Abstract
Estrogen replacement therapy in women has demonstrated a protective effect in the development of colonic carcinomas. Gender-related differences in the development of colonic carcinomas have also been reported. Estrogen receptor beta (ERβ) is expressed in colon carcinomas and has demonstrated prognostic value in colon cancer patients. This study investigated an ERβ 3’ non-coding polymorphism associated with transcriptional activity to determine clinical outcome in patients with metastatic colon cancer. Genomic DNA from 318 metastatic colon cancer patients, 177 males and 141 females, were collected from 1992 to 2003. These patients were analyzed for CA repeat polymorphism of the ERβ gene. Gender-related survival differences were associated with an ERβ (CA)n repeat polymorphism (P for interaction=0.003, the likelihood ratio test). Female patients with any short <22 (CA)n repeat alleles had shorter overall survival compared to female patients that had both long ≥22 (CA)n repeat alleles. In the male patients the opposite overall survival difference was found. This study supports the role of an ERβ (CA)n repeat polymorphism as a prognostic marker in metastatic colon cancer; however, this prognostic factor had opposite implications based on gender.
Keywords: Colon cancer, gender, ERβ, polymorphisms, ERβ CA repeat polymorphism, clinical outcome
Introduction
Colorectal cancer is the second most common cause of cancer-related death in the United States. In 2009, an estimated 106,100 new cases will be diagnosed and 49,920 deaths will occur1. Recent evidence suggests that there are gender-related differences in the development of colonic carcinomas. Women of all ages are less likely to develop colon cancer 2–4. Animal models have also shown that male rats exposed to experimental carcinogens have a higher risk of developing colon cancer compared to female rats 5, 6. Large comprehensive studies such as the Women’s Health Initiative (WHI) have shown conclusively that postmenopausal women treated with estrogen replacement therapy have a significant reduction in their relative risk of developing colon cancer 7, 8. The molecular mechanisms by which estrogen replacement therapy exerts its protective effect against colon cancer are not understood. However, the presence of sex hormones and their receptors in the colon may influence the development of colon cancer differently in men and women.
Estrogen receptor beta (ERβ) is expressed in colonic tissue 9. Loss of ERβ expression has been linked to malignant transformation 10 and advanced Dukes stage 11. Colon cancer cell lines expressing ERβ treated with estrogen have shown induction of apoptosis 12. ERβ expression in the colon is also associated with regulation of important prognostic markers, such as thymidylate synthase (TS), survivin, telomerase 13, APC tumor-suppressor gene 14 and p38/MAPK 15. Multiple review articles indicate that loss of ERβ expression is a common step in the development of colonic carcinoma 16–18.
Within the ERβ gene, located on chromosome 14q22–32, is a polymorphic dinucleotide (CA)n repeat in the 3’ non-coding region. In vitro studies of vector insertion of short (CA)n repeats show an association with increased ERβ protein expression 19. Women with increased numbers of ERβ CA repeats have a 6-fold increase in risk of developing colon cancer 20. The number of CA repeats in the ERβ polymorphism is also associated with higher bone mineral density in premenopausal women 21 as well as with increased androgen hormone levels and increased sex steroid binding globulin levels 22, 23. Based on this information, we tested the hypothesis that genetic variation at the ERβ locus may be associated with gender-related survival in patients with metastatic colon cancer.
Materials and Methods
Eligible Patients
A total of 318 patients with metastatic colon cancer treated at the University of Southern California/Norris Comprehensive Cancer Center (USC/NCCC) or the Los Angeles County+University of Southern California Medical Center (LAC+USCMC) between 1992 and 2003 were eligible for the present study. This population included only metastatic or recurrent colon cancer patients. All patients in this study signed informed consents and enrolled in protocols designed to study the molecular determinants of colon cancer. These protocols permitted blood collection (protocol 0S-99-10) and/or tissue collection (protocol 0S-00-15).
All enrolled patients were followed with an institutional database. Patient information was collected through database review and retrospective chart review when additional patient information was necessary. A large number of the patients (220/318= 69%) were initially treated at an outside institution until, because of failure to respond to prior treatment, they were referred to USC/NCCC or LAC+USCMC for future treatments. The end-point of this study is overall survival. The overall survival was determined by calculating the difference between the date of first treatment at USC/NCCC or LAC+USC and the date of last follow-up appointment or date of death from disease.
All 318 patients were enrolled in at least one chemotherapy clinical trial while being treated at this institution (USC/NCCC or LAC/USCMC). All patients were treated with 5-fluorouracil (5-FU) based chemotherapy regimens. Response to chemotherapy was not investigated as an end point for this study. This is a heavily pretreated cohort with 20 patients (6%) treated with one line of chemotherapy, 19 patients (6%) treated with two different chemotherapy regimens, 183 patents (58%) treated with three different chemotherapy regimens, and 96 patients (30%) treated with four or more chemotherapy regimens. Although, the treatment regimens varied among patients, the majority of the patients were exposed to similar chemotherapies. All 318 patients (100%) received treatment with 5-fluorouracil (5-FU), 298 patients (94%) received treatment with 5-FU/Irinotecan (CPT-11) and 279 patients (88%) received treatment with 5-FU/oxaliplatin.
Laboratory Methods
DNA extraction
Peripheral blood and paraffin embedded tissue samples were collected from each patient. Genomic DNA was extracted from whole blood or paraffinized tissue using the QiaAmp kit (Qiagen, Valencia, CA). Genomic DNA was obtained from peripheral blood for 314 of the patients. There were four samples where peripheral blood was not available and, therefore, genomic DNA was obtained from paraffin embedded tissue. These 318 genomic DNA samples were used to analyze the ERβ (CA)n repeat polymorphism.
Genotyping of ERβ (CA)n Repeat Polymorphism
The polymerase chain reaction (PCR) was performed with oligonucleotide primers (5’-GGTAAACCATGGTCTGTACC-3’ and 5’-AACAAAATGTTGAATGAGTGGG-3’) designed to amplify a polymorphic (CA)n repeat in the flanking region of the human ERβ gene. The reaction was carried out in a final PCR volume of 15µL containing 100 ng of genomic DNA, 200 µM dNTP’s, 1.0 µM 5’ 33P-γATP end-labeled reverse primer, 1.0 µM unlabeled forward primer, 0.75 U Taq polymerase (Perkin Elmer) and PCR buffer (10 mM Tris-HCl pH 8.3, 50 mM KCl, 1.5 mM MgCl2). The reaction was carried out for 35 cycles with 94°C (1min) denaturation, 60°C (1min) annealing, and 72°C (1min) extension. The reaction products were separated on a 6% denaturing polyacrylamide DNA sequencing gel, vacuum blotted for 1 hour at 80°C and exposed to an XAR film (Eastman-Kodak Co., Rochester, NY) for 6 to 8 hours. The exact number of the CA repeats was confirmed by direct sequencing.
ERβ intratumoral mRNA Gene Expression
In our cohort of 318 patients there were only a total of 64 patients with available paraffin-embedded tumor blocks. All 64 patients with available paraffin-embedded tumor blocks were analyzed for ERβ intratumoral mRNA Gene Expression. Paraffin-embedded tumor blocks were reviewed for quality and tumor content. For each sample Microdissection of intratumoral tissue was extracted, RNA isolation and cDNA synthesis was done followed by real-time PCR quantification of mRNA expression as described 24.
Statistical Analysis
The primary end-point of this study is overall survival. The overall survival was defined as the time period between the date of first treatment at USC/NCCC or LAC+USC for their metastatic disease and date of death or the date of last follow-up appointment. Patients who were still alive at the last follow-up were censored at that time.
The associations between sex and other baseline demographic and clinical characteristics were examined using contingency tables and the χ2 test or when appropriate, Fisher’s exact test. The ERβ (CA)n repeat polymorphism was categorized into two groups using the most frequent (CA)n as a cut-off point: any alleles with <22 (CA)n and both alleles with ≥ 22 (CA)n in the similar way as previous studies 22, 23, 25. The differences in expression levels of two ERβ isoforms, ERβ1 and ERβ5, by ERβ (CA)n repeat polymorphism and sex were examined using the Mann-Whitney U test. The associations of ERβ (CA)n repeat polymorphisms with overall survival were analyzed individually using Kaplan-Meier plots and the log-rank test. In the univariate analysis, the estimate of relative risk (RR) with 95% confidence intervals (CI) was based on the log-rank test 26, 27. Multivariate analysis using Cox proportional hazards regression model was performed to evaluate the association between ERβ polymorphism and overall survival when adjusted for baseline characteristics. Interactions between sex and ERβ polymorphism were examined using stratified models and were tested by comparing corresponding likelihood ratio statistics between the baseline and nested Cox proportional hazards models that included the multiplicative product terms 28.
Results
This population contained a total of 318 patients, 141 female (44%) and 177 male (56%). Within the two populations, males and females, there were no statistically significant variations with regard to age, racial/ethnic make-up, location of the primary tumor, or location of first metastatic site. The median age at the time of diagnosis was 58 years old with a range of 25 to 86 years of age. The racial/ethnic distribution of study participants was 234 Caucasian (73%), 43 Asian (14%), 24 Hispanic (8%), 15 Black (5%) and 2 Native American (1%). The location of the primary tumor within the colon was as follows: 144 left-sided tumors (45%), 124 right-sided tumors (39%) and 50 (16%) where the side was unknown. The location of the first metastatic site for 156 (49%) patients was the liver, for 56 (18%) intra-abdominal sites, for 46 (15%) other (lung, bone, stomach, spleen, pancreas or gallbladder) metastatic sites and there were 57 (18%) patients who presented with two or more metastatic sites at the on-set of metastatic disease (Table 1).
Table 1.
Demographic and baseline clinical information by sex.
| Total | Female | Male | P value* | |
|---|---|---|---|---|
| N | 318 | 141 | 177 | |
| Age, median (range) | 58 (25–86) | 57 (25–82) | 59 (25–86) | |
| ≤ 39 | 28 (9%) | 16 (11%) | 12 (7%) | |
| 40–59 | 144 (45%) | 66 (47%) | 78 (44%) | 0.23 |
| 60+ | 146 (46%) | 59 (42%) | 87 (49%) | |
| Ethnic | ||||
| Caucasian | 234 (73%) | 103 (73%) | 131 (74%) | |
| Asian | 43 (14%) | 24 (17%) | 19 (11%) | |
| Black | 15 (5%) | 6 (4%) | 9 (5%) | 0.27 |
| Hispanic | 24 (8%) | 7 (5%) | 17 (10%) | |
| Native American | 2 (1%) | 1 (1%) | 1 (1%) | |
| Side of tumor | ||||
| Left | 144 (54%) | 62 (50%) | 82 (57%) | |
| Right | 124 (46%) | 62 (50%) | 62 (43%) | 0.27 |
| Unknown | 50 | 17 | 33 | |
| Number of first metastatic sites | ||||
| 1 | ||||
| Liver | 156 (49%) | 65 (46%) | 91 (52%) | |
| Intra-abdominal | 56 (18%) | 28 (20%) | 28 (16%) | 0.60 |
| Other | 46 (15%) | 19 (14%) | 27 (15%) | |
| 2+ | 57 (18%) | 28 (20%) | 29 (17) | |
| Unknown | 3 | 1 | 2 | |
Based on the χ2 test or Fisher’s exact test.
Overall survival differences in this population were not associated with gender differences, racial/ethnic distribution, location of primary tumor, or location of first metastatic lesion. The ERβ (CA)n repeat polymorphism was not associated with the age of on-set of metastatic disease, location of primary tumor, or location of first metastatic lesion (data not shown).
ERβ (CA)n Repeat Polymorphism
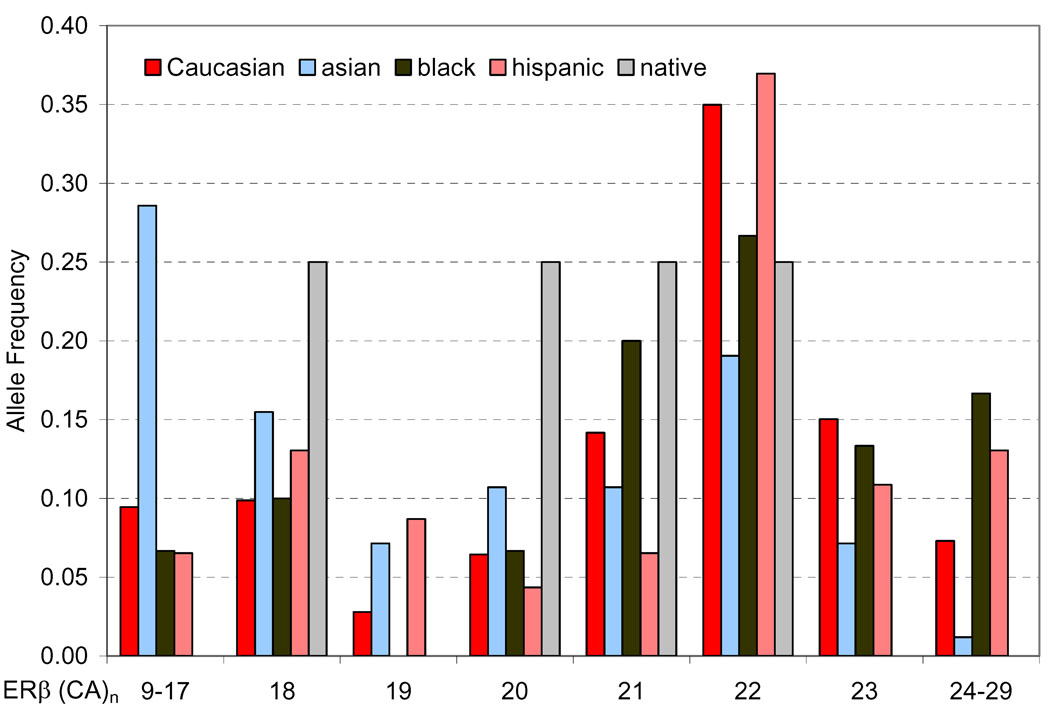
The extracted genomic DNA was evaluated for the ERβ (CA)n repeat polymorphism and the assay was successful in 315 of the 318 cases. There were three cases where the genomic DNA was either consumed or degraded. Eighteen different alleles of the ERβ gene polymorphism were identified, comprising PCR products of 137–177 base pairs which is 9–29 (CA)n repeats (Figure 1). The most predominant allele was the 22 (CA)n repeat (163 base pairs). Asians were more likely to carry shorter (CA)n repeats of the ERβ gene compared to other ethnic groups (P<0.001, Figure 1). The frequency of ERβ (CA)n repeat allelic distributions were similar to other studies done in Caucasian populations 22, 25, 29.
Figure 1.
Allelic Distribution of ERβ (CA)n repeats by ethnicity
The ERβ (CA)n repeats were divided into two subgroups. The cut-off for these groups was 22 ER-beta (CA) repeats, which was determined by previous publications 22, 25, 29. That is, patients with fewer than 22 repeats were in the first subgroup, and patients with at least 22 repeats were in the second subgroup. Twenty-two repeats was chosen as the cutoff because it was the most frequent allele, and has been used as a cutoff in previous studies. This approach was used to evaluate the effect that the polymorphism had on overall survival among all 315 study participants; 71% (224/315) of the patients had at least one short allele <22 (CA)n repeat, while 29% (91/315) of the patients had two long allele ≥22 (CA)n repeats. There was no difference with regard to the allelic distribution among genders. Without separation of the patients according to gender there were no statistically significant associations (Table 2); however, when the patient population was separated by gender, the (CA)n repeat genotypes were associated with overall survival (OS) (Table 2).
Table 2.
Association of Polymorphisms of ERβ (CA)n repeat by Overall Survival and Gender.
| Univariate analysis | |||
|---|---|---|---|
| All patients |
|||
| ER β (CA)n repeat | N (%) | Median survival (95% CI), mo |
RR (95% CI) |
| Any (CA)n <22 | 224 (71%) | 14.0 (11.9–16.2) | 1 (Reference) |
| Both (CA)n ≥ 22 | 91 (29%) | 13.4 (10.3–15.6) | 1.10 (0.85, 1.43) |
| Log-rank P | 0.48 | ||
| Female | Male | |||||
|---|---|---|---|---|---|---|
| EER β (CA)n repeat |
Median survival (95% CI), mo |
RR (95% CI) | N (%) | Median survival (95% CI), mo |
RR (95% CI) | |
| Any (CA)n <22 | 101(72%) | 14.1(12.0–17.9) | 1(Reference) | 123(70%) | 13.6(10.1–16.8) | 1 (Reference) |
| Both (CA)n ≥ 22 | 39(28%) | 18.4(14.7–26.6) | 0.69(0.45, 1.06) | 52(30%) | 10.3(7.9–13.1) | 1.51 (1.07, 2.12) |
| Log-rank P | 0.083 | 0.014 | ||||
|
P for interaction of Female vs Male |
0.003 | |||||
| Multivariate analysis | |||
|---|---|---|---|
| All patients |
Female |
Male |
|
| ER β (CA)n repeat | RR (95% CI)* | RR (95% CI)* | RR (95% CI)* |
| Any (CA)n <22 | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Both (CA)n ≥ 22 | 0.99 (0.75, 1.30) | 0.62 (0.39, 1.00) | 1.34 (0.94, 1.90) |
| Adjusted P* | 0.93 | 0.05 | 0.11 |
| P for interaction† | 0.005 | ||
Based on Cox proportional hazards model; adjusted by age and previous treatments and stratified by race; P values were based on Wald test in the multivariate model.
Based on the likelihood ratio test; adjusted by age and previous treatments and stratified by race.
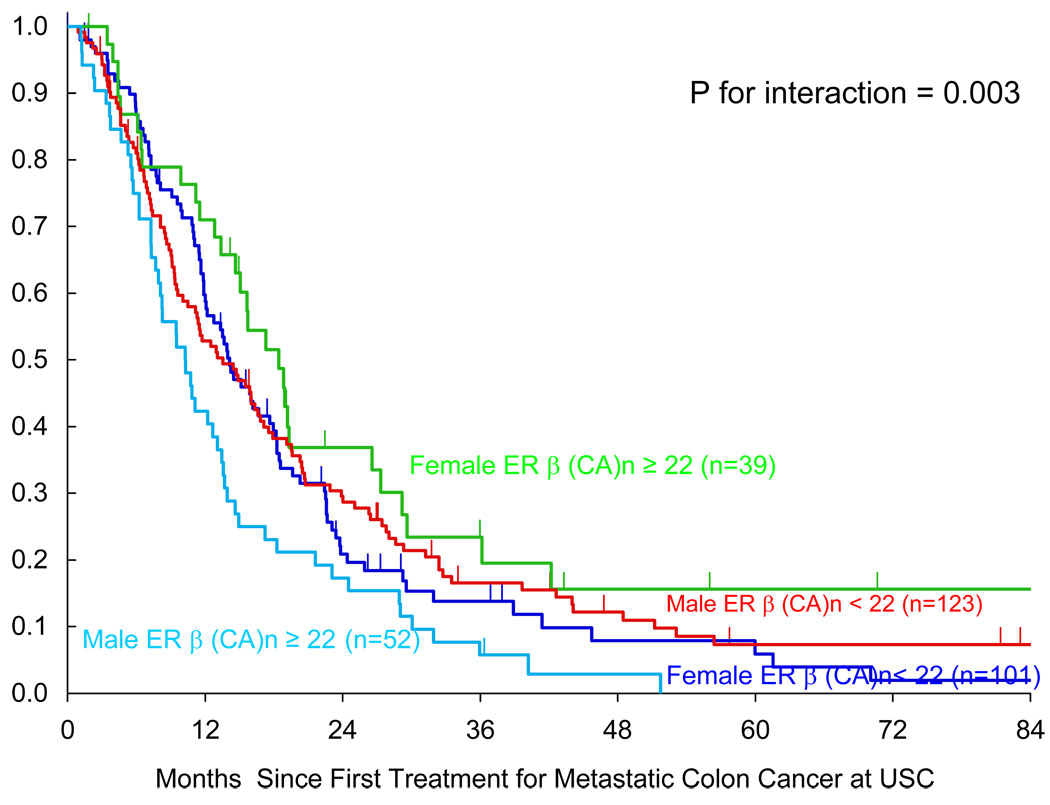
Female patients with any short <22 (CA)n repeat alleles (n=101) had shorter OS (median OS =14.1 months) compared to the female patient group that had two long ≥22 (CA)n repeat alleles (n=39)(median OS=18.4 months)(p= 0.05, multivariate analysis)(Table 2). In the male patients the opposite overall survival (OS) difference was found. The male patients with any short <22 (CA)n repeat allele (n=123) had a longer overall survival (median OS=13.6 months) compared with males that had two long ≥22 (CA)n repeat alleles (n=52)(median OS=10.3 months)(P=0.014, univariate analysis)(Table 2). Therefore, as a prognostic factor this ERβ (CA)n repeat polymorphism had opposite implications based on gender (P for interaction = 0.003).
Patients with two long ≥22 (CA)n repeat alleles showed a large shift in OS based on whether the patient was male (median OS=10.3 months)(n=52) or female (median OS=18.4 months)(n=39)(Table 2)(Fig. 2). However, patients with any short <22 (CA)n repeat allele had a median OS that was similar; males 13.6 months OS (n=123) and females 14.1 months OS (n=101)(Table 2).
Figure 2.
Gender Related Overall Survival for ERβ long alleles ≥22 and short <22 (CA)n repeats.
Intertumoral mRNA gene expression of ERβ levels in association with ERβ (CA)n Repeat Polymorphism
The intertumoral mRNA gene expression of ERβ levels were associated with the ERβ (CA)n repeat polymorphism. There was a significant difference in ERβ1 expression values by ERβ (CA)n repeat polymorphism (P = 0.043, Mann-Whitney test). The tumors with any short <22 (CA)n repeat alleles had higher intratumoral ERβ1 mRNA expression value.
A total of 64 patients had paraffin-embedded tumor blocks available. 47 patients had at least one short <22 (CA)n repeat allele. These 47 patients had a median mRNA expression level of 1.88 (range, 0.01 to 195.09). This was compared to the 17 patients with both long ≥22 (CA)n repeat alleles which had a median mRNA expression level of 1.14 (range, 0.01 to 4.47). Therefore, the tumors with any short <22 (CA)n repeat alleles had higher ERβ1 expression level (median 1.88) compared to both long ≥22 (CA)n repeat alleles which had a lower expression level of 1.14 (P = 0.043, Mann-Whitney test).
ERβ1, ERβ2, ERβ3, ERβ4 or ERβ5 expression values revealed no associated differences of intratumoral mRNA expression based on gender (P = 0.24). Although ERβ1 expression values were associated with ERβ (CA)n repeat polymorphism (P = 0.043, Mann-Whitney test), there were no similar significant associations with ERβ (CA)n repeat polymorphism and ERβ2, ERβ3, ERβ4 or ERβ5 expression values.
AR Polymorphism analysis and ERβ Haplotype
We also analyzed a CA repeat polymorphism in the androgen receptor, as well as ERβ haplotype. Neither marker was significantly associated with clinical outcome (data not shown).
Discussion
This study has investigated genetic variation in the sex steroid hormone receptor ERβ in metastatic colon cancer patients. Gender-related survival differences were associated with an ERβ (CA)n repeat polymorphism (P for interaction=0.003, the likelihood ratio test). Female patients with any short <22 (CA)n repeat allele had shorter overall survival compared to female patients that had two long ≥22 (CA)n repeat alleles. In the male patients the opposite overall survival difference was found.
Our patient population had a frequency of ERβ (CA)n repeat allelic distributions similar to that of other study populations (Figure 1)22, 25, 29. Demographic information for age, ethnicity, and location of tumor was collected for our patient population and no associations were found (Table 1). This indicates that our findings are not due to a sampling error.
Evidence is accumulating to support the role of sex steroid hormones in the development of colonic carcinomas. One explanation is the known protective effect that estrogen has against the development of colon cancer. It has been clearly demonstrated that menopausal women treated with estrogen replacement therapy have a significant reduction in both their relative risk and their relative rate of developing colon cancer 7, 8. In addition, women of all ages are less likely to develop colon cancer 2–4. Generally speaking females have higher levels of estrogens and lower levels of androgens; the opposite is true in males.
In this population overall survival differences associated with the ERβ polymorphism were only observed when the population was separated by gender. This could imply that ERβ has a fundamentally different role among genders. One study by Foley et al found that selective loss of ERβ protein expression led to malignant transformation in colonic tissues 10. ERβ is the major estrogen receptor expressed in the colon 9 and multiple review articles indicate that loss of ERβ expression is a common step in the development of colonic carcinoma 16–18. This information indicates that the presence of ERβ expression in the colon has a protective effect. Combine this information with the known protective effect of estrogen replacement therapy in menopausal women and there is strong indication that both estrogen hormone and the presence of ERβ have a protective effect on the development of colonic carcinoma.
The ERβ (CA)n repeat polymorphism has functional significance. One study by Ugai et al found, in vitro insertion of short (CA)n repeats showed an association with increased ERβ protein expression 19. In addition, our study has shown that patients with any short <22 (CA)n repeat alleles had a higher intratumoral ERβ1 gene expression value compared to patients with homozygous long ≥22 (CA)n repeat alleles (p-value = 0.043). To our knowledge this is the first study to link this ERβ (CA)n repeat polymorphism to ERβ mRNA expression. Both our study and the study by Ugai et al indicates that, short <22 (CA)n repeat alleles have higher ERβ expression and the homozygous long ≥22 (CA)n repeat alleles have lower relative ERβ expression. The mechanism of how this ERβ (CA)n repeat polymorphism effects the ERβ mRNA expression is not known and further in vitro and in vivo studies are indicated.
The ERβ (CA)n repeat polymorphism is clinically significant. This ERβ (CA)n repeat polymorphism has been positively associated with a diverse range of conditions including hormonal regulation 22, 23, bone mineral density 21, 30–32, Alzheimer's disease 29, and blood pressure 33. In addition, epidemiologic studies have shown women with increased numbers of ERβ CA repeat polymorphisms have a 6-fold increase in risk of developing colon cancer 20.
In our study of the ERβ polymorphism in the male population the findings were consistent with our hypothesis. The male population that had two long ≥22 (CA)n repeat alleles associated with low relative ERβ expression had worse overall survival (OS) (median OS=10.3 months), compared to male patients with any short <22 (CA)n repeat allele (median OS=13.6 months) (Table 2). Previous data has established the protective effect of ERβ against the development of colon cancer 16–18. Our data in the male population is consistent with this ERβ protective effect and showed that after the development of colon cancer males with increased ERβ expression had a better prognosis. Our data indicates that the short (CA)n ERβ polymorphism, which has increased ERβ expression, is a marker of more favorable clinical outcome in males with colon cancer. It is possible that in males ERβ acts as an apoptotic factor in both normal and neoplastic colonic mucosa. Additional studies are needed to better characterize this effect.
Our study found that female patients that had two long ≥22 (CA)n repeat alleles, associated with low relative ERβ expression, had a better overall survival (OS) (median OS=18.4 months) compared to female patients with any short <22 (CA)n repeat allele which is associated with increased relative ERβ expression (median OS =14.1 months) (Table 2). Given the protective effect of the relative expression of ERβ on the development of colonic carcinoma, the female population had exactly the opposite overall survival from what would have been expected.
The patients with two long ≥22 (CA)n repeat alleles have lower relative ERβ expression 19. Since expression of ERβ has a protective effect on the development of colonic carcinoma 16–18, the long repeats should have worse overall survival. However, this was not seen in the females. Although there is an established protective effect of ERβ against the development of colon cancer 16–18, our data would indicate that after the development of colon cancer, increased ERβ expression in female patients is a marker of poor prognosis. The short (CA)n repeat ERβ polymorphism is associated with increased ERβ expression and is a marker for poor prognosis in females with colon cancer. It is possible that while ERβ acts as an apoptotic factor in female normal colonic mucosa, after neoplastic transformation an alteration in ERβ may occur to allow ERβ to function as a neoplastic growth factor. The molecular mechanisms of ERβ function in tumors are currently unclear. Future studies are needed to explore molecular function of ERβ polymorphisms in colonic neoplasms and to confirm our findings.
Our data, that a functional ERβ polymorphism is associated with opposite prognostic implications based on gender, is consistent with other studies. Opposite prognostic implications in relation to ERβ expression in men and women have been demonstrated in lung cancer 34. Furthermore, our previous findings showed the same opposite effect of two functional polymorphisms of epidermal growth factor receptor (EGFR) in predicting clinical outcome based on gender in the same patient cohort 35. In this study the males followed the expected prognostic implication that EGFR polymorphisms are associated with increased expression of EGFR and have a worse clinical outcome. However, the female patients with the same polymorphisms had exactly the opposite effect.
Another explanation for women not exhibiting the expected ERβ protective effect could have to do with intermediates that result in different activation in males and females. For example, EGFR has been shown to interact in an opposite fashion based on sex-specific steroid signaling 36. EGFR has been shown to have bidirectional signaling with both estrogen 37 and androgen receptors 38, 39. If tumor specific expression of ERβ and EGFR in the colon were inversely related, then regulation of estrogen’s protective effect would also be inversely related. This might contribute to the unexpected inverse survival benefit observed in our female patient population. Future studies are necessary to explore the possible molecular interactions that may exist between sex hormone pathways involving ERβ and EGFR in the colon.
Our data may have implications for treatment. There have been indications that men and women respond differently to treatment for colon cancer. A phase III clinical trial (EORTC 05963) in metastatic colorectal cancer found gender was associated with a treatment-specific inversion of overall survival benefit among males and females 40. In animal models of known positive ERβ colon cancer, known selective estrogen receptor modulator (SERM) has been shown to interact with ERβ protein and mRNA expression to give a chemopreventative affect 41. This may indicate that treatment of human colon cancer may be responsive to SERMs. Lung cancer, like colon cancer, has expression of primary ERβ and has large numbers of patients represented from both genders. In a lung cancer clinical trial, fulvestrant, an anti-estrogen, has demonstrated anti-tumor activity and clinical responses to this treatment 42.
In summary, this study supports the role of a 3’ non-coding (CA)n repeat polymorphism of the ERβ gene as a prognostic marker in metastatic colon cancer. The ERβ (CA)n repeat polymorphic variant had opposite prognostic implications based on gender. To our knowledge, this is the first study that shows a relationship between ERβ polymorphisms and gender-related survival in colon cancer. However, these preliminary findings still need to be replicated in larger studies, as well as, additional confirmatory in vitro and in vivo functional investigations of this polymorphism.
Acknowledgements
The authors would like to thank Ivonne Villalobos for assistance with manuscript preparation.
This investigation was supported by grants from the National Institutes of Health (5 K24CA827540, 5P30CA14089-271), the San Pedro Guild Research Fund and the Dhont Family Foundation. The research was done in the Sharon A. Carpenter Laboratory at USC/Norris Comprehensive Cancer Center.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ries LA, Wingo PA, Miller DS, Howe HL, Weir HK, Rosenberg HM, et al. The annual report to the nation on the status of cancer, 1973–1997, with a special section on colorectal cancer. Cancer. 2000;88(10):2398–2424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.DeCosse JJ, Ngoi SS, Jacobson JS, Cennerazzo WJ. Gender and colorectal cancer. Eur J Cancer Prev. 1993;2(2):105–115. doi: 10.1097/00008469-199303000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Wingo PA, Ries LA, Rosenberg HM, Miller DS, Edwards BK. Cancer incidence and mortality, 1973–1995: a report card for the U.S. Cancer. 1998;82(6):1197–1207. doi: 10.1002/(sici)1097-0142(19980315)82:6<1197::aid-cncr26>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 5.Gershbein LL. Action of estrogen and adrenocorticoids on adenocarcinoma induction by 1,2-dimethylhydrazine in male rats. Res Commun Chem Pathol Pharmacol. 1993;81(1):117–120. [PubMed] [Google Scholar]
- 6.Ochiai M, Watanabe M, Kushida H, Wakabayashi K, Sugimura T, Nagao M. DNA adduct formation, cell proliferation and aberrant crypt focus formation induced by PhIP in male and female rat colon with relevance to carcinogenesis. Carcinogenesis. 1996;17(1):95–98. doi: 10.1093/carcin/17.1.95. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Grodstein F, Newcomb PA, Stampfer MJ. Postmenopausal hormone therapy and the risk of colorectal cancer: a review and meta-analysis. Am J Med. 1999;106(5):574–582. doi: 10.1016/s0002-9343(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 9.Fiorelli G, Picariello L, Martineti V, Tonelli F, Brandi ML. Functional estrogen receptor beta in colon cancer cells. Biochem Biophys Res Commun. 1999;261(2):521–527. doi: 10.1006/bbrc.1999.1062. [DOI] [PubMed] [Google Scholar]
- 10.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW. Selective loss of estrogen receptor beta in malignant human colon. Cancer Res. 2000;60(2):245–248. [PubMed] [Google Scholar]
- 11.Jassam N, Bell SM, Speirs V, Quirke P. Loss of expression of oestrogen receptor beta in colon cancer and its association with Dukes' staging. Oncol Rep. 2005;14(1):17–21. [PubMed] [Google Scholar]
- 12.Qiu Y, Waters CE, Lewis AE, Langman MJ, Eggo MC. Oestrogen-induced apoptosis in colonocytes expressing oestrogen receptor beta. J Endocrinol. 2002;174(3):369–377. doi: 10.1677/joe.0.1740369. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama Y, Sakamoto H, Satoh K, Yamamoto T. Tamoxifen and gonadal steroids inhibit colon cancer growth in association with inhibition of thymidylate synthase, survivin and telomerase expression through estrogen receptor beta mediated system. Cancer Lett. 2000;161(1):63–71. doi: 10.1016/s0304-3835(00)00600-5. [DOI] [PubMed] [Google Scholar]
- 14.Cho NL, Javid SH, Carothers AM, Redston M, Bertagnolli MM. Estrogen receptors alpha and beta are inhibitory modifiers of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res. 2007;67(5):2366–2372. doi: 10.1158/0008-5472.CAN-06-3026. [DOI] [PubMed] [Google Scholar]
- 15.Caiazza F, Galluzzo P, Lorenzetti S, Marino M. 17Beta-estradiol induces ERbeta up-regulation via p38/MAPK activation in colon cancer cells. Biochem Biophys Res Commun. 2007;359(1):102–107. doi: 10.1016/j.bbrc.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 16.Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9(4):385–391. doi: 10.1016/S1470-2045(08)70100-1. [DOI] [PubMed] [Google Scholar]
- 17.Barone M, Tanzi S, Lofano K, Scavo MP, Guido R, Demarinis L, et al. Estrogens, phytoestrogens and colorectal neoproliferative lesions. Genes Nutr. 2008;3(1):7–13. doi: 10.1007/s12263-008-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11(3):537–551. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ugai K, Nishimura K, Fukino K, Nakamura T, Ueno K. Functional analysis of transcriptional activity of cytosine and adenine (CA) repeats polymorphism in the estrogen receptor beta gene. J Toxicol Sci. 2008;33(2):237–240. doi: 10.2131/jts.33.237. [DOI] [PubMed] [Google Scholar]
- 20.Slattery ML, Sweeney C, Murtaugh M, Ma KN, Wolff RK, Potter JD, et al. Associations between ERalpha, ERbeta, and AR genotypes and colon and rectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2936–2942. doi: 10.1158/1055-9965.EPI-05-0514. [DOI] [PubMed] [Google Scholar]
- 21.Lau HH, Ho AY, Luk KD, Kung AW. Estrogen receptor beta gene polymorphisms are associated with higher bone mineral density in premenopausal, but not postmenopausal southern Chinese women. Bone. 2002;31(2):276–281. doi: 10.1016/s8756-3282(02)00827-x. [DOI] [PubMed] [Google Scholar]
- 22.Westberg L, Baghaei F, Rosmond R, Hellstrand M, Landen M, Jansson M, et al. Polymorphisms of the androgen receptor gene and the estrogen receptor beta gene are associated with androgen levels in women. J Clin Endocrinol Metab. 2001;86(6):2562–2568. doi: 10.1210/jcem.86.6.7614. [DOI] [PubMed] [Google Scholar]
- 23.Westberg L, Ho HP, Baghaei F, Nilsson S, Melke J, Rosmond R, et al. Polymorphisms in oestrogen and progesterone receptor genes: possible influence on prolactin levels in women. Clin Endocrinol (Oxf) 2004;61(2):216–223. doi: 10.1111/j.1365-2265.2004.02083.x. [DOI] [PubMed] [Google Scholar]
- 24.Vallbohmer D, Iqbal S, Yang DY, Rhodes KE, Zhang W, Gordon M, et al. Molecular determinants of irinotecan efficacy. Int J Cancer. 2006;119(10):2435–2442. doi: 10.1002/ijc.22129. [DOI] [PubMed] [Google Scholar]
- 25.Setiawan VW, S EH, G AC, D JH, Vivo ID. Estrogen receptor beta (ESR2 ) polymorphisms and endometrial cancer (United States) Cancer Causes Control. 2004;15(6):627–633. doi: 10.1023/B:CACO.0000036170.28502.5f. [DOI] [PubMed] [Google Scholar]
- 26.Gebhardt F, Zanker KS, Brandt B. Modulation of epidermal growth factor receptor gene transcription by a polymorphic dinucleotide repeat in intron 1. J Biol Chem. 1999;274(19):13176–13180. doi: 10.1074/jbc.274.19.13176. [DOI] [PubMed] [Google Scholar]
- 27.Berry G, Kitchin RM, Mock PA. A comparison of two simple hazard ratio estimators based on the logrank test. Stat Med. 1991;10(5):749–755. doi: 10.1002/sim.4780100510. [DOI] [PubMed] [Google Scholar]
- 28.Rothman KJ. Modern Epidemiology. Philadelphia: Lippincott - Raven; 1998. [Google Scholar]
- 29.Forsell C, Enmark E, Axelman K, Blomberg M, Wahlund LO, Gustafsson JA, et al. Investigations of a CA repeat in the oestrogen receptor beta gene in patients with Alzheimer's disease. Eur J Hum Genet. 2001;9(10):802–804. doi: 10.1038/sj.ejhg.5200714. [DOI] [PubMed] [Google Scholar]
- 30.Scariano JK, Simplicio SG, Montoya GD, Garry PJ, Baumgartner RN. Estrogen receptor beta dinucleotide (CA) repeat polymorphism is significantly associated with bone mineral density in postmenopausal women. Calcif Tissue Int. 2004;74(6):501–508. doi: 10.1007/s00223-003-0170-x. [DOI] [PubMed] [Google Scholar]
- 31.Shearman AM, Karasik D, Gruenthal KM, Demissie S, Cupples LA, Housman DE, et al. Estrogen receptor beta polymorphisms are associated with bone mass in women and men: the Framingham Study. J Bone Miner Res. 2004;19(5):773–781. doi: 10.1359/JBMR.0301258. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa S, Hosoi T, Shiraki M, Orimo H, Emi M, Muramatsu M, et al. Association of estrogen receptor beta gene polymorphism with bone mineral density. Biochem Biophys Res Commun. 2000;269(2):537–541. doi: 10.1006/bbrc.2000.2285. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa S, Emi M, Shiraki M, Hosoi T, Ouchi Y, Inoue S. Association of estrogen receptor beta (ESR2) gene polymorphism with blood pressure. J Hum Genet. 2000;45(6):327–330. doi: 10.1007/s100380070002. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz AG, Prysak GM, Murphy V, Lonardo F, Pass H, Schwartz J, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res. 2005;11(20):7280–7287. doi: 10.1158/1078-0432.CCR-05-0498. [DOI] [PubMed] [Google Scholar]
- 35.Press OA, Zhang W, Gordon MA, Yang D, Lurje G, Iqbal S, et al. Gender-related survival differences associated with EGFR polymorphisms in metastatic colon cancer. Cancer Res. 2008;68(8):3037–3042. doi: 10.1158/0008-5472.CAN-07-2718. [DOI] [PubMed] [Google Scholar]
- 36.Hitosugi T, Sasaki K, Sato M, Suzuki Y, Umezawa Y. Epidermal growth factor directs sex-specific steroid signaling through Src activation. J Biol Chem. 2007;282(14):10697–10706. doi: 10.1074/jbc.M610444200. [DOI] [PubMed] [Google Scholar]
- 37.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17(3):309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 38.Bonaccorsi L, Carloni V, Muratori M, Formigli L, Zecchi S, Forti G, et al. EGF receptor (EGFR) signaling promoting invasion is disrupted in androgen-sensitive prostate cancer cells by an interaction between EGFR and androgen receptor (AR) Int J Cancer. 2004;112(1):78–86. doi: 10.1002/ijc.20362. [DOI] [PubMed] [Google Scholar]
- 39.Bonaccorsi L, Muratori M, Carloni V, Marchiani S, Formigli L, Forti G, et al. The androgen receptor associates with the epidermal growth factor receptor in androgen-sensitive prostate cancer cells. Steroids. 2004;69(8–9):549–552. doi: 10.1016/j.steroids.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 40.Giacchetti S, Bjarnason G, Garufi C, Genet D, Iacobelli S, Tampellini M, et al. Phase III trial comparing 4-day chronomodulated therapy versus 2-day conventional delivery of fluorouracil, leucovorin, and oxaliplatin as first-line chemotherapy of metastatic colorectal cancer: the European Organisation for Research and Treatment of Cancer Chronotherapy Group. J Clin Oncol. 2006;24(22):3562–3569. doi: 10.1200/JCO.2006.06.1440. [DOI] [PubMed] [Google Scholar]
- 41.Janakiram NB, Steele VE, Rao CV. Estrogen receptor-beta as a potential target for colon cancer prevention: chemoprevention of azoxymethane-induced colon carcinogenesis by raloxifene in F344 rats. Cancer Prev Res (Phila Pa) 2009;2(1):52–59. doi: 10.1158/1940-6207.CAPR-08-0140. [DOI] [PubMed] [Google Scholar]
- 42.Traynor AM, Schiller JH, Stabile LP, Kolesar JM, Eickhoff JC, Dacic S, et al. Pilot study of gefitinib and fulvestrant in the treatment of post-menopausal women with advanced non-small cell lung cancer. Lung Cancer. 2009;64(1):51–59. doi: 10.1016/j.lungcan.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]