, Worthington J, Ollier WE. Complement C4B null alleles status confers risk for systematic lupus erythematosus in a Spanish population. Eur. J. Immunogenet. 1998;25:317–320. doi: 10.1046/j.1365-2370.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 41.Lhotta K, Wurzner R, Rumpelt HJ, Eder P, Mayer G. Membranous nephropathy in a patient with hereditary complete complement C4 deficiency. Nephrol. Dial. Transplant. 2004;19:990–993. doi: 10.1093/ndt/gfh008. [DOI] [PubMed] [Google Scholar]
- 42.Licht C, Fremeaux-Bacchi V. Hereditary and acquired complement dysregulation in membranoproliferative glomerulonephritis. Thromb. Haemost. 2009;101:271–278. [PubMed] [Google Scholar]
- 43.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 2002;31(4):424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 44.Aitman TJ, Dong R, Vyse TJ, Norsworthy PJ, Johnson MD, Smith J, Mangion J, Roberton-Lowe C, Marshall AJ, Petretto E, Hodges MD, Bhangal G, Patel SG, Sheehan-Rooney K, Duda M, Cook PR, Evans DJ, Domin J, Flint J, Boyle JJ, Pusey CD, Cook HT. Copy number polymorphism in Fcgr3 predisposes to glomerulonephritis in rats and humans. Nature. 2006;439:851–855. doi: 10.1038/nature04489. [DOI] [PubMed] [Google Scholar]
- 45.Levin A. Management of membranoproliferative glomerulonephritis: evidence-based recommendations. Kidney Int. 1999 Suppl 70:S41–S46. doi: 10.1046/j.1523-1755.1999.07006.x. [DOI] [PubMed] [Google Scholar]
- 46.Faedda R, Satta A, Tanda F, Pirisi M, Bartoli E. Immunosuppressive treatment of membranoproliferative glomerulonephritis. Nephron. 1994;67:59–65. doi: 10.1159/000187889. [DOI] [PubMed] [Google Scholar]
- 47.Cattran DC, Cardella CJ, Roscoe JM, Charron RC, Rance PC, Ritchie SM, Corey PN. Results of a controlled drug trial in membranoproliferative glomerulonephritis. Kidney Int. 1985;27:436–441. doi: 10.1038/ki.1985.28. [DOI] [PubMed] [Google Scholar]
- 48.Lhotta K, Wurzner R, Konig P. Glomerular deposition of mannose-binding lectin in human glomerulonephritis. Nephrol. Dial. Transplant. 1999;14:881–886. doi: 10.1093/ndt/14.4.881. [DOI] [PubMed] [Google Scholar]
- 49.van Bruggen MC, Walgreen B, Rijke TP, Berden JH. Attenuation of murine lupus nephritis by mycophenolate mofetil. J. Am. Soc. Nephrol. 1998;9:1407–1415. doi: 10.1681/ASN.V981407. [DOI] [PubMed] [Google Scholar]
- 50.Wu YL, Yang Y, Chung EK, Zhou B, Kitzmiller KJ, Savelli SL, Nagaraja HN, Birmingham DJ, Tsao BP, Rovin BH, Hebert LA, Yu CY. Phenotypes, genotypes and disease susceptibility associated with gene copy number variations: complement C4 CNVs in European American healthy subjects and those with systemic lupus erythematosus. Cytogenet. Genome Res. 2008;123:131–141. doi: 10.1159/000184700. [DOI] [PMC free article] [PubMed] [Google Scholar]