Abstract
Objective
Binge-eating involves an abnormal motivation for highly palatable food in that these foods are repeatedly consumed despite their binge-triggering effects and life-affecting consequences associated with binge-eating. We determined if rats identified as binge-eating prone (BEP) similarly display abnormal motivation for palatable food.
Method
Food-sated BEP and binge-eating resistant (BER) rats were given voluntary access to palatable food paired with increasing intensity of footshock. Later, they were exposed to a period of cyclic caloric restriction-refeeding.
Results
BEPs consumed significantly more and tolerated higher levels of footshock for palatable food than BERs. Cyclic restriction-refeeding increased BERs' tolerance of shock for palatable food.
Discussion
Previously observed parallels of the rat BEP model to human binge-eating can now be extended to include an abnormal motivation for palatable food. This model should prove useful in identifying specific genes that interact with the nutritional environment to mediate binge-eating and may point to novel physiological targets to treat compulsive overeating.
Keywords: BED, obesity, rats, motivation, footshock, compulsive overeating, compulsivity, emotional eating, caloric restriction, dieting, bulimia
Binge-eating is characterized by the compulsion to seek out and consume large quantities of food in a discrete time period (1). While the macronutrient composition of binges is often similar to normal meals (2), it is highly palatable food that is greatly craved and preferred during binges. These are foods that are typically high in sucrose and fat and, because they are calorie-dense, are commonly “forbidden” between binges (3-7). The motivation to repeatedly seek out and consume palatable food can be construed as abnormal given the many consequences that result from ingesting these foods. For example, palatable foods are known to trigger binges (7, 8), and they contribute to weight gain and ensuing preoccupation with weight gain (7-8). Binges lead to worsening body image, low self-esteem, mood disturbances, increased perceived life stress, and adverse medical consequences (9-13). Repeatedly returning to intake of palatable foods with full knowledge that a binge, along with worsening of binge-eating symptoms and consequences, is likely to follow cannot be regarded as adaptive.
Animal models are valuable in that they aid in identifying the physiological underpinnings of complex human behaviors, of which binge-eating is certainly an example. The validity of an animal model of binge-eating is in part contingent on the number of clinical features it reproduces. One feature not previously investigated in these models is the compulsive nature of eating palatable food despite aversive consequences. Consuming significantly more palatable food can imply an increased motivation for that food. However, tolerating punishment for it is stronger evidence of abnormal motivation for palatable foods. Therefore, the main goal of this study was to ascertain whether binge-eating prone (BEP) rats are also characterized by heightened motivation for palatable food, as defined by the voluntary tolerance of punishment for a particular palatable food. Here, the voluntary punishment was incrementing levels of electrical foot shock delivered immediately after the retrieval of a highly palatable food. The voluntary nature of this behavior was ensured by allowing the rat freedom to enter and escape the palatable food-baited alley at any time, by providing plain rat chow in an alley free of shock, and by not restricting the rat of any food intake prior to being placed into the food choice alley. A second aim of the study was to determine the extent to which exposure to a brief history of cyclic caloric restriction followed by ad lib refeeding changes the motivation of BEP and BER rats to tolerate footshock for palatable food. Cyclic caloric restriction was designed to simulate restrictive dieting which is common among many with binge-eating disorders, including bulimia nervosa and binge eating disorder (10, 14-16).
Our BEP/BER model is based on indentifying inherent and stable differences in consumption of palatable food in rats in a discrete, 1-4hr, period of time (17). Rats of the same age and sex generally consume very similar amounts of standard rat chow, their maintenance diet. However, they can vary vastly in how much palatable food they consume when given a choice between these foods and chow. BEP rats are those that consistently consume > 40% more palatable food than those rats consistently eating the least amount of palatable food (BER rats). BEPs are not simply “big eaters,” because they do not overeat on their standard and less palatable maintenance diet of rat chow. Hence, BEPs require palatable food to trigger an abnormal response to their food intake, likely reflecting a gene x environment interaction on their feeding behavior, an interaction that is likely also present in human binge-eating. Since the BEPs do not compensate for their greater intake of palatable food by eating less chow, their total food intake is also greater than that of BERs.
Besides eating larger amounts of food in a discrete and same period of time as BER rats, the BEP rats also display other behaviors that are characteristic of human binge-eating. These are described elsewhere (17), but briefly, they include: 1) eating beyond satiety, since they consume as much food after a period of food deprivation as when sated; 2) forsaking healthier chow for palatable food when stressed (BERs do the opposite, forsaking palatable food over more nutritious chow when stressed); 3) rebounding more quickly from stress-induced hypophagia (any stress-induced hypophagia is no longer apparent in BEPs within one hour), and 4) BEP status does not always predict obesity (10, 18-22). When placed on a steady high-fat diet, just as many BEPs as BERs develop obesity and as many of each group resist obesity (17). Similarly, among humans that binge-eat, not all develop obesity because some will compensate in various ways, typically in maladaptive ways, to resist obesity (1). BEPs and BERs that resist obesity do so by voluntarily eating less of the high-fat diet (17).
The present study used the BEP/BER model to further validate its use in binge-eating research by determining if BEPs were also characterized by an abnormal motivation for palatable food. We tested the hypothesis that rats assigned BEP status would retrieve and consume more palatable food despite experiencing incrementing levels of footshock for doing so. We secondly hypothesized that BEP and BER rats subjected to a history of cyclic caloric restriction-refeeding, a simulation of human dieting, would seek out and consume more palatable food despite the aversive consequence of footshock. Hence, it was predicted that experience with caloric restriction would turn BERs into more BEP-like rats and, in BEPs, the experience would increase their motivation for palatable food to an even greater extent. Lastly, we measured the food intake of BEPs and BERs when they had access to the previously shock-paired palatable food for the first time in the safe confines of their home cage vs. the shock-delivering maze. We predicted that BERs would consume as much of this palatable food as BEPs due to increased incentive value produced by its former unattainable nature, a simulation of “forbidden” food.
Method
Subjects
N = 52, young adult (90-day old) female Sprague-Dawley rats were pair-housed in standard bedded cages under a 12-hr light/dark cycle (lights out at 1100 hrs) with access to ad libitum chow and water during 2 weeks of acclimation to the colony. Following this, 4 feeding tests were conducted wherein all rats were given ad lib access to a choice of chow and a palatable food, Oreo Double Stuf cookies (Nabisco, East Hanover, NJ), for a 24 hr period. Each feeding test was followed by at least 3-5 days of only chow, such that their access to the palatable food was intermittent. During the 4 feeding tests, the foods were given just prior to lights out and amounts consumed were measured after 4 hrs. For each test, a median kcal score was determined. The rats were tentatively categorized as either BEP or BER for each test, depending on whether they ate more or less than the median score, respectively. The final assignment of BEP status for the study was given to the N=10 that consumed the highest mean palatable food kcals and that were consistently categorized as BEPs across the 4 tests. Final BER status assignment went to those N=10 that consumed the lowest mean palatable food kcals and that were consistently categorized as having BER status in all 4 tests. Their BEP/BER phenotype remained stable over time, as will be seen in Exp. 3. Past studies using the BEP/BER categorization also provided strong evidence that the phenotypes persist over time and after exposure to various experimental manipulations [17]. Intermittent chow-only tests also confirmed no difference in chow intake between groups, indicating that BEPs and BERs were not simply ‘big’ or ‘small’ eaters, respectively.
Diets
The rats had access to ad lib Purina rat chow (Harlan Teklad Global Diets, Indianapolis, IN) throughout the study, except where noted. Double Stuf Oreo cookies (original flavor; Nabisco, East Hanover, NJ) were used as the palatable food to assign BEP/BER status. This palatable food is high in fat and carbohydrate (sucrose) composition which is typical of ingredients in palatable foods that are craved and over consumed during human binges (3-7). Oreo cookies have also been used successfully in other rat models of binge-eating and were originally used to develop the BEP/BER model (8, 17, 23-27). M&M Candies (Mars, Inc., McLean, VA), also high in fat and sucrose content, served as the palatable food in the footshock maze to measure the rats' motivation for this food. Only in the maze could the rats consume M&Ms. The only exception was at the very end of the study (Exp. 4), as described below. M&Ms, and not Oreos, were used in the maze to establish a distinct association between the rewarding properties of this palatable food and the consequence of footshock. A previous study confirmed that, like Oreos, both BEPs and BERs preferred M&Ms over chow, but BEPs by definition consumed significantly more M&M kcals than did BERs under normal conditions (17). The M&Ms were also a practical choice for the maze because they were small enough to be contained in the alley feeder and could be easily retrieved, carried away, and eaten within the alleys of the maze.
Footshock Maze
The maze consisted of two alleys of a Coulbourn Instruments Habitest System (Allentown, PA). At the end of each of these 70 × 9.5 cm enclosed, transparent alleys was a food hopper, one containing premeasured chow pellets and the other premeasured M&Ms. Only the arm baited with M&Ms was rigged to deliver scrambled electric shock through metal bars on the alley floor. The on/off lever to deliver current was manually operated, but each level of shock voltage was pre-set to ensure accuracy before the rats were placed in the maze. Separating the two alleys of the maze was a covered hub that was always free of shock. The alleys were positioned so that animals were free to roam all sections of the maze throughout the duration of each test session. In this way, even the section of the maze paired with footshock was escapable at all times.
Statistical Analyses
Four experiments are described below with corresponding results after each description. In Exp. 1, separate one-way ANOVAs analyzed the effect of BEP/BER status on M&Ms kcals consumed and retrieved at each level of shock. If rats did not tolerate a certain level of shock, a value of zero was assigned. A chi-square analysis was used to determine if more rats from one of the groups tolerated each level of shock. Exp. 2 used a 2×2 factorial design (history of cyclic caloric restriction-refeeding or lack of cyclic caloric restriction-refeeding × BEP or BER). One-way ANOVAs were used to explore main effects and interactions of these factors on the same dependent variables as in Exp.1. For Exp. 3 and 4, intake of Oreo kcals (Exp. 3) and M&M kcals (Exp. 4) by BEP vs. BER rats in the home cage were analyzed using separate one-way ANOVAs. For all tests, alpha was set at p < 0.05 for significance. Results are reported as group means ± SEM. All procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Experiments & Results
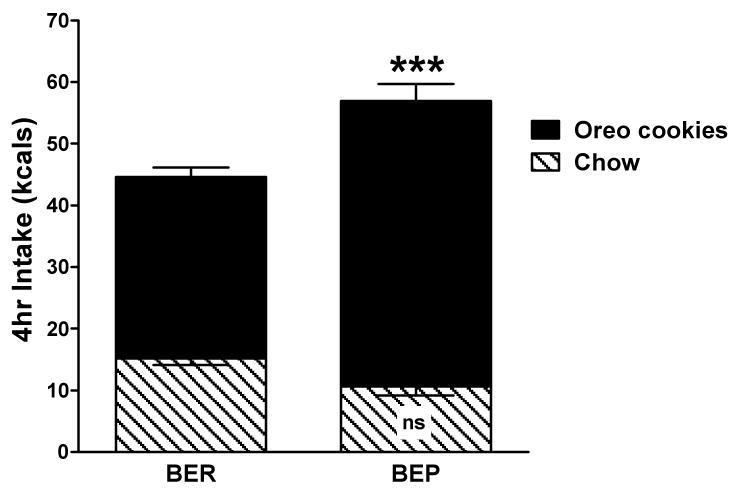
BEP/BER status was confirmed prior to all the experiments. The mean median split value of palatable food intake across 4 feeding tests using the initial 52 rats was 35 kcals/4 hrs. As expected, there were no differences in the amount of chow eaten between BEPs and BERs, regardless of whether chow was given alone or with the cookies. Averaged across the 4 feeding tests, BEP rats ate 64% more palatable food kcals than did BERs, p < 0.001 (Figure 1). Also as expected, due to the intermittent vs. constant access to palatable food, there were no differences in BEP and BER body weights at the end of the feeding tests (BEP = 171.90 ± 1.7 g vs. BER = 168.13 ± 2.0 g; not shown). Intermittent access to palatable food was designed to simulate the intermittent (vs. constant) intake of palatable foods that is typical in humans with binge-eating patterns (1).
Figure 1.
The mean consumption of chow (hatched bars) and Oreo cookies (dark bars) across four 4-hr feeding tests used to assign binge-eating prone (BEP) and binge-eating resistant (BER) rats; *** = p < 0.001 BEP vs. BER intake.
Experiment 1: Motivation for Palatable Food in BEP vs. BER Rats
Procedure: Acclimation to Food Choice Shock Maze
Rats were transported from the animal colony to the laboratory at app. 1200 hrs in their home cages with ad lib water and chow so that they were sated at the time of testing. All procedures in the maze occurred in the dark under red light. Each rat was placed into the maze in an order counterbalanced for group status (BEP/BER) for 10 min/day until acclimated. “Acclimation” was defined as the rat taking ≥ 1 bite of an M&M during the first minute in the maze. Each was allowed as many trials as needed to reach acclimation. Once acclimated, individual rats moved on to the testing phase of the experiment. Acclimation trials and testing sessions occurred 3 dys/week, with at least 1 non-testing day in between.
Results
The number of trials required to acclimate to the maze ranged from 2 to 10 trials. There were no differences between BEP and BER rats in the number of trials required to acclimate [F(2, 29) = 1.04, p = 0.37]. The mean number of trials to acclimate was 3.13 ± 0.3.
Procedure: Test of Motivation for palatable food Despite Consequences
On the first test day, which followed acclimation, no footshock was administered after M&M retrievals. This allowed a measure of baseline palatable food consumption in 10 min for each rat, as well as a way to confirm the rats' acclimation to the maze. If the rat failed to consume a bite of palatable food in < 1 minute, it was moved back to the acclimation phase until it once again passed. On the second testing day, the lowest level of shock (0.10 mA) was administered for 3 sec immediately following retrieval of an M&M. A “retrieval” was defined as the complete removal of an M&M from the food hopper by either paw or mouth. This level of shock was repeated for as many times as the rat returned and retrieved an M&M during a 10 min session. In each 10 min session thereafter, the shock level was increased by 0.05 mA increments until the rat no longer made an M&M retrieval. On the test day following this failure to retrieve, the rat received another chance to do so at the previously administered level of shock. If the rat again failed to retrieve palatable food, it was no longer placed in the maze on testing days but was instead kept in its home cage with chow for the remainder of this phase of the study. Willingness to tolerate footshock for M&Ms under sated conditions and amidst access to chow in an adjacent arm that was free of shock was our operational definition of motivation for palatable food.
When placed into the maze, the rats were always pointed toward the center hub which was not baited with food or wired to footshock. This ensured that the animals would not be biased toward either of the food choice alleys. Two research assistants were always present during the experiment. One assistant placed rats into the maze and administered footshock via a manually operated trigger; another, who was blind to group status, recorded the behaviors of the animals. Between animals, the apparatus was cleaned with chlorohexine. The number of M&M retrievals and the total amount of M&Ms consumed (in kcals) at each shock level, as well as the highest shock level tolerated (i.e., the highest level at which each was still willing to brave for more M&Ms) were recorded for each rat in each testing trial.
Results
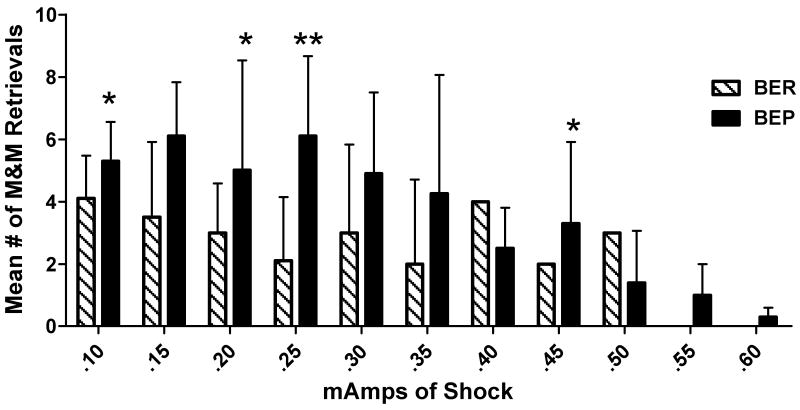
Despite considerable variance within the groups of N=10, there was a significant overall difference in the number of M&M retrievals between groups. BEPs made more retrievals (4.30 ± 0.4) than BERs (2.75 ± 0.3) with all shock levels combined, [F(2, 29) = 4.58, p < 0.02]. At each shock level (Figure 2), group performances varied. BEP retrieval numbers were not statistically different from BERs' at the lowest level (0.10 mA), at 0.15 mA level, or at 0.20 mA footshock. However, as shock intensity increased to 0.25 mA, BEPs made significantly more retrievals than BERs (6.10 ± 0.8 vs. 2.11 ± 0.7, respectively), [F(2, 29) = 6.48, p < 0.01]. At 0.45 mA, BEPs continued to make more palatable food retrievals than BER rats, [F(2,29) = 4.42, p < 0.05]. Only BEPs continued to make retrievals beyond the 0.50 mA level of footshock (Fig. 2).
Figure 2.
Mean number of M&M retrievals made by binge-eating prone (BEP) and binge-eating resistant (BER) rats during a 10 minute session in the maze at each shock level; * = p < 0.05; ** = p < 0.01.
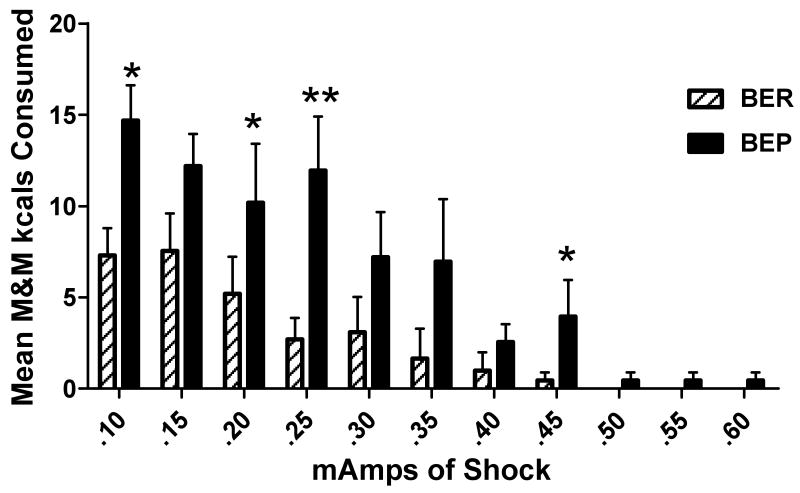
BEPs also consumed more palatable food than BERs across all shock levels combined [F(1, 19) = 6.35, p < 0.05]. As shown in Figure 3, BEPs consumed significantly more than BERs at the 0.10 mA shock level (14.7 ± 1.9 kcals vs. 7.3 ± 1.5 kcals, respectively), [F(2, 29) = 4.08, p < 0.05], the 0.20 mA level, [F(2, 29) = 3.29, p = 0.05], the 0.25 mA level (BEP = 11.95 ± 3.0 kcals vs. BER = 2.7 ± 1.2 kcals), [F(2, 29) = 6.11, p < 0.01], and the 0.45 mA level [F(2, 29) = 3.34, p = 0.05].
Figure 3.
Mean amount of M&M kcals consumed by binge-eating prone (BEP) and binge-eating resistant (BER) rats during a 10 minute session in the maze at each shock level; * = p < 0.05; ** = p < 0.01.
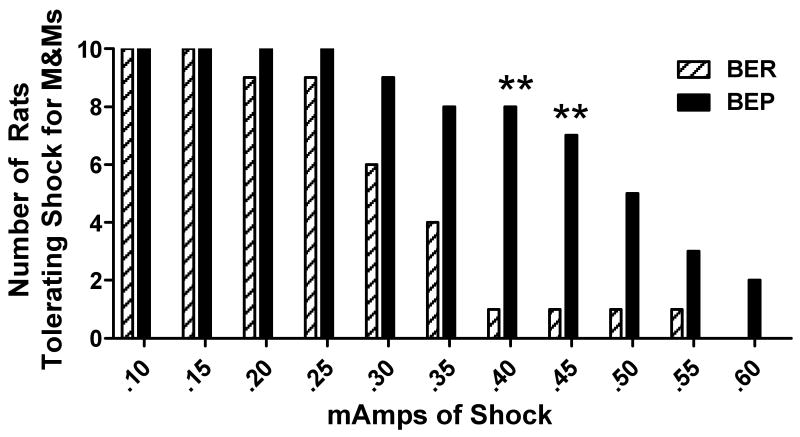
Finally, as shock levels increased, the number of BER rats that tolerated shock for palatable food decreased, while the number of BEPs tolerating incrementing levels remained virtually the same (Figure 4). At the 0.40 mA level, more BEP vs. BER rats tolerated shock for M&Ms (N = 8, or 80%, of BEPs vs. only 1, or 10%, of BERs), [X2 (2, N = 30) = 10.05, p < 0.01]. At the 0.45 mA level, the results were similar with N = 7 (70%) of BEP rats and N = 1 (10%) of BER rats tolerating shock [X2 (2, N = 30) = 9.30, p < 0.01]. Beyond the 0.45 mA level, the N per group that remained was too low to detect significance; however, the trend for a greater number of BEPs vs. BERs tolerating shock for M&Ms continued. As to the highest shock level tolerated by each group, there was a clear difference between the groups [F(2, 29) = 6.02, p < 0.01]. BEPs were willing to tolerate a much higher, and statistically significant, level of footshock as a negative consequence for making palatable food retrievals compared to BERs (0.42 ± 0.04 mA vs. 0.26 ± 0.03 mA, respectively).
Figure 4.
Absolute number of binge-eating prone (BEP) and binge-eating resistant (BER) rats that were willing to tolerate footshock for M&Ms at each shock level; ** = p < 0.01.
Experiment 2: Effect of a History of Cyclic Caloric Restriction-Refeeding on Motivation for Palatable Food in BEP vs. BER Rats
Procedure: Cyclic Caloric Restriction-Refeeding Protocol
After Exp. 1, half of the rats within each BEP and BER group were assigned to experience either a history with or a history without cyclic caloric restriction-refeeding. These subgroups were matched for levels of shock tolerated. The cyclic caloric restriction-refeeding protocol was the same used in previous experiments with other rat models of binge-eating (8, 23-28) and is outlined in Table 1. A total of five 11-day restriction-refeeding “cycles” were imposed on the groups receiving a history of cyclic caloric restriction-refeeding. Five days on ad lib chow followed the last refeeding day of the 5th cycle. Body weights were monitored throughout. After the last restriction cycle, the mean body weight of those with a history of restriction-refeeding was 334.43 ± 8.9 g, vs. 348.32 ± 11.8 g. of those without such a history. This difference was not significant [F(1, 29) = 0.885, p = 0.36], and any trends to weigh less were evenly distributed among BEP and BER rats. At that time, testing in the shock maze proceeded as in Exp. 1. sans the acclimation period. As in Exp. 1, the first day of testing in the maze did not include footshock.
TABLE 1. A typical 11-day restriction-refeeding “cycle” of the caloric restriction-refeeding protocol.
| Groups | Restriction (Days 1-5) |
Refeeding with palatable food (Days 6-7) |
Refeeding without palatable food (Days 8-11) |
|---|---|---|---|
| BEP w/ Restriction-Refeeding |
66% of chow* | Ad lib chow + Palatable Food |
Ad lib chow |
| BEP w/out Restriction-Refeeding |
Ad lib chow | Ad lib chow + Palatable Food |
Ad lib chow |
| BER w/ Restriction-Refeeding |
66% of chow* | Ad lib chow + Palatable Food |
Ad lib chow |
| BER w/out Restriction-Refeeding |
Ad lib chow | Ad lib chow + Palatable Food |
Ad lib chow |
Palatable Food = Oreo Double Stuf Cookies;
% of chow = determined from the mean 24-hr chow intake of all rats prior to beginning the first cycle. For the last (fifth) cycle, the % of chow given was limited to 50%, not 66%, of the mean.
Results
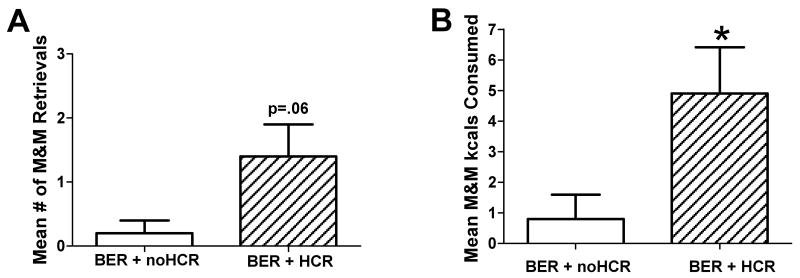
There were no significant main effects due to group (BEP/BER) or experience with restriction-refeeding, nor interaction effects on the number of M&Ms retrieved, consumed, or level of shock tolerated when the rats were placed back into the maze. A confound with this design and explanation for this lack of difference is that all of the animals' last experience in the maze was with a level of shock aversive enough to preclude an M&M retrieval. Hence, all rats were highly hesitant to enter the alley despite the fact that no shock was being delivered on this first trial back in the maze. There was, however, a trend for BERs with a history of caloric restriction-refeeding to make more retrievals than BERs without this experience (Figure 5A; 1.40 ± 0.5 vs. 0.20 ± 0.2, respectively), [F(1, 9) = 4.80, p = 0.06]. They also consumed significantly more palatable food than their non-restricted counterparts (Figure 5B; 4.9 ± 1.5 kcals vs. 0.8 ± 0.8 kcals, respectively), [F(1, 9) = 5.70, p < 0.05]. No significant difference was revealed between BEPs with and without history of cyclic caloric restriction-refeeding, both of which retrieved a mean of only 0.8 ± 0.4 M&Ms and consumed a mean 2.6 ± 1.8 palatable food kcals (not shown).
Figure 5.
Behavior of binge-eating resistant (BER) rats with and without a history of cyclic caloric restriction-refeeding-refeeding (HCR and no-HCR) when placed back into the shock maze, but without shock. This followed Exp. 1 where the same rats experienced levels of footshock that were too aversive to tolerate for M&Ms. (A) Trend for BERs with a HCR to retrieve more M&Ms than BERs with no-HCR. (B) BERs with a HCR consumed more M&Ms than BERs with no-HCR; *p<0.05.
Experiment 3: Stability of BEP and BER Status
Procedure
To determine if exposure to footshock in the alley with a different palatable food (M&Ms vs. Oreos), history of cyclic caloric restriction-refeeding, or mere passage of time altered the rats BEP/BER status, following Exp. 2 the rats were given the same Oreo cookie + chow feeding test in their home cages that was used to classify them as BEP or BER prior to Exp. 1.
Results
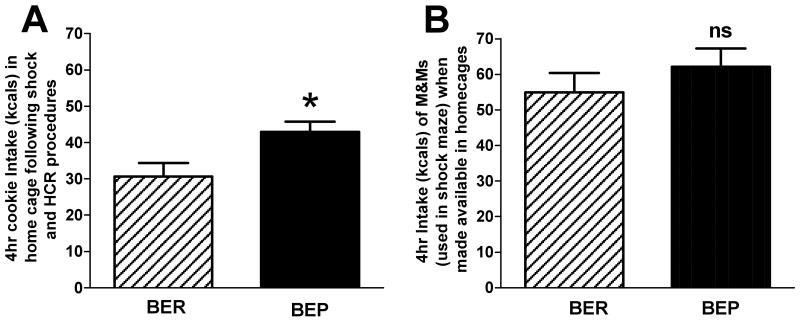
Despite exposure to the aforementioned manipulations, the home cage feeding test using Oreo cookies revealed that the BEP and BER assignments remained stable. BEPs consumed significantly more palatable food kcals than the BER group (42. 99 ± 2.7 kcals vs. 30.67 ± 3.7 kcals, respectively), [F(1, 19) = 7.24, p < 0.05]; Figure 6A.
Figure 6.
(A) The mean consumption of Oreo kcals and chow in the home cage by BEP and BER groups following prior experience with a different palatable food (M&Ms), with footshock, and with the history of cyclic caloric restriction-refeeding (HCR) protocol; * = p < 0.05. (B) The mean consumption of M&M kcals, once only available in the shock maze, now given for the first time in home cages.
Experiment 4: Response of BEP vs. BER Rats to Free Access of a Palatable Food Previously Associated with Choice-Conflict Stress
Procedure
Following Exp. 3, the rats were maintained only on chow and water for three days in their home cages. They were then given pre-measured amounts of chow and M&Ms at dark onset, and intakes were measured after 4 hrs. This was the first and only time they received M&Ms in the safe (i.e., shock-free) environment of their home cages.
Results
As shown in Figure 6B, and in contrast to the Oreo feeding tests, BER rats ate as many M&M kcals as did BEP rats (BEP = 62.10 ± 5.2 kcals vs. BER = 54.90 ± 5.5 kcals), [F(1, 19) = 0.90, p = 0.36, ns].
Discussion
The main finding of the study was that rats classified as binge-eating prone (BEP), for their increased food intake in the presence of palatable food, not only consumed more of this food but were also willing to tolerate higher levels of footshock to retrieve and consume it compared to binge-eating resistant rats (BERs). This was observed in the rats despite their sated condition and despite the presence of chow, free of shock, in an adjacent arm of the maze. The data collected during their acclimation to the maze suggests that this difference in motivation between the groups was not caused by differences in anxiety, motor ability, or learning capacity, since BEPs did not differ from BERs in the required number of exposures to the maze or time in the maze to retrieve the palatable food for the first time.
A subsequent experiment imposed the rats with a brief cyclic history of food restriction and refeeding meant to simulate human-like dieting. Given that this study was confounded by the rats' last experience in the maze, which was with a highly aversive level of footshock (one too aversive to tolerate for palatable food), and given the low Ns from sub-diving BEP/BER groups so that half of each would experience cyclic caloric restriction-refeeding (N=5/group), we must regard the results as pilot data for further exploration. Nonetheless, despite these shortcomings, the results suggest that in otherwise non-binge-eating prone rats (BERs), a history of human-like dieting may alter their motivation for palatable food. We previously reported that a history of cyclic caloric restriction-refeeding is a necessary trigger of binge-eating in stressed rats (8, 23-25, 27), a phenomenon now observed in other laboratories using rats and mice (33-34). In the present study, prior experience in the shock maze could be regarded as stressful. Among the BER group, only those with a history of cyclic caloric restriction-refeeding showed a trend to retrieve and consume significantly more palatable food than BERs without this history. Therefore, despite normal body weights and satiety following restriction-refeeding protocol, stress combined with this history may have once again served to augment food intake, even in rats not disposed to binge-eat (in BERs).
BEPs did not respond in kind to the experience of cyclic caloric restriction-refeeding. One reason for this may be that, compared to BERs, their last experience in the maze was with a much higher level of shock than that experienced by BERs. However, another explanation is that BEPs are dispositionally unaffected by periods of caloric restriction. We previously reported that under a state of acute food deprivation, one producing hunger (as evidenced by overeating of BERs after the same acute food deprivation), BEPs did not consume any more palatable food than they did when not deprived of food (17). That is, BEPs consume as much palatable food when sated as when hungry. This suggests that, when confronted with palatable food, BEPs are eating for reasons outside of metabolic need. Hence, their motivation for palatable food as tested here may be unaffected by a prior history of cyclic caloric restriction-refeeding. In this way, BEPs may be more representative of individuals with binge eating disorder (BED) and obesity resulting from compulsive overeating, since in these conditions, a history of dieting is not always present (1). Bulimia nervosa may be more accurately modeled by BERs with a history of cyclic caloric restriction-refeeding. These are rats that typically eat less palatable food than BEPs (a mode of self-restriction) but that can be changed to consume more after a bout of caloric restriction or ‘dieting’ (1). To optimally use this model to study these clinical subgroups, it will first be necessary to repeat this experiment with a larger number of animals that are subjected to a history of cyclic caloric restriction-refeeding protocol prior to being tested for palatable food motivation in the shock maze.
In the final test, when all rats had access to the consequence-associated M&Ms in the safety of their home cages for the first time, BERs ate as much of this palatable food as BEPs. This could not be due to a change in their BEP/BER status, since the Oreo test confirmed that BERs still ate less than BEPs. One could argue that BERs simply preferred or liked M&Ms more than BEPs did and, therefore, ate more of them when shock was no longer a threat. We cannot definitively rule out this possibility, but it is not a likely explanation given that BEPs were willing to tolerate higher shock intensities for this type of palatable food. An alternate possibility for increased consumption of M&Ms by BERs to match that of BEPs is that the M&Ms were now accessible free of shock and previous association between this food and aversive footshock could have increased the salience and appetitive quality of this food when freely accessible. Fig. 6 shows that even BEPs ate more palatable food kcals than usual when they ate M&Ms vs. Oreos. This increased intake likely reached a ceiling effect in BEPs. As for BERs, increased kcal intake in the presence of shock-free M&Ms (vs. Oreos) may have also been due to an increased salience and appetitive nature of this food from its previous forbidden-like quality. Dieters voluntarily restrain from highly palatable foods, and it is well-established that restraint from these foods increases their saliency and appetitive nature (35). Recently our lab reported that even non-food cues associated with palatable food are enough to elicit overeating in rats (36). A caveat to these conclusions, however, is that a test of M&M intake in the home cage was only performed once. We do not know if, with repetition, BERs would revert back to their typical BER status, consuming less M&M kcals than BEPs. It is possible that a subset of the BERs might have continued this BEP-like pattern with previously forbidden palatable food. This would suggest interesting genetic diversity within the BER phenotype. There is, however, evidence to suspect that BERs would have eventually returned to eating less M&Ms than BEPs, despite their now free access. This is based on the stubborn nature of the BEP/BER phenotype that we have observed in past studies. The BEP/BER phenotypes persist over time, across various experiences with hunger, with footshock, and with exposures to different kinds of palatable foods (see ref.17 for these tests). Furthermore, K. Klump and colleagues recently found that even ovariectomy did not abolish BEP/BER status in post-pubertal rats. Interestingly, female rats do not significantly converge into BEP/BER groups until puberty, raising a potentially critical role of reproductive hormones on the onset of binge-eating (personal communication, Oct. 2009).
Despite the limitations in the secondary tests conducted in this study, the main finding of increased motivation for palatable food in BEP compared to BER rats is important for several reasons. First, it extends the BEP phenotype to include an aspect that is understudied in binge-eating animal models yet is very salient in clinical binge-eating. Secondly, the action of non-food deprived, normal-weight rats to tolerate high levels of electric shock for a favored food should not be regarded as anything but strikingly abnormal and a powerful testament of motivation. This is emphasized by the fact that, in the past, we obtained similar behavior in rats but only when injected centrally with peptide YY, a powerful orexigenic (37). Other studies using footshock to test for motivation in rats involved, not food, but drugs of abuse (38, 39). Hence, this study is unique in that untreated (drug-naïve) rats were found to willingly tolerate aversive levels of footshock, not for rewarding drugs but rewarding food. Thirdly, the results highlight the powerful role of palatable foods to trigger binge-eating. Given our observations in rodents, the power of palatable foods to motivate feeding even in the face of punishment may be more biologically rooted than contingent on complex cognitive processes (e.g., calorie-counting or cognitive disinihibition). This has important implications for treatment strategies and the prevention of relapse in humans with binge-eating disorders who must repeatedly encounter these substances in today's hedonic food environment.
Gene x environment interaction studies of human eating disorders are rare and very much needed (40). The BEP/BER animal model of binge-eating represents a gene x environment interaction, with the environmental factor being the presence of palatable food. Palatable food has an effect on BEP rats that it does not have in BER rats. Our palatable food-centric environment is not likely to change. By identifying the genes that predispose some humans to react differently to palatable foods should guide novel treatments for bulimia, BED, and obesity caused by eating in the absence of hunger. These are treatments aimed at curbing an abnormal motivation for palatable foods and may also be effective on abnormal motivation for other appetitive stimuli (e.g., sex, alcohol, illicit substances, gambling). The BEP/BER model can be used as a vehicle to these gene discoveries.
Acknowledgments
We are grateful to Drs. Paul Blanton and Kristine Lokken for their guidance and advice on translational aspects of this study. We also thank the following students for assisting with laboratory maintenance and data collection: Michel Thomas, Jennie Yang, Mary Holsten, Taylor Johnson, Adrianne McCullars, and Jillian Woodruff. This research was supported by NIH grant DK066007 (MMB).
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Kissileff HR, Walsh BT, Kral JG, Cassidy SM. Laboratory studies of eating behavior in women with bulimia. Physiol Behav. 1986;38:563–570. doi: 10.1016/0031-9384(86)90426-9. [DOI] [PubMed] [Google Scholar]
- 3.Gendall KA, Sullivan PE, Joyce PR, Carter FA, Bulik CM. The nutrient intake of women with bulimia nervosa. Int J Eat Disord. 1997;21:115–127. doi: 10.1002/(sici)1098-108x(199703)21:2<115::aid-eat2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.de Castro JM, Bellisle F, Dalix AM. Palatability and intake relationships in free-living humans: measurement and characterization in the French. Physiol Behav. 2000;68:271–277. doi: 10.1016/s0031-9384(99)00166-3. [DOI] [PubMed] [Google Scholar]
- 5.Yeomans MR, Blundell JE, Leshem M. Palatability: response to nutritional need or need-free stimulation of appetite? Br J Nutr. 2004;92:3–14. doi: 10.1079/bjn20041134. [DOI] [PubMed] [Google Scholar]
- 6.Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol. 2008;295:R1066–1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kales EF. Macronutrient analysis of binge eating in bulimia. Physiol Behav. 1990;48:837–840. doi: 10.1016/0031-9384(90)90236-w. [DOI] [PubMed] [Google Scholar]
- 8.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 9.Hagan MM, Shuman ES, Oswald KD, Corcoran KJ, Profitt JH, Blackburn K, et al. Incidence of chaotic eating behaviors in binge-eating disorder: contributing factors. Behav Med. 2002;28:99–105. doi: 10.1080/08964280209596048. [DOI] [PubMed] [Google Scholar]
- 10.Polivy J, H CP. Etiology of binge eating: Psychological mechanisms. In: Fairburn CGW, Terence G, editors. Binge eating: Nature, assessment, and treatment. New York: The Guilford Press; 1996. pp. 173–205. [Google Scholar]
- 11.Wilfley DE, Wilson GT, Agras WS. The clinical significance of binge eating disorder. Int J Eat Disord. 2003;34:96–106. doi: 10.1002/eat.10209. [DOI] [PubMed] [Google Scholar]
- 12.Bulik CM, Reichborn-Kjennerud T. Medical morbidity in binge eating disorder. Int J Eat Disord. 2003;34:39–46. doi: 10.1002/eat.10204. [DOI] [PubMed] [Google Scholar]
- 13.Williams PM, Goodie J, Motsinger CD. Treating eating disorders in primary care. Am Fam Physician. 2008;77:187–95. [PubMed] [Google Scholar]
- 14.Abbott DW, de Zwaan M, Mussell MP, Raymond NC, Seim HC, Crow SJ, et al. Onset of binge eating and dieting in overweight women: implications for etiology, associated features and treatment. J Psychosom Res. 1998;44:367–374. doi: 10.1016/s0022-3999(97)00261-4. [DOI] [PubMed] [Google Scholar]
- 15.Stice E, Agras WS. Subtyping bulimic women along dietary restraint and negative affect dimensions. J Consult Clin Psychol. 1999;67:460–469. doi: 10.1037//0022-006x.67.4.460. [DOI] [PubMed] [Google Scholar]
- 16.Stice E, Agras WS, Telch CF, Halmi KA, Mitchell JE, Wilson T. Subtyping binge eating-disordered women along dieting and negative affect dimensions. Int J Eat Disord. 2001;30:11–27. doi: 10.1002/eat.1050. [DOI] [PubMed] [Google Scholar]
- 17.Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without binge-eating. Int J Obesity. 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 18.Gluck ME. Stress response and binge eating disorder. Appetite. 2006;46:26–30. doi: 10.1016/j.appet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Goldfield GS, Adamo KB, Rutherford J, Legg C. Stress and the relative reinforcing value of food in female binge eaters. Physiol Behav. 2008;93:579–587. doi: 10.1016/j.physbeh.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Pike KM, Wilfley D, Hilbert A, Fairburn CG, Dohm FA, Striegel-Moore RH. Antecedent life events of binge-eating disorder. Psychiatry Res. 2006;142:19–29. doi: 10.1016/j.psychres.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Striegel-Moore RH, Dohm FA, Kraemer HC, Schreiber GB, Taylor CB, Daniels SR. Risk factors for binge-eating disorders: an exploratory study. Int J Eat Disord. 2007;40:481–487. doi: 10.1002/eat.20400. [DOI] [PubMed] [Google Scholar]
- 22.Wolff GE, Crosby RD, Roberts JA, Wittrock DA. Differences in daily stress, mood, coping, and eating behavior in binge eating and nonbinge eating college women. Addict Behav. 2000;25:205–216. doi: 10.1016/s0306-4603(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 23.Artiga AI, Viana JB, Maldonado CR, Chandler-Laney PC, Oswald KD, Boggiano MM. Body composition and endocrine status of long-term stress-induced binge-eating rats. Physiol Behav. 2007;91:424–431. doi: 10.1016/j.physbeh.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 25.Placidi RJ, Chandler PC, Oswald KD, Maldonado C, Wauford PK, Boggiano MM. Stress and hunger alter the anorectic efficacy of fluoxetine in binge-eating rats with a history of caloric restriction. Int J Eat Disord. 2004;36:328–341. doi: 10.1002/eat.20044. [DOI] [PubMed] [Google Scholar]
- 26.Chandler-Laney PC, Castaneda E, Viana JB, Oswald KD, Maldonado CR, Boggiano MM. A history of human-like dieting alters serotonergic control of feeding and neurochemical balance in a rat model of binge-eating. Int J Eat Disord. 2007;40:136–142. doi: 10.1002/eat.20349. [DOI] [PubMed] [Google Scholar]
- 27.Boggiano MM, Chandler PC, Viana JB, Oswald KD, Maldonado CR, Wauford PK. Combined dieting and stress evoke exaggerated responses to opioids in binge-eating rats. Behav Neurosci. 2005;119:1207–1214. doi: 10.1037/0735-7044.119.5.1207. [DOI] [PubMed] [Google Scholar]
- 28.Chandler-Laney PC, Castaneda E, Pritchett CE, Smith ML, Giddings M, Artiga AI, et al. A history of caloric restriction induces neurochemical and behavioral changes in rats consistent with models of depression. Pharmacol Biochem Behav. 2007;87:104–114. doi: 10.1016/j.pbb.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boeka AG, Lokken KL. The Iowa gambling task as a measure of decision making in women with bulimia nervosa. J Int Neuropsychol Soc. 2006;12:741–745. doi: 10.1017/S1355617706060887. [DOI] [PubMed] [Google Scholar]
- 30.Cassin SE, von Ranson KM. Is binge eating experienced as an addiction? Appetite. 2007;49:687–690. doi: 10.1016/j.appet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Rieger E, Wilfley DE, Stein RI, Marino V, Crow SJ. A comparison of quality of life in obese individuals with and without binge eating disorder. Int J Eat Disord. 2005;37:234–240. doi: 10.1002/eat.20101. [DOI] [PubMed] [Google Scholar]
- 32.Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence Appetite. 2009;53:1–8. doi: 10.1016/j.appet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Hancock SD, Menard JL, Olmstead MC. Variations in maternal care influence vulnerability to stress-induced binge eating in female rats. Physio Behav. 2005;85:430–439. doi: 10.1016/j.physbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Consoli D, Contarino A, Tabarin A, Drago F. Binge-like eating in mice. Int J Eat Disord. 2009;42:402–408. doi: 10.1002/eat.20637. [DOI] [PubMed] [Google Scholar]
- 35.Papies EK, Stroebe W, Aarts H. The allure of forbidden food: On the role of attention in self-regulation. J Exp Social Psych. 2008;44:1283–1292. [Google Scholar]
- 36.Boggiano MM, Dorsey J, Thomas JM, Murdaugh D. The Pavlovian power of palatable food: lessons for weight-loss adherence from a new rodent model of cue-induced overeating. Int J Obesity. 2009;33:693–701. doi: 10.1038/ijo.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagan MM, Moss DE. Effect of peptide YY (PYY) on food-associated conflict. Physiol Behav. 1995;58:731–735. doi: 10.1016/0031-9384(95)00100-w. [DOI] [PubMed] [Google Scholar]
- 38.Robinson TE. Neuroscience. Addicted rats. Science. 2004;305:951–953. doi: 10.1126/science.1102496. [DOI] [PubMed] [Google Scholar]
- 39.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 40.Bulik CM. Exploring the gene-environment nexus in eating disorders. J Psychiatry Neurosci. 2005;30:335–339. [PMC free article] [PubMed] [Google Scholar]