Abstract
Toll-like receptors (TLRs) are essential components of the innate immune system, and their ligands are important activators of neutrophils. Bruton’s tyrosine kinase (Btk) has been reported to mediate signaling through toll-like receptors (TLRs) in many cell types, however, the role of Btk in TLR activation of neutrophils remains unclear. Impaired TLR-induced neutrophil function was found in mice with loss of Btk and in humans with TLR-signaling defects, but the integrity of TLR pathways in X-linked agammaglobulinemia (XLA) neutrophils has not been assessed. In this study LPS (TLR4) or an imidazoquinoline compound (TLR7/8) activated XLA neutrophil shedding of surface CD62L, and phosphorylated MAP kinases p38, JNK and ERK. TLR activation also induced normal respiratory burst and retarded apoptosis for XLA neutrophils, comparable to normal controls. These data demonstrate that the loss of Btk in XLA neutrophils does not impair functional responses to TLR signals.
Keywords: Bruton’s tyrosine kinase, BTK, X-Linked Agammaglobulinemia, XLA, Toll-like receptors, TLR, neutrophils, respiratory burst
Introduction
X-linked agammaglobulinemia (XLA) is a primary immunodeficiency disease resulting from mutations in a cytoplasmic tyrosine kinase, Bruton’s Tyrosine Kinase (Btk). The defect is characterized by a block in B cell maturation leading to a loss of peripheral blood B cells and severely reduced serum immunoglobulins[1, 2]. Prior to receiving replacement immune globulin (Ig), patients with XLA experience invasive pyogenic infections, resulting in significant morbidity and mortality[3–5]. Interestingly, although Btk expression is abundant in neutrophils as well as many other cells of hematopoietic lineage, patients maintained on sufficient Ig therapy are generally healthy[6, 7], suggesting that Btk is either dispensable outside the B-cell compartment, or that compensatory kinases maintain normal functions in other cells [8]. However, a number of recent studies have demonstrated that Btk plays an important, or possibly essential, role in signaling through several Toll-like receptors (TLRs) which recognize conserved pathogen-associated molecular patterns such as lipopolysaccharides (LPS), bacterial DNA, or single-stranded viral RNA (ssRNA)[9–19]. These observations have suggested that in addition to the loss of humoral immunity, subjects with XLA might have additional innate immune defects.
The first suggestion that Btk might be important in TLR signaling came from work in Xid mice in which a point mutation in the pleckstrin homology domain of the Btk gene results in loss of Btk function. LPS induced production of TNF-α and IL-1β was found impaired in these mice and Xid macrophages and neutrophils had reduced generation of reactive oxygen intermediates and defective LPS clearance[16, 20, 21]. Further work in Btk deficient mice and the human monocyte line THP1 showed that TLR2, 4, 7, 8 and 9 ligands phosphorylated Btk, and that Btk could be co-immunoprecipitated with myeloid differentiation primary response gene (88) (MyD88), toll-interleukin 1 receptor domain containing adaptor protein (TIRAP, also known as the MyD88 adaptor-like protein, or Mal), and Interleukin-1 receptor-associated kinase 1 (IRAK1), key components of the TLR signaling complex[9, 12, 22, 23].
TLRs have selected patterns of distribution on immune cells, with the final effector functions differing depending on the cell type. Neutrophils, the most abundant immune cell and first responders to infection, express most of the TLRs[24]. When neutrophil TLRs bind their ligands, signaling pathways are activated which triggers the shedding of surface L-selectin, upregulation of surface integrins, priming of respiratory burst, increased cytokine production and phagocytosis, and slowed progression to apoptosis [24].
Taking advantage of TLR induced changes in adhesion molecules, von Bernuth et al. showed the impaired shedding of L-selectin was characteristic of subjects with mutations in NF-κB essential modulator (NEMO) or IL-1R associated kinase (IRAK-4); this test was suggested for clinical screening for these defects. Neutrophils of these subjects had previously been shown to have decreased LPS-induced NADPH oxidase activation, impaired superoxide production [25] and defective neutrophil migration and phagocytosis [26]. However, if Btk is integral to TLR signaling in neutrophils, subjects with XLA should show similar neutrophil defects. XLA patients occasionally are found to be neutropenic, typically coinciding with the severe infection that suggests the presence of an immunodeficiency and potentially, neutrophil dysfunction [4, 27], however, neutropenia is not a characteristic of XLA patients on sufficient Ig replacement. To date no study has directly assessed TLR induction of pro-inflammatory signaling pathways and effector functions in primary XLA neutrophils. The present study assesses the activation and downstream activity of TLR4 and TLR7/8 effector functions, to examine whether the distinct signaling pathways activated by extracellular and endosomal TLRs are intact.
Methods and Materials
Patients and controls
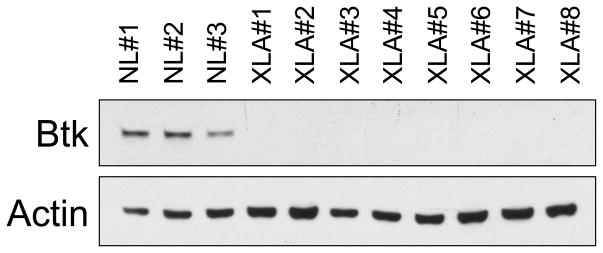
This study included 8 XLA patients of age 12 to 43 years. All patients were profoundly hypogammaglobulinemic, had fewer than 0.1% B cells in peripheral blood. Mutational analyses to confirm the diagnosis of XLA were kindly performed by Dr M. E. Conley. Lack of Btk expression by these patients was confirmed by western blot as described below (Figure 1). All subjects were well at the time of the study and on replacement Ig in standard doses; blood samples were collected before monthly infusions. Control subjects were healthy adult volunteers. These studies were done using an Institutional Review Board approved protocol and informed consent.
Figure 1. Btk expression in XLA cells.
Leukocytes of 8 XLA patients examined by western blot, demonstrated no Btk expression in contrast to cells of 3 normal controls.
Neutrophil isolation
Whole blood was mixed 1:1 with 3% dextran (MP Biomedicals, Solon, OH) in PBS and erythrocytes allowed to sediment for 30 minutes. The leukocyte rich supernatant was then applied to a Ficoll-Hypaque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation. PBMCs were removed, and remaining erythrocytes in the pelleted mixture were lysed by hypotonic NaCl treatment. Purified neutrophils were resuspended in Hanks’ Balanced Salt Solution (HBSS, Gibco, Carlsbad, CA) supplemented with 10% Fetal Calf Serum, 1% penicillin/streptomycin.
TLR induced CD62L Shedding
The assay for neutrophil CD62L shedding was performed as described by von Bernuth et al. Briefly, 100 L of heparinized whole blood was incubated for 1 hour at 37°C alone or in the presence of the of a water-soluble R848, imidazoquinoline compound TLR7/8 ligand CL097 (0.1 μg/mL–5.0μg/mL) (Invivogen, San Diego, CA), LPS (1ng/mL-1μg/mL) (Invivogen) or as a control, phorbol 12-myristate 13-acetate (PMA) (1ng/mL-1μg/mL) (Sigma, St. Louis, MO). In the control, CL097, and PMA treated cells 10μg/mL polymixin B (Sigma) was added before treatment with TLR ligand. Erythrocytes were then lysed in (1.3M NH4Cl, 100mM KHCO3, 1mM EDTA). Remaining leukocytes were incubated for 15 minutes on ice in PBS+2% fetal calf serum with FITC-conjugated monoclonal anti-CD62L BD Biosciences, San Diego, CA) or isotype control, washed three times in PBS/FCS; CD62L surface expression was analyzed via flow cytometry on a FACSCalibur (BD Biosciences). Granulocytes were gated according to forward-scatter/sideways-scatter and the mean fluorescence intensity (MFI) of CD62L surface expression determined.
Western blot
Neutrophils in complete HBSS media were plated at 5×106 cells/mL and allowed to rest for 6 hours at 37°C. After 1 hour, neutrophils were incubated for an optimized time of 5 minutes with the optimized concentrations of 2.5μg/mL CL097 or 100ng/mL LPS, and subsequently centrifuged at 4°C and lysed with lysis buffer (1% Triton X-100, 20mM Tris [pH 7.4], 40mM NaCl, 5mM EDTA, 50mM NaF, 30mM Na4P2O7) supplemented with protease and phosphatase inhibitors (Thermo Scientific, Rockford, IL, USA). Protein was quantified with the Micro BCA Protein Assay Kit (Thermo Scientific), and subsequently fractionated on 12% SDS-PAGE gels (Thermo Scientific), transferred to nitrocellulose membrane (Whatman, Dassel, Germany) and developed with rabbit-anti-phospho-p38 MAPK R&D Systems, Minneapolis, MN), rabbit-anti-phospho-p44-42MAPK (ERK1/2) (Cell Signaling), rabbit-anti-phospho SAPK/JNK (Cell Signaling), goat-anti-Btk (Santa Cruz Biotechnology, Santa Cruz, CA), and mouse-anti-β-actin (Cell Signaling), followed by incubation in the appropriate horseradish peroxidase-conjugated secondary antibody (SantaCruz Biotechnology) and protein visualized using Immobilon Western HRP Substrate (Millipore, Billerica, MA).
Respiratory burst
To examine respiratory burst by a cytochrome C reduction assay, XLA or control neutrophils were washed and resuspended in 10mM glucose in PBS at a concentration of 2×107 neutrophils/mL. Neutrophils were combined 1:9 with stimulation buffer (138mM NaCl, 2.7mM KCl, 0.6mM CaCl2, 1.0mM MgCl2, 5mM glucose, 10mM NaH2PO4/Na2HPO4, pH 7.4), and cytochrome C from equine heart (Sigma) was added to a concentration of 0.1mM. Cells were plated in a 96 well plate in the presence or absence of 50μg/mL superoxide dismutase (SOD, MP Biomedicals). Neutrophils were stimulated with 2.5μg/mL CL097, 100ng/mL LPS or 10ng/mL PMA for 20 minutes and absorbance of reduced cytochrome C read at 550nM absorbance. SOD-inhibitable cytochrome C reduction was assessed by subtracting absorbance in samples pretreated with SOD, which corrects for non-superoxide mediated reduction. Results are given relative to the baseline respiratory activity.
Production of superoxide radicals was also quantified using the dihydrorhodamine (DHR) assay. For this, neutrophils in suspension in HBSS complete media were incubated with 5μg/mL DHR (EMD Darmstadt, Germany) and allowed to rest at 37°C for 30 min. Samples were treated with TLR ligands as above for 90 minutes. Neutrophils were subsequently washed in PBS and intracellular rhodamine visualized on FACScalibur. Data is presented as the mean fluorescence of rhodamine in activated samples, divided by the mean fluorescence of untreated cells.
TLR induced Cell survival
Effective TLR activation prolongs the life span of neutrophils, delaying apoptosis [28]. To examine this effect in Btk deficient cells, neutrophils were plated in HBSS complete media at 2×106 cells/mL and treated with 2.5μg/mL CL097, 100ng/mL LPS or 20ng/mL GM-CSF as a positive control, (eBioscience, San Diego, CA). After 48 hours cells, were washed with PBS, stained for Annexin 5 and propidium iodide (PI) (BD Pharmingen, San Diego, CA) and assessed by flow cytometry.
Statistical Analysis
Statistical analysis was performed using PRISM 4.0 (GraphPad). An unpaired two-tailed t-test was used for one-to-one comparisons of individual treatment groups to the correlating no-treatment controls, or comparing within a treatment group for differences between control and XLA patient cellular responses.
Results
Shedding of CD62
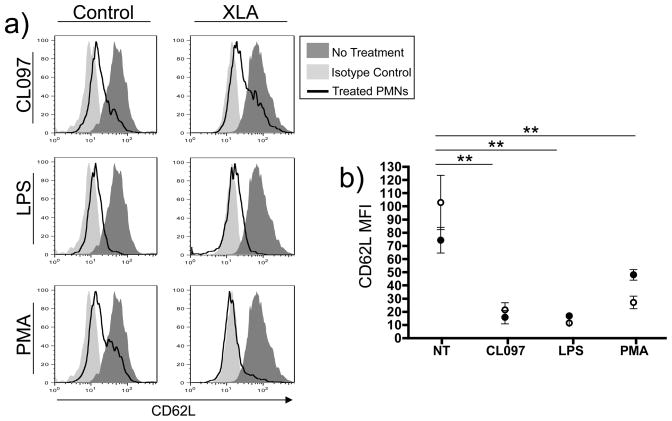
As shedding of leukocyte surface CD62L occurs upon successful TLR activation, we treated blood from XLA patients and normal controls, with optimized concentrations of LPS, CL097 or as a control, PMA to assess whether this early marker of TLR signaling was intact. Neutrophils of XLA subjects exhibited the same loss of neutrophil surface CD62L as control neutrophils when exposed to these ligands, suggesting that TLR4 and TLR7/8 signaling activation is retained (Figure 2a, b).
Figure 2. Neutrophil CD62L shedding.
Whole blood was treated with TLR ligands CL097 (2.5μg/mL) or LPS 100ng/mL or PMA 10ng/mL for 1 hour, followed by RBC lysis and analysis of CD62L shedding from the surface of neutrophils by FACS. Representative histograms of gated granulocytes from a control and one XLA subject, demonstrated loss of CD62L with TLR activation (a). The MFI of FACS analyses from XLA subjects (open circles, n=6) and controls (solid circles, n=6) is represented graphically (b). An unpaired two-tailed t-test used to individually compare CD62L expression on untreated XLA cells with treatment groups. ** denotes a p value of <0.005.
MAP kinase activation
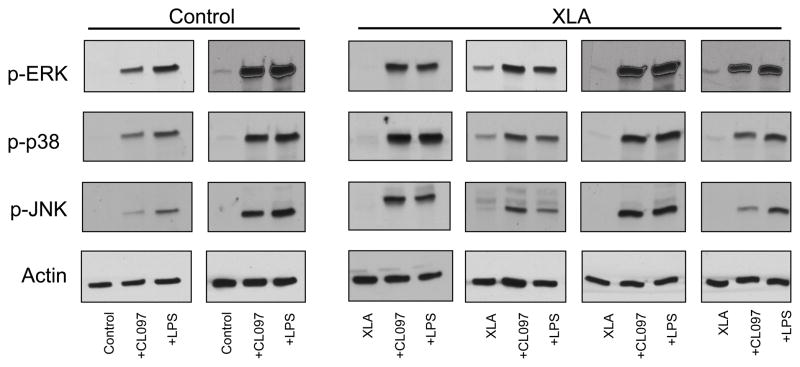
TLRs activation also leads to phosphorylation of the MAP kinase families p38, ERK, and JNK in neutrophils, all of which are involved in mediating effector functions; thus we assessed whether these MAP kinases required Btk to attain an activated phosphorylated state. LPS and CL097 treatment stimulated an increase in phosphorylation of p38, ERK, and JNK in both control (n=6) and XLA neutrophils (n=6) Figure 3 shows representative data for one XLA subject and a control.
Figure 3. MAPK signaling.
Neutrophils isolated from blood of controls (n=6) or XLA subjects (n=6) were treated for 5 minutes with CL097 (2.5μg/mL) or LPS (100ng/mL), and phosphorylation of MAP kinases p38, JNK and ERK was assessed by western blot. Representative results for two controls and four XLA subject are shown in comparison to β-actin.
TLR induced Neutrophil oxidative burst
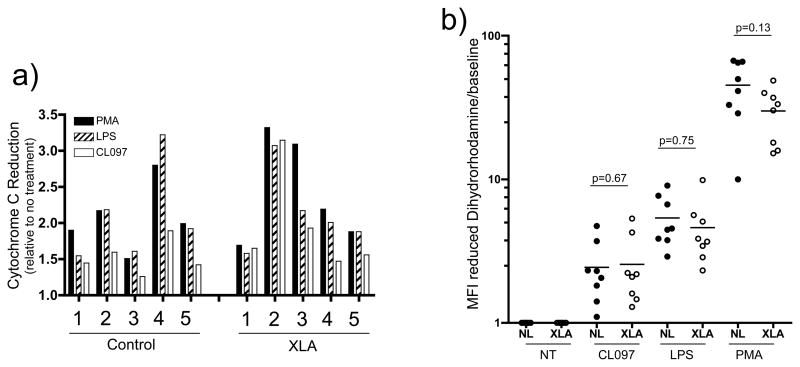
As TLR signaling pathways in neutrophils appeared intact, TLR effector functions were then assessed. TLR activation of neutrophils normally leads to priming the cells for the release of reactive oxygen species needed to kill phagocytosed pathogens[25, 29]. To examine priming of respiratory burst, isolated XLA or control neutrophils were incubated with cytochrome C and stimulated with LPS, CL097 or, as a control, PMA. The level of cytochrome C reduction by the superoxide radicals produced following neutrophil activation was then assessed. As shown in Figure 4a induction of superoxide production in XLA neutrophils was comparable to control neutrophils.
Figure 4. Figure 4a and 4b: TLR induction of respiratory burst.
Isolated neutrophils from normal controls (n=5) or XLA subjects (n=5) were first incubated with cytochrome C, in the presence or absence of SOD, and treated for 20 minutes with CL097 (2.5μg/mL), LPS (100ng/mL) or PMA (10ng/mL). (a) Reduction of cytochrome C by the superoxide radicals produced by neutrophils was assessed at 550nM absorbance. (b) Isolated neutrophils from normal controls (solid circles, n=8) and XLA subjects (open circles, n=8) were incubated with dihydrorhodamine and treated as in Figure 4a, and reduction of dihydrorhodamine to rhodamine was assessed by FACS. These results are represented graphically as MFI of the rhodamine signal. p-values shown are from a two-tailed t-test comparing control and XLA responses within each treatment group.
To further investigate TLR induced respiratory burst, PMA, CL097, or LPS stimulated neutrophils were examined for dihydrorhodamine reduction. Figure 4b shows the mean fluorescence intensity (MFI) of activated cells, relative to baseline fluorescence in untreated cells. Both XLA and control neutrophils demonstrated a significant and similar induction of reactive oxygen species when incubated with CL097 and LPS. (As expected, the level of PMA activation for both XLA and control neutrophils was greater than either TLR activation.) There was no statistically significant difference between control and XLA rhodamine levels within each treatment group.
TLR and delayed apoptosis
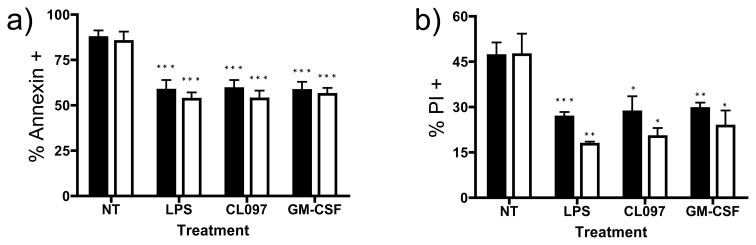
TLR agonists promote the survival of activated neutrophils [28]. To test whether TLR activation prolongs the survival of Btk deficient neutrophils, isolated neutrophils from controls and XLA patients were incubated with LPS, CL097 or as a positive control, GM-CSF, which also prolongs neutrophil survival[30]. For both control and XLA neutrophils, treatment with LPS and CLO97 significantly reduced the amount of neutrophil staining by annexin V (Figure 5a) and incorporation of PI (Figure 5b) showing that this facet of TLR function was also retained in XLA neutrophils. GM-CSF, as expected, also reduced markers of apoptosis and cell death.
Figure 5. Assessing neutrophil apoptosis.
Isolated neutrophils from normal controls (solid bars, n=4) or XLA subjects (open bars, n=4) were cultured for 48 hours with CL097 (2.5μg/mL), LPS (100ng/mL) or GM-CSF (20ng/mL), and induction of apoptosis assessed by Annexin V, (5a) or late apoptosis PI, (5b). Data is represented as a percentage of Annexin or PI positive cells. P values denoted represent two-tailed t-test comparison of each treatment to the untreated control neutrophils. No differences were seen between normal and XLA neutrophils in any treatment group. Noted significance (*p<0.05, **p<0.01, ***p<0.005) represents comparison to untreated neutrophils from same patient or control subject.
Discussion
Based predominantly on mouse models and cell lines, a number of previous studies have concluded that Btk is a key component of selected cell-specific TLR signaling pathways[9–11, 14, 17, 31]. LPS stimulated Xid or Btk −/− murine macrophages displayed impaired production of TNF-α and IL-1β [17, 20], IL-10[17], impaired AP-1 and NF-κB activation[10, 12, 17], as well as diminished production of reactive oxygen intermediates[16]. On the other hand, studies on human XLA dendritic cells, monocytes and macrophages have provided contradictory results, showing both reduced[18, 22, 32] or normal TLR cytokine secretion responses[33, 34].
TLR pathways are crucial to activation of a variety of inflammatory cells including neutrophils, and it is not surprising that neutrophil function might be impaired in patients with mutations in IRAK4 or NEMO, key signaling intermediates in the NF-κB pathway activated by TLR ligation[25, 26]. The original observation that an individual with IRAK4 deficiency had defective CD62L shedding[35], led to the suggestion of a clinical test to screen for TLR defects[36]. While L-selectin shedding was clearly abnormal in subjects with IRAK-4 and NEMO defects, the neutrophils of XLA subjects examined here demonstrated normal loss of CD62L upon TLR activation, suggesting that this marker of neutrophil activation is Btk-independent. To further assess TLR-induced signaling, activation of p38, ERK, and JNK was assessed, as TLR activation of these pathways regulates important neutrophil functions such as adhesion, migration, activation of NF-κB signaling, and downstream cytokine release[26, 37, 38]. However, there was no difference in activation of these kinases in XLA neutrophils compared with controls. Turning to an essential downstream effector function, respiratory burst, we found that TLR activated XLA neutrophils were also comparable to controls. In addition, XLA neutrophils treated with TLR agonists demonstrated delayed apoptosis, further confirming the fidelity of TLR activation and signaling pathways in the absence of Btk. Other TLR-induced effector functions, such as phagocytosis or chemokine production, have not been investigated in XLA neutrophils, so further studies are warranted.
The results of these studies appear to be in concert with the clinical phenotype of XLA subjects, as patients maintained on adequate Ig-replacement therapy do not have infections suggestive of innate immune defects[25, 36, 39, 40]. While XLA patients may be neutropenic during acute infections[27], which might suggest accelerated apoptosis or increased susceptibility to stress, XLA neutrophils displayed normally enhanced survival on culture with TLR ligands. However, it remains possible that sufficient replacement Ig could ameliorate the outcomes of any Btk related TLR defects. Possibly in concert with this, Btk−/− mice challenged with LPS had defective neutrophil mediated clearance, which was reversed by infusions with murine IgM natural antibody [21].
A possible explanation for why Btk deficiency does not have significant clinical effects outside the B cell compartment is that other members of the Tec kinase family may able to compensate for Btk deficiency. The Xid and Btk−/− mice have a much less severe phenotype than XLA, and one group has demonstrated this is due to compensatory activity of the homologous Tec kinase[41]. Human neutrophils are known to also express Tec as well as Bmx, another Tec kinase family member, and Btk, Tec and Bmx are often activated in concert upon neutrophil stimulation. It is possible that these kinases are able to compensate for the loss of Btk in XLA neutrophils [42]. We conclude that as for mast cells[31], Btk appears dispensable for essential TLR induced functions in neutrophils.
Acknowledgments
This work was supported by the National Institutes of Health, AI 101093, AI-467320, AI-48693, NIAID Contract 03-22, The Jeffrey Modell Foundation, and the David S Gottesman Immunology Chair. Baxter Healthcare also supports a study on the demographics of immune deficiency in New York State.
Abbreviations
- XLA
X-linked agammaglobulinemia
- Btk
Bruton’s tyrosine kinase
- Ig
immunoglobulin
- TLR
Toll-like receptor
- LPS
lipopolysaccharide
- MFI
mean fluorescence intensity
- DHR
dihydrorhodamine 123
- SOD
superoxide dismutase
- PMA
phorbol 12-myristate 13-acetate
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HBSS
Hanks balanced salt solution
- ssRNA
single-stranded RNA
- PI
propidium iodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conley ME. B cells in patients with X-linked agammaglobulinemia. Journal of Immunology. 1985;134:3070–3074. [PubMed] [Google Scholar]
- 2.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [see comment][erratum appears in Nature 1993 Jul 22;364(6435):362] [DOI] [PubMed] [Google Scholar]
- 3.Conley ME. Genetics of hypogammaglobulinemia: what do we really know? Curr Opin Immunol. 2009;21:466–471. doi: 10.1016/j.coi.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrar JE, Rohrer J, Conley ME. Neutropenia in X-linked agammaglobulinemia. Clinical Immunology & Immunopathology. 1996;81:271–276. doi: 10.1006/clin.1996.0188. [DOI] [PubMed] [Google Scholar]
- 5.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, Conley ME, Cunningham-Rundles C, Ochs HD. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine. 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 6.Howard V, Greene JM, Pahwa S, Winkelstein JA, Boyle JM, Kocak M, Conley ME. The health status and quality of life of adults with X-linked agammaglobulinemia. Clin Immunol. 2006;118:201–208. doi: 10.1016/j.clim.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Winkelstein JA, Conley ME, James C, Howard V, Boyle J. Adults with X-linked agammaglobulinemia: impact of disease on daily lives, quality of life, educational and socioeconomic status, knowledge of inheritance, and reproductive attitudes. Medicine. 2008;87:253–258. doi: 10.1097/MD.0b013e318187ed81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu Y, Kung HJ. Signaling network of the Btk family kinases. Oncogene. 2000;19:5651–5661. doi: 10.1038/sj.onc.1203958. [DOI] [PubMed] [Google Scholar]
- 9.Doyle SL, Jefferies CA, Feighery C, O’Neill LA. Signaling by Toll-like receptors 8 and 9 requires Bruton’s tyrosine kinase. Journal of Biological Chemistry. 2007;282:36953–36960. doi: 10.1074/jbc.M707682200. [DOI] [PubMed] [Google Scholar]
- 10.Doyle SL, Jefferies CA, O’Neill LA. Bruton’s tyrosine kinase is involved in p65-mediated transactivation and phosphorylation of p65 on serine 536 during NFkappaB activation by lipopolysaccharide. Journal of Biological Chemistry. 2005;280:23496–23501. doi: 10.1074/jbc.C500053200. [DOI] [PubMed] [Google Scholar]
- 11.Hasan M, Lopez-Herrera G, Blomberg KE, Lindvall JM, Berglof A, Smith CI, Vargas L. Defective Toll-like receptor 9-mediated cytokine production in B cells from Bruton’s tyrosine kinase-deficient mice. Immunology. 2008;123:239–249. doi: 10.1111/j.1365-2567.2007.02693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jefferies CA, Doyle S, Brunner C, Dunne A, Brint E, Wietek C, Walch E, Wirth T, O’Neill LA. Bruton’s tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. Journal of Biological Chemistry. 2003;278:26258–26264. doi: 10.1074/jbc.M301484200. [DOI] [PubMed] [Google Scholar]
- 13.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 14.Lee KG, Xu S, Wong ET, Tergaonkar V, Lam KP. Bruton’s tyrosine kinase separately regulates NFkappaB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B Cells. Journal of Biological Chemistry. 2008;283:11189–11198. doi: 10.1074/jbc.M708516200. [DOI] [PubMed] [Google Scholar]
- 15.Liljeroos M, Vuolteenaho R, Morath S, Hartung T, Hallman M, Ojaniemi M. Bruton’s tyrosine kinase together with PI 3-kinase are part of Toll-like receptor 2 multiprotein complex and mediate LTA induced Toll-like receptor 2 responses in macrophages. Cellular Signalling. 2007;19:625–633. doi: 10.1016/j.cellsig.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Mangla A, Khare A, Vineeth V, Panday NN, Mukhopadhyay A, Ravindran B, Bal V, George A, Rath S. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood. 2004;104:1191–1197. doi: 10.1182/blood-2004-01-0207. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt NW, Thieu VT, Mann BA, Ahyi AN, Kaplan MH. Bruton’s tyrosine kinase is required for TLR-induced IL-10 production. Journal of Immunology. 2006;177:7203–7210. doi: 10.4049/jimmunol.177.10.7203. [DOI] [PubMed] [Google Scholar]
- 18.Sochorova K, Horvath R, Rozkova D, Litzman J, Bartunkova J, Sediva A, Spisek R. Impaired Toll-like receptor 8-mediated IL-6 and TNF-alpha production in antigen-presenting cells from patients with X-linked agammaglobulinemia. Blood. 2007;109:2553–2556. doi: 10.1182/blood-2006-07-037960. [DOI] [PubMed] [Google Scholar]
- 19.Taneichi H, Kanegane H, Sira MM, Futatani T, Agematsu K, Sako M, Kaneko H, Kondo N, Kaisho T, Miyawaki T. Toll-like receptor signaling is impaired in dendritic cells from patients with X-linked agammaglobulinemia. Clinical Immunology. 2008;126:148–154. doi: 10.1016/j.clim.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Mukhopadhyay S, Mohanty M, Mangla A, George A, Bal V, Rath S, Ravindran B. Macrophage effector functions controlled by Bruton’s tyrosine kinase are more crucial than the cytokine balance of T cell responses for microfilarial clearance. J Immunol. 2002;168:2914–2921. doi: 10.4049/jimmunol.168.6.2914. [DOI] [PubMed] [Google Scholar]
- 21.Reid RR, Prodeus AP, Khan W, Hsu T, Rosen FS, Carroll MC. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J Immunol. 1997;159:970–975. [PubMed] [Google Scholar]
- 22.Horwood NJ, Page TH, McDaid JP, Palmer CD, Campbell J, Mahon T, Brennan FM, Webster D, Foxwell BM. Bruton’s tyrosine kinase is required for TLR2 and TLR4-induced TNF, but not IL-6, production. Journal of Immunology. 2006;176:3635–3641. doi: 10.4049/jimmunol.176.6.3635. [DOI] [PubMed] [Google Scholar]
- 23.Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 25.Singh A, Zarember KA, Kuhns DB, Gallin JI. Impaired priming and activation of the neutrophil NADPH oxidase in patients with IRAK4 or NEMO deficiency. J Immunol. 2009;182:6410–6417. doi: 10.4049/jimmunol.0802512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouma G, Doffinger R, Patel SY, Peskett E, Sinclair JC, Barcenas-Morales G, Cerron-Gutierrez L, Kumararatne DS, Davies EG, Thrasher AJ, Burns SO. Impaired neutrophil migration and phagocytosis in IRAK-4 deficiency. Br J Haematol. 2009;147:153–156. doi: 10.1111/j.1365-2141.2009.07838.x. [DOI] [PubMed] [Google Scholar]
- 27.Kozlowski C, Evans DI. Neutropenia associated with X-linked agammaglobulinaemia. J Clin Pathol. 1991;44:388–390. doi: 10.1136/jcp.44.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 29.Elbim C, Lizard G. Flow cytometric investigation of neutrophil oxidative burst and apoptosis in physiological and pathological situations. Cytometry A. 2009;75:475–481. doi: 10.1002/cyto.a.20726. [DOI] [PubMed] [Google Scholar]
- 30.Klein JB, Rane MJ, Scherzer JA, Coxon PY, Kettritz R, Mathiesen JM, Buridi A, McLeish KR. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J Immunol. 2000;164:4286–4291. doi: 10.4049/jimmunol.164.8.4286. [DOI] [PubMed] [Google Scholar]
- 31.Zorn CN, Keck S, Hendriks RW, Leitges M, Freudenberg MA, Huber M. Bruton’s tyrosine kinase is dispensable for the Toll-like receptor-mediated activation of mast cells. Cell Signal. 2009;21:79–86. doi: 10.1016/j.cellsig.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Horwood NJ, Mahon T, McDaid JP, Campbell J, Mano H, Brennan FM, Webster D, Foxwell BM. Bruton’s tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production. Journal of Experimental Medicine. 2003;197:1603–1611. doi: 10.1084/jem.20021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jyonouchi H, Geng L, Toruner GA, Vinekar K, Feng D, Fitzgerald-Bocarsly P. Monozygous twins with a microdeletion syndrome involving BTK, DDP1, and two other genes; evidence of intact dendritic cell development and TLR responses. European journal of pediatrics. 2008;167:317–321. doi: 10.1007/s00431-007-0493-0. [DOI] [PubMed] [Google Scholar]
- 34.Perez de Diego R, Lopez-Granados E, Pozo M, Rodriguez C, Sabina P, Ferreira A, Fontan G, Garcia-Rodriguez MC, Alemany S. Bruton’s tyrosine kinase is not essential for LPS-induced activation of human monocytes. Journal of Allergy & Clinical Immunology. 2006;117:1462–1469. doi: 10.1016/j.jaci.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 35.Kuhns DB, Long Priel DA, Gallin JI. Loss of L-selectin (CD62L) on human neutrophils following exudation in vivo. Cell Immunol. 1995;164:306–310. doi: 10.1006/cimm.1995.1174. [DOI] [PubMed] [Google Scholar]
- 36.von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, Vasconcelos J, Sirvent N, Guedes M, Vitor AB, Herrero-Mata MJ, Arostegui JI, Rodrigo C, Alsina L, Ruiz-Ortiz E, Juan M, Fortuny C, Yague J, Anton J, Pascal M, Chang HH, Janniere L, Rose Y, Garty BZ, Chapel H, Issekutz A, Marodi L, Rodriguez-Gallego C, Banchereau J, Abel L, Li X, Chaussabel D, Puel A, Casanova JL. Pyogenic bacterial infections in humans with MyD88 deficiency. Science. 2008;321:691–696. doi: 10.1126/science.1158298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aomatsu K, Kato T, Fujita H, Hato F, Oshitani N, Kamata N, Tamura T, Arakawa T, Kitagawa S. Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases. Immunology. 2008;123:171–180. doi: 10.1111/j.1365-2567.2007.02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabroe I, Dower SK, Whyte MK. The role of Toll-like receptors in the regulation of neutrophil migration, activation, and apoptosis. Clin Infect Dis. 2005;41(Suppl 7):S421–426. doi: 10.1086/431992. [DOI] [PubMed] [Google Scholar]
- 39.Kim TW, Staschke K, Bulek K, Yao J, Peters K, Oh KH, Vandenburg Y, Xiao H, Qian W, Hamilton T, Min B, Sen G, Gilmour R, Li X. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med. 2007;204:1025–1036. doi: 10.1084/jem.20061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orange JS, Jain A, Ballas ZK, Schneider LC, Geha RS, Bonilla FA. The presentation and natural history of immunodeficiency caused by nuclear factor kappaB essential modulator mutation. J Allergy Clin Immunol. 2004;113:725–733. doi: 10.1016/j.jaci.2004.01.762. [DOI] [PubMed] [Google Scholar]
- 41.Ellmeier W, Jung S, Sunshine MJ, Hatam F, Xu Y, Baltimore D, Mano H, Littman DR. Severe B cell deficiency in mice lacking the tec kinase family members Tec and Btk. Journal of Experimental Medicine. 2000;192:1611–1624. doi: 10.1084/jem.192.11.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lachance G, Levasseur S, Naccache PH. Chemotactic factor-induced recruitment and activation of Tec family kinases in human neutrophils. Implication of phosphatidynositol 3-kinases. Journal of Biological Chemistry. 2002;277:21537–21541. doi: 10.1074/jbc.M201903200. [DOI] [PubMed] [Google Scholar]