Abstract
Diabetes mellitus (DM) is the leading co-morbidity in septic patients but its impact upon survival and immuno-inflammatory signalling in sepsis is undetermined. We investigated the effect of untreated DM upon survival and immuno-inflammatory responses in the acute phase (days 1–5) of murine polymicrobial sepsis using the AKITA model of Type 1 diabetes. Diabetic female C57BL/6-Ins2Akita (AKITA) and C57BL/6 wild-type (WT) mice underwent cecal ligation and puncture (CLP), blood (20μl) was sampled for 5 days and survival monitored for 28 days. By day 5, all 8 AKITA mice expired compared to 10 out of 28 deaths in WT mice. Blood glucose declined post-CLP in all groups (most dramatically in AKITAs by 75%). To compare the evolution of inflammatory profiles, mice were retrospectively divided based on outcome into AKITA, WT-Died and WT-Survived (within days 1–5). Hypoglycemia developed in all groups which resolved in WT-Survived (97mg/dl at 96h) but intensified in WT-Died and AKITAs (approx. 30mg/dl). Dramatic increases in both pro-and anti-inflammatory cytokines were observed in WT-Died (i.e. IL-6: 38.2±17.8 ng/ml at 24h), which contrasted with a lack of pre-lethal cytokine response in AKITA mice (IL-6: 4.3±3.4 ng/ml at 24h). A pre-lethal composite cytokine score was calculated on values obtained 24h prior to death. This score was 3 fold lower for pro-inflammatory cytokines and 6-fold lower for anti-inflammatory mediators in the AKITA mice compared to the WT-Died mice but identical to the composite score in WT-Survived. These data demonstrate that untreated type I diabetes mellitus severely exacerbates sepsis mortality without inducing a pre-lethal release of systemic pro- or anti-inflammatory cytokines.
Keywords: Glucose, Hyperglycemia, CLP, Comorbidity, Cytokine receptors, Stratification, Inflammation
INTRODUCTION
Recent estimates demonstrate that approximately 171 million people worldwide suffer from diabetes (1) and as many as fifty percent of diabetic patients may remain undiagnosed due to lack of symptoms (2). Sepsis is one of the leading causes of death in intensive care units (ICUs) and is frequently complicated by a number of co-existing conditions including type 1 and 2 diabetes mellitus (DM) (3). Despite the frequent presence of co-morbid conditions, pre-clinical sepsis studies typically rely on normal animals lacking clinically relevant comorbidities such as diabetes or chronic renal failure. The dismal failure of several treatment trials which extrapolated data from flawed sepsis surrogates (4) underscores the need for better pre-clinical models of sepsis that would combine septicemia with the common comorbidities such as DM.
The autosomal dominant point mutation Mody (Cys/Tyr) in the Ins2 insulin gene of AKITA mice serves as a well-established, insulin-dependent model for investigating type 1 human diabetes (5). The Mody mutation causes a primary defect in protein processing that renders pancreatic β cells incapable of insulinsecretion resulting in hyperglycemia (5). The diabetic phenotype is apparent as early as 4 weeks after birth resulting in significant elevations in blood glucose by 7 weeks of age in either gender (5–6). Classic early symptoms of hyperglycemia (polydipsia and polyuria) are not accompanied by obesity, infertility and atherosclerosis. If allowed to progress for several weeks, the AKITA mutation constitutes one of the best available investigative platforms for diabetic (type 1) nephropathy in mice (7). In chronic conditions diabetic cardiomyopathy (8), autonomic neuropathy (9) and retinal degeneration (10) has been reported in mature AKITA mice.
Diabetes increases susceptibility to common infections e.g. in the lower respiratory tract, urinary tract, skin and mucous membranes (11–12). Diabetics, regardless of type, also have a higher risk for bloodstream infections (13). While insulin exerts strong anti-inflammatory signaling (14), hyperglycemia triggers robust pro-inflammatory responses demonstrable in vivo and in vitro (15). Type 1 and 2 diabetic patients display increased concentrations of circulating pro-inflammatory cytokines (16) and in a recent prospective study elevated levels of C-reactive protein and IL-6 were predictive for type 2 diabetes in healthy middle-aged women (17). Pre-existing diabetes was also shown to influence the risk of sepsis-related organ dysfunction. When compared to severe sepsis patients without diabetes, diabetic subjects were less likely to develop respiratory failure but were more likely to develop renal failure (18).
Septic patients constitute up to 28% of all hospital admissions (19) and the gastrointestinal tract is the second leading source (after respiratory) of sepsis (19). Although diabetes is a leading co-morbidity in septic patients, clinical and experimental studies evaluating its impact upon sepsis are rare. Using the AKITA mutation and the CLP model of abdominal sepsis, we investigated the influence of pre-existing type 1 diabetes upon the progression of sepsis. Specifically, in this study we examined the impact of untreated diabetes upon long-term survival and, using the outcome as the reference point, we retrospectively assessed the evolution of septic inflammatory response in the acute phase (days 1–5) of CLP sepsis.
MATERIALS AND METHODS
Animals
Nine-week-old female C57BL/6-Ins2Akita (AKITA) mice on the C57BL/6 backgroundand C57BL/6 control mice were purchased from The Jackson Laboratories. All animal procedures were approved by the Institutional Animal Care and Use Committee of Boston University School of Medicine. To eliminate gender-related variability, only female mice were included in the study. All animals had unrestricted access to food and water throughout the entire study period (including the baseline sampling time-point).
Sepsis model
The murine model of CLP arguably constitutes the best surrogate for abdominal poly-microbial human sepsis (20). We followed the original CLP protocol by the Chaudry group (21) with modifications including analgesia (buprenorphine, 0.1 mg/kg twice daily for 3 days), antibiotic therapy (Imipenem, 25 mg/kg twice daily for 5 days) and fluid resuscitation (Lactated Ringers, 1ml/mouse twice daily for 5 days). Inhalation anesthesia (Isofluorane, 2 vol.%) via rubber mask was used for all surgeries. The ligated cecum was double-punctured (through and through) with 23ga needle and CLP was performed in small sets of animals at a time to assure the experimental reproducibility. All animals were followed for 28 days or until death, whichever occurred first. Death was used as an end-point for all mice, any mice recognized as agonal were sacrificed. Following institutional recommendations, sham surgeries were not performed to reduce the total number of mice required for the study, since the intent was to examine the effect of diabetes on sepsis, not surgery.
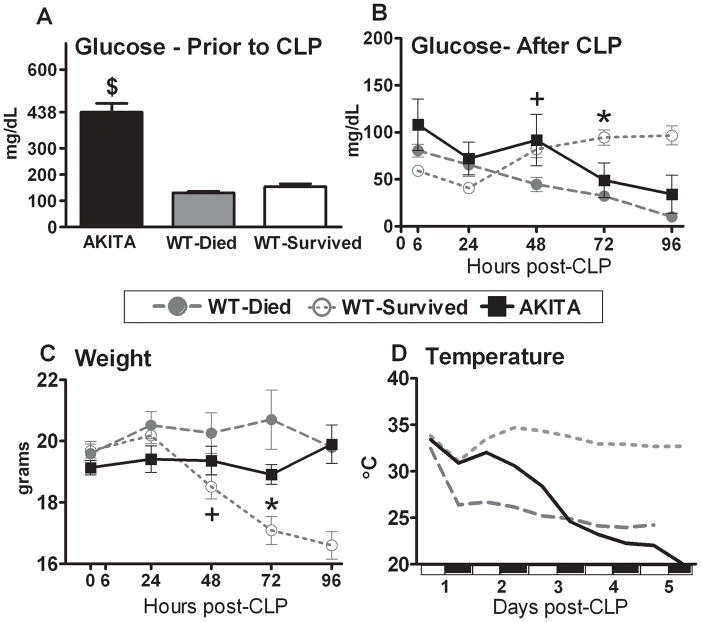
In order to eliminate any DM-related organ/metabolic dysfunction typically developing in later stages of the disease, the AKITA mice were subjected to CLP no later than two weeks after the onset of lasting hyperglycemia (blood glucose ≥300dl/ml). With the exception of the elevated blood glucose (Fig. 2A), AKITA mice were healthy and well hydrated prior to CLP, as evidenced by the identical (compared to WT mice) average body weight (Fig. 2C) and temperature (Fig. 2D), although the urine output was not specifically tested. This was simultaneously corroborated by the detailed clinical examination that failed to reveal any signs of illness typical for rodents (e.g. inadequate grooming, isolation, enophthalmia, abnormal skin-grip test).
FIG. 2. Pre-CLP (panel A) and post-CLP (panel B) temporal profiles of blood glucose, body weight (panel C), temperature (panel D) in diabetic (AKITA) and non-diabetic wild type (WT) mice in the acute phase (days 1–5) of sepsis.
Non-diabetic animals were further divided into two groups based on outcome: WT-Died (dead within days 1–5) and WT-Survived (alive after day 5). Parameters were measured either repeatedly (panels A–C) or continuously (panel D) until day 5 post-CLP (see statistical section for distribution of n). The white and black bars on the x-axis of panel D indicate the light cycle of the room. Data are mean ± SEM except for panel D where only mean values are plotted. $ P < 0.0001 between AKITA and WT mice; + P < 0.05 between WT-Died and WT-Survived; * P < 0.05 between WT-Survived and remaining groups.
Sampling
The vena submandibularis was punctured with 25 gauge needle and 20μl blood was drawn into a pipette tip rinsed with EDTA (169 mM tripotassium salt). No mice were sacrificed at the time of sampling. Blood was collected 6 hours post-CLP and at 24-hour intervals for the first 5 days or until death. The 5-day cut-off was selected based on dissimilar mechanism(s) of death between the acute (days 1–5) vs. chronic (days 6–28 post-CLP) sepsis (22). The last individual cytokine measurement represents an animal that died within the 24h after sampling. Samples were immediately diluted 1:10 in phosphate-buffered saline with a 1:50 dilution of EDTA, centrifuged (5min/1000G at 4°C), the diluted plasma removed and stored at −20° C until analysis.
Temperature and body weight and movement recordings
The core body temperature and gross motor activity in all animals was recorded continuously using mini-mitter (G2 E-Mitter model) transponders (Mini-Mitter Company, Inc, Sunriver, OR) implanted intraperitonealy during surgery. All mini-mitters were implanted in the upper right quadrant, opposite the CLP surgery site. Body weight was recorded once daily.
Glucose measurement
Circulating glucose concentrations were determined directly in undiluted blood obtained using the Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN). The blood glucose monitoring system employed here was not influenced by low hematocrit in the 20–44% range.
Hematology
Following blood collection, the cell pellet was immediately re-suspended in 480 μl of Hemavet solution (CDC Technologies, Oxford, CT). A complete blood count including differential was performed in a Hemavet 1500 (CDC Technologies, Oxford, CT).
Microarray immunoassay
The primary (capture) antibodies (against 20 cytokines) were spotted into 96-well microtiter plates followed by blocking the plates (23). Samples were then incubated with a biotinylated secondary antibody, then streptavidin conjugated to an infrared (IR) fluorophore. The plates are then read with the Odyssey IR imaging system (LI-COR Biosciences, Lincoln, NE).
Statistical Analysis
The Kaplan-Meier 28 day-survival (Fig. 1) was assessed by the log-rank test. Data displaying Gaussian distribution were analyzed on the original scale by the one-way ANOVA followed by the Tukey post-hoc test. Abnormally distributed values were analyzed by the Kruskall-Wallis test followed by the Dunns post-hoc analysis (3 groups) or Wilcoxon rank sum test (2 groups).
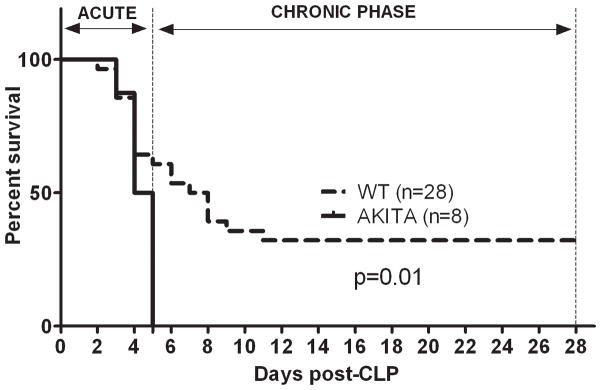
FIG. 1. Sepsis-induced mortality is exacerbated in diabetic (AKITA) mice in comparison to non-diabetic wild type (WT) animals.
CLP was performed with a 23-gauge needle to produce 40% mortality in WT mice in the acute phase of sepsis. Mortality was monitored for 28 days. The deaths were separated into two groups: acute (days 1–5) or chronic (days 6–28). In AKITAs, 100% mortality was reached by day 5. This was significantly higher (p<0.01 log rank survival) compared to WT mice. n=8 AKITAs; n=28 WT mice.
For dying animals (AKITA and WT-Died), every data point in Figs. 2–5 represents the average concentration of repetitive measurements taken from the animals that died within days 1–5. The following data distribution applies for the AKITA and WT-Died: at 0, 6 and 24h time-point n=8 and 11, at 48h n=8 and 7, at 72h n=7 and 5, at 96h n=4 and 0, respectively (applicable to all figures). For the WT-Survived, the average concentration values at each time point are based on repetitive measurements taken from the same animals (n=17; applicable to all figures). In tables all parameters (Figs. 2–5) were analyzed using a one-way ANOVA model fitted at the selected time point (i.e. 6h, 24h, 48h, 72h and 96h post-CLP) rather than using repeated measures ANOVA across all five time-points. The one-way ANOVA model was used given that the differences among groups (not the time trends in separate groups) were the primary endpoints and given the reduction in sample size (AKITA and WT-Died) across time related to the different groups. Given the severe reduction of n at 96 hour post-CLP, statistical evaluation was not performed for this time-point. Sample size distribution in tables 1 and 2: WT-Survived n=17; WT-Died n=11; AKITA n=8. For average inflammatory scores (Fig. 6), all marker values were normalized (each mediator individually) to the median value of that specific cytokine. The normalized values were then added for each animal across all pro- and anti-inflammatory cytokines for each group. Cytokine levels below the limit of detection were assigned a value equal to one-half of the lower limit of detection in the standard curve. At time zero, a below detection limit (bdl) value was assigned to the group whenever more than 50% of the measured samples fell below the bdl. The lower limit of detection for cytokines and cytokine inhibitors ranged between 3–132 pg/ml. Cytokine values collected from surviving WT animals (alive after day 5 post-CLP) were sampled for comparison on the same post-CLP day as the moribund mice. Data are graphed as the mean ± standard error of the means (SEM) wherever applicable. All tests were carried out using Prism 5 (GraphPad Software Inc., San Diego, CA).
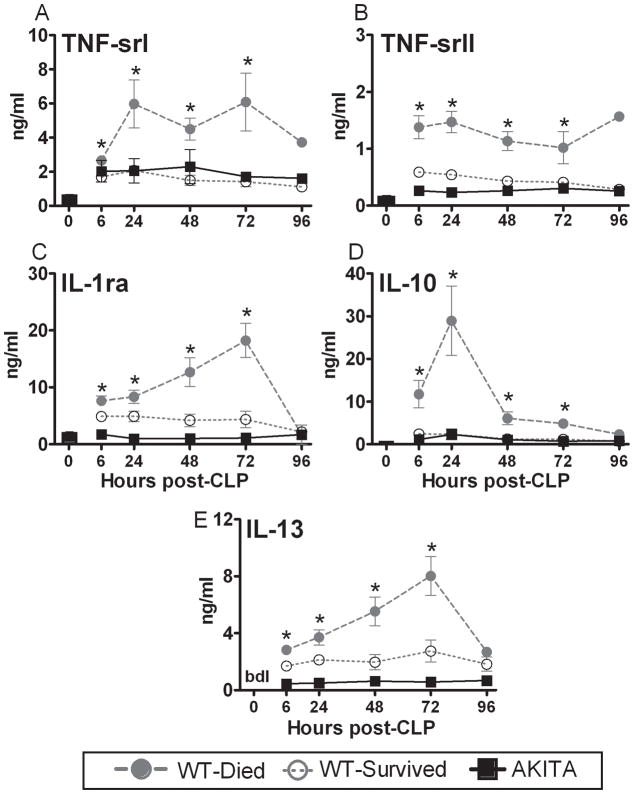
FIG. 5. Temporal profiles of anti-inflammatory plasma cytokines in diabetic (AKITA) and non-diabetic wild type (WT) mice in the acute phase (days 1–5) of sepsis.
Non-diabetic animals were additionally divided into two groups based on outcome: WT-Died (dead within days 1–5) and WT-Survived (alive within days 1–5). All parameters were measured at 24h intervals post-CLP (see statistical section for distribution of n). For WT-Died and AKITA groups, values plotted at each time-point were obtained within 24 hours of death. Bars represent the group average value at 0h post-CLP. bdl: below detectable limit; Data are mean ± SEM. * P < 0.05 between WT-Died and remaining groups; # P < 0.05 between AKITA and remaining groups.
TABLE 1.
Comparison of plasma pro-inflammatory cytokine concentrations (ng/ml) in AKITA and Wild Type (WT) mice 24 hours prior to death*
| CYTOKINE (ng/ml) | WT-Survived | WT-Died | AKITA |
|---|---|---|---|
| Interleukin (IL)-1β | 1.1 ± 0.2 | 3.4 ± 0.6† | 0.4 ± 0.1 |
| IL-2 | 0.5 ± 0.1 | 0.7 ± 0.1‡ | 0.1 ± 0.01 |
| IL-5 | 0.3 ± 0.1 | 0.8 ± 0.1† | 0.1 ± 0.02 |
| IL-6 | 1.5 ± 0.7 | 38.2 ± 17.8† | 4.3 ± 3.4 |
| IL-12 | 0.4 ± 0.1 | 1.6 ± 0.3 † | 0.1 ±0.03 |
| IL-17 | 0.1 ± 0.03 | 0.5 ± 0.07† | 0.1 ± 0.02 |
| TNFα | 0.1 ± 0.02 | 0.3 ± 0.05† | 0.1 ± 0.01 |
| IFNγ | 0.7 ± 0.2 | 3.3 ± 0.4† | 0.2 ± 0.1 |
| ICAM-1 | 6.7 ± 1.1 | 18.1 ± 2.5† | 7.6 ± 1.2 |
| MIP-1α | 5.5 ± 0.8 | 13.7 ± 1.1† | 5.1 ± 1.3 |
| MIP-2 | 2.1 ± 0.9 | 68.8 ± 21.9† | 5.7 ± 2.9 |
| MCP-1 | 4.1 ± 1.2 | 36.6 ± 5.8† | 9.2 ± 2.5 |
| Eotaxin | 2.4 ± 0.5§ | 15.4 ± 5.5 | 28.9 ± 13.9 |
| Eotaxin-2 | 1.9 ± 0.3 | 5.6 ± 0.8† | 2.3 ± 0.5 |
The pooled pre-lethal cytokine values were collected within 24 hours of death from all mice (i.e. WT-Died, AKITA groups) that died between days 1–5 post-CLP (acute sepsis). Dying mice were matched with the WT-survived animals (n=18) of the same post-CLP day as the moribund mouse.
p<0.01 WT-Died versus WT-Survived and AKITA;
p<0.01 WT-Died versus AKITA;
p<0.001 WT-Survived versus WT-Died and AKITA. Data are mean ± SEM.
TABLE 2.
Comparison of plasma anti-inflammatory cytokine concentrations (ng/ml) in AKITA and Wild Type (WT) mice 24 hours prior to death*
| CYTOKINE (ng/ml) | WT-Survived | WT-Died | AKITA |
|---|---|---|---|
| Interleukin (IL)-1ra | 3.8 ± 1.0 | 13.8 ± 2.4† | 1.5 ± 0.4 |
| IL-4 | 0.5 ± 0.08 | 1.1 ± 0.2† | 0.2 ± 0.05 |
| IL-10 | 1.3 ± 0.3 | 17.0 ± 7.6† | 1.4 ± 0.5 |
| IL-13 | 2.1 ± 0.4 | 6.0 ± 1.1† | 0.7 ± 0.2 |
| TNF-srI | 1.6 ± 0.3 | 7.3 ± 1.5† | 2.7 ± 1.0 |
| TNF-srII | 0.4 ± 0.1 | 1.4 ± 0.2† | 0.4 ± 0.1 |
The pooled pre-lethal cytokine values were collected from mice dying between days 1–5 post-CLP (acute sepsis). Dying mice were matched with the WT-survived animals (n=18) of the same post-CLP day as the moribund mouse.
p<0.01 WT-Died versus WT-Survived and AKITA. Data are mean ± SEM.
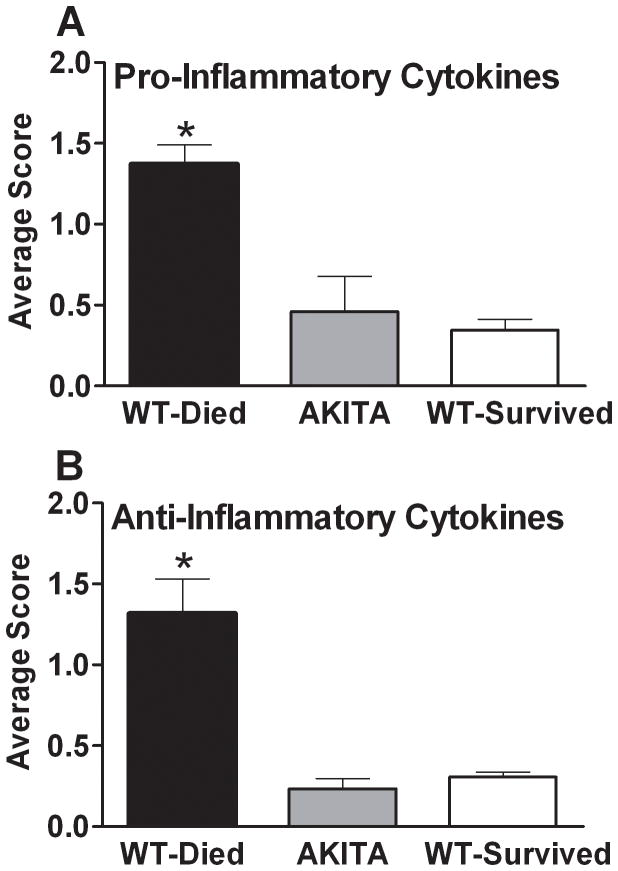
FIG. 6. Composite Inflammatory Score in diabetic (AKITA) and non-diabetic wild type (WT) mice in the acute phase of sepsis (days 1–5).
Non-diabetic animals were further divided into two groups based on outcome: WT-Died (dead within days 1–5) and WT-Survived (alive after day 5). All pro-inflammatory (Panel A) and anti-inflammatory (Panel B) cytokine values were normalized (see statistical section) to generate a composite score for each group. Data are mean ± SEM. * P < 0.05 between WT-Died and remaining groups.
RESULTS
Mortality after CLP-dependent sepsis
To determine the potential impact of pre-existing diabetes upon mortality in experimental sepsis, mice were subjected to a CLP protocol that produced approximately 50% mortality in WT mice at the end of acute sepsis (day 5 post-CLP). In the AKITA mice, CLP-induced sepsis caused 100% (8/8) mortality, whereas in the WT group, only 40% (10/28) of mice died by day 5 post-CLP. All survivors were monitored for 28 days, since lethality also occurs in the chronic sepsis (defined as the period from day 6 to 28 after CLP) (22). An additional 28% mortality took place in the chronic phase bringing the total difference in 28 day survival between AKITA and WT animals to 60% at day 5 and 32% at day 28 post-CLP (both p=0.01). The median survival (MS) was 4.5 days for AKITAs and 7.5 days for WT mice with the MS ratio of 1.7 (95% confidence interval: 1.3 to 2.2).
Changes in circulating glucose during the early sepsis
To define the pre-lethal fluctuations of critical parameters in diabetic and normal animals in the acute phase of sepsis, mice were retrospectively divided into three groups based on outcome: AKITA (all died within 5 days, no survivors), WT-Died (dead within days 1–5) and WT-Survived (alive after day 5). Such comparisons were feasible since mice were not sacrificed in order to collect blood. Prior to CLP, AKITA mice displayed hyperglycemia (blood glucose = 438±34 vs. 145±7 mg/dL in WT, Fig. 2A). Within 6 hours post CLP, the circulating glucose fell by 75% in the AKITA mice compared to a 38% in WT-Died and a 62% decrease in the WT-Survived. After 48 hours a gradual recovery of circulating glucose was apparent in the surviving mice (reaching 62% of normal glucose level by 96h post-CLP), whereas virtually identical hypoglycemia preceded WT and AKITA deaths. Overall, the pre-lethal circulating glucose profiles in AKITA mice followed a similar trajectory as the WT-Died, with a similar outcome.
Changes in physical parameters during the early sepsis
The acute phase of CLP sepsis is characterized by a consistent weight loss in survivors as contrasted with a body weight gain in non-survivors. The average pre-CLP body weight was virtually identical in WT and AKITA mice. A gradual weight loss was observed in WT survivors as early as 48 hours that continued until day 5 post-CLP (Fig. 2C). In contrast, WT-Died and AKITA mice retained and/or gained the pre-CLP body mass in a similar fashion throughout the entire period of acute sepsis.
Murine sepsis produces hypothermia and abolishes the diurnal pattern of gross motor activity. A distinct outcome-dependent separation of temperature profiles occurred as early as day 1 post-CLP (Fig. 2D). The body temperature of surviving mice recovered by day 2 while a gradual temperature decrease was observed in all non-surviving mice. Interestingly, during the first 48 hours, the pre-lethal hypothermia in AKITAs was not as severe as that observed in WT-Died mice (p<0.05 between 12–36h post-CLP for AKITA vs. WT-Died). From day 3 onward, however, the body temperature profiles in both non-surviving groups (AKITA and WT) were virtually identical and significantly lower than WT survivors (P<0.05). The changes in gross motor activity among groups appeared to follow their respective temperature profiles: from day 3 onward the motor activity of AKITA mice gradually deteriorated and followed the movement pattern (consistently poor from the onset of sepsis) of WT non-survivors (data not shown).
Changes in hematologic parameters during the early sepsis
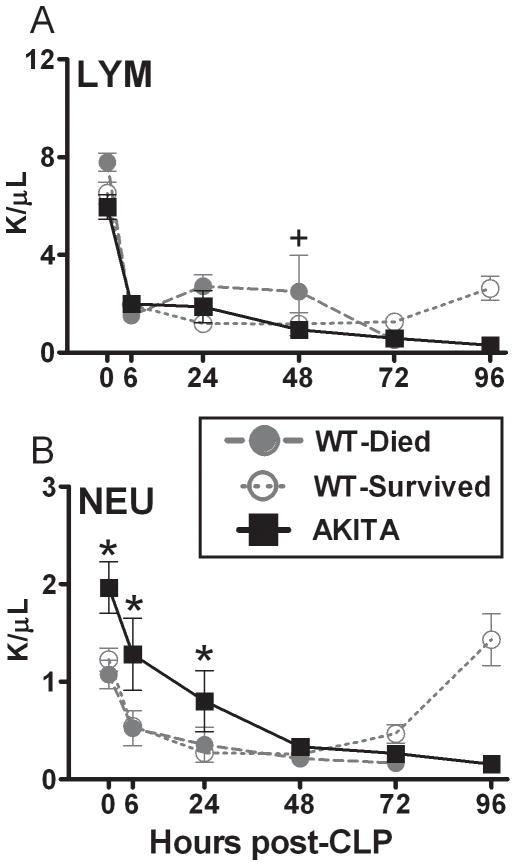
Lymphopenia, neutropenia and mild anemia are frequent findings in the acute phase of CLP sepsis. In all animals CLP induced a gradual decrease in the levels of red blood cells (RBC) and hemoglobin (Hb); the decline was identical for all groups (38% for RBC and 41% for Hb at day 5 compared to day 0, data not shown). All three groups of mice also showed a dramatic drop in lymphocyte and neutrophil counts within the first 6 hours of sepsis. After retrospectively separating the mice based on outcome (WT mice), the circulating lymphocyte counts in the AKITA mice followed the profile of the WT-died group (Fig. 3A). In AKITA mice, after the initial decline, a gradual decrease in circulating lymphocytes continued until the end of the observation period (96h post-CLP). For circulating neutrophils, both AKITA and WT mice had a similar gradual sepsis-induced decrease. Of note, the hyperglycemic AKITA mice had a greater number of circulating neutrophils (compared to normoglycemic mice) both prior and early after (0–24h) the septic challenge. From 72h onward, there was a clear recovery of lymphocytes and neutrophils in WT survivors compared to their lasting depression in the moribund AKITAs and WT-Died mice. Based on the data from figures 2 and 3, the diabetic mice had similar derangements as the WT mice that died, specifically hypoglycemia, no loss of body weight, hypothermia, lymphopenia and neutropenia.
FIG. 3. Temporal profiles of lymphocytes (LYM; panel A) and neutrophils (NEU; panel B) in diabetic (AKITA) and non-diabetic wild type (WT) mice in the acute phase (days 1–5) of sepsis.
Non-diabetic animals were additionally divided into two groups based on outcome: WT-Died (dead within days 1–5) and WT-Survived (alive after day 5). All parameters were measured (counts per μl blood) at 24h intervals post-CLP (see statistical section for distribution of n). Data are mean ± SEM. + P < 0.05 between WT-Died and remaining groups; # P < 0.05 between AKITA and WT-Survived by one sample Wilcoxon Signed Rank Test.; * P < 0.05 between AKITA and WT mice.
Changes in pro-inflammatory cytokines during the early sepsis
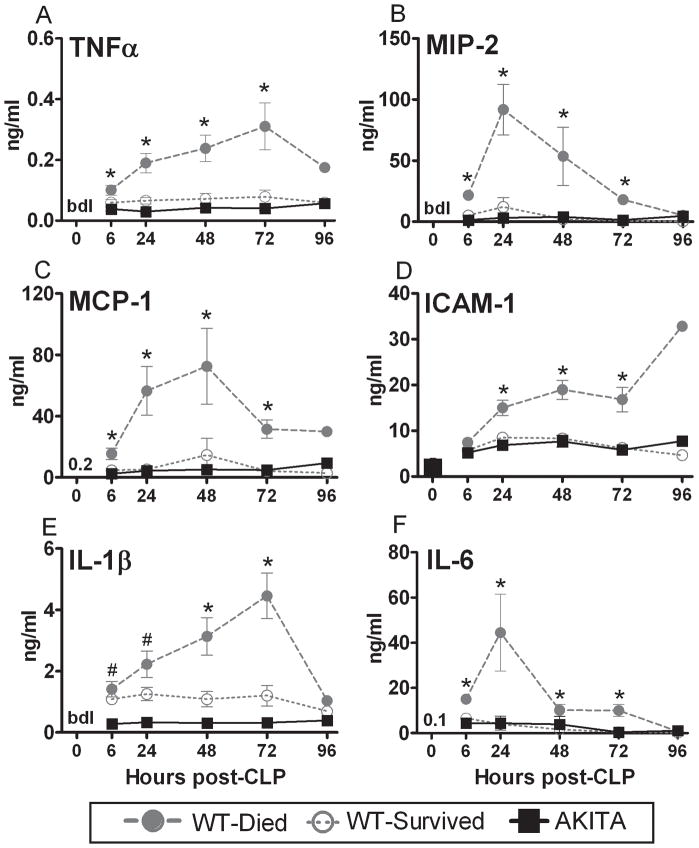
Given that virtually all mice dying in the acute sepsis mount an exuberant inflammatory response, we examined whether the early deaths in AKITAs were driven by a similar hyper-inflammatory response. Twenty circulating pro-and anti-inflammatory cytokines were measured at 24-hour intervals in all mice until day 5 or death (Figures 4 and 5). Virtually none of the pro-inflammatory cytokines became elevated in the AKITA mice even though these mice had similar mortality and physiologic changes as the wild-type mice who died. This contrasts with the significant inflammatory response produced by WT-Died animals who exhibited higher levels of IL-6 and MIP-2 (both more than 11 fold increased) compared to the diabetic or WT surviving mice. Cytokine profiles in the AKITA were nearly superimposable with cytokine profiles recorded in WT survivors throughout the entire 5-day observation period.
FIG. 4. Temporal profiles of pro-inflammatory plasma cytokines in diabetic (AKITA) and non-diabetic wild type (WT) mice in the acute phase (days 1–5) of sepsis.
Non-diabetic animals were additionally divided into two groups based on outcome: WT-Died (dead within days 1–5) and WT-Survived (alive within days 1–5). All parameters were measured in 24h intervals post-CLP (see statistical section for distribution of n). For WT-Died and AKITA groups, values plotted at each time-point were obtained within 24 hours of death. Data are mean ± SEM. Bar and numerals represent the group average value at 0h post-CLP. bdl: below detectable limit; * P < 0.05 between WT-Died and remaining groups; # P < 0.05 between AKITA and WT mice.
Changes in anti-inflammatory cytokines during the early sepsis
Even though all AKITA mice died by day 5, their circulating anti-inflammatory markers were dramatically lower compared to the levels in WT-Died animals. Similar to the pro-inflammatory response, the profiles of cytokine inhibitors in AKITA mice were either identical (IL-10 and TNF srI) or slightly lower (TNS-srII, IL-1ra, and IL-13) compared to the post-CLP profiles recorded in WT-Survived mice (Fig. 5A–E). These temporal changes in the anti-inflammatory cytokines mirrored those observed with the pro-inflammatory markers,
Pre-lethal profiles of pro-and anti-inflammatory cytokines
In all the previous figures, data were depicted as a kinetic profile using the time of CLP surgery as a reference point, yet the exact onset of sepsis is typically not known at the time of admission to the ICU. In such patients efforts are directed towards preventing them from reaching another, undesirable reference time point, death. We evaluated the predictive potential of the inflammatory response recorded directly before death (not earlier than 24h prior) in septic mice with type 1 diabetes as a pre-existing comorbidity. Only cytokine values collected within 24 hours of death (for deaths occurring on any post-sepsis day 1–5) were included. The entire cytokine set was then analyzed using the time of death as the reference point (rather than the onset of sepsis). All dying mice were matched with the WT-survived animals (the WT-Survived group) of the same post-CLP day as the moribund mouse.
Pro-inflammatory (Table 1) and anti-inflammatory (Table 2) concentrations of circulating cytokines recorded (within 24 hours of death and in matching survivors) in AKITA and WT mice were compared. The comparison revealed that regardless of the day of death or the type of inflammatory marker, pre-lethal concentrations of virtually all cytokines in AKITA mice were significantly lower than in the WT-Died mice. Stated more directly, the outcome was the same, death, but the inflammatory profile was substantially different.
To illustrate the global, outcome-correlated inflammatory profiles among the three groups, all afore mentioned cytokine and cytokine inhibitor values were normalized (see statistical section) to generate a composite score for both the pro- and anti-inflammatory response (Fig. 6). The composite inflammatory score in AKITA mice was several folds lower when compared to the WT-Died (3 fold for the pro-inflammatory and 6 fold for the anti-inflammatory profile). At the same time, the AKITA score was virtually identical to the score for the WT-Survived mice.
DISCUSSION
The most important finding of this study was the failure of diabetic mice to mount a substantial inflammatory response to sepsis, despite 100% mortality. It is appropriate to claim that this effect was not related to any of the reported diabetic complications (7–10), given that AKITAs mice used in the study were relatively young and were enrolled relatively shortly after becoming hyperglycemic. Pre-existing diabetes translated into a profound and wide-ranging suppression of the usual robust post-CLP systemic cytokine release. While low-intensity inflammation typically occurs in chronic diabetes, little is known about potential alterations in rapid cytokine responses to an acute infectious challenge. Among women with genitourinary tract infections, females with diabetes had a lower concentration of IL-6 in the urine and their whole blood/monocytes secreted markedly less pro-inflammatory cytokines upon stimulation with LPS compared to healthy subjects (24). Interestingly, this suppression was much more pronounced in patients with type 1 diabetes compared to the type 2 cohort.
In animal models of inflammation, pre-existing diabetes typically blunts either a local or systemic response. Following intratracheal LPS challenge, type 1 diabetic (alloxan-induced) rats had lower lung lavage concentrations of TNFα, IL-1β and IL-10 compared to healthy animals (25). In two other rodent models of type 1 diabetes, different inflammatory stimuli (thioglycollate or glycogen) failed to strongly elicit leukocyte recruitment with diminished expression of cytokines either in exudates or peritoneal lavage fluid (26;27). However, the depressed cytokine response described in our study stands in contrast to the data from another CLP/diabetes study. In Goto-Kakizaki type 2 diabetic rats, the circulating levels of both IL-6 and IL-10 were markedly elevated at 20h post-CLP compared to WT rats (28). It is unclear whether this contrasting effect was equally widespread and influenced outcome, given that no other time-points, inflammatory markers and/or survival were examined in this study. Since the immuno-inflammatory characteristics of rat and mouse CLP models are comparable (29) the disparity in the post-CLP cytokine expression is likely attributable to the difference in the type of diabetes in these models (type 1 vs. type 2).
Based on the data presented here it is not clear whether such a diminished capacity of AKITA mice in mounting of an appropriate inflammatory response is true for other (inflammatory) stimuli. The only other existing AKITA study investigating cytokine response to an intraperitoneal inflammatory challenge (zymosan injection), showed a markedly higher concentration of IL-6, KC and MCP-1 (compared to WT) in the peritoneal lavage fluid (PLF) at 2 hours post-zymosan (6). After we tested concentration of these cytokines at later time-points in the same set of mice, this initial effect proved to be short-lasting. At 4 hours, WT mice had several folds higher PLF concentrations of IL-6, KC, and MCP-1 than AKITAs (Supplemental figure 1). This suggests that in general AKITA mice produce significantly fewer cytokines compared to the WT mice, although additional models of inflammation need to be tested to confirm that.
We demonstrated the lack of correlation between the activation of the inflammatory response and early mortality of AKITA mice. In sepsis, failure to mount an adequate response to infectious stimuli is as disastrous as an immuno-inflammatory overreaction (30). Both clinical trials and animal studies showed that utter elimination of circulating inflammatory mediators (e.g. TNF, IL-1β) exacerbates sepsis mortality (4). This overwhelming “cytokine failure” observed in diabetic AKITA mice likely contributes to the underlying mechanism of their 100% mortality. Furthermore, it is possible that this meager cytokine response reflects a state of prevailing immunoparalysis developing after an overpowering infectious stimulus. Limitations of the non-lethal monitoring implemented in this study precluded us from determining the scale (systemic vs. local) and the potential source(s) (humoral vs. cellular) of this impaired systemic inflammatory response in AKITAs. We also revealed that the cytokine profile in moribund diabetic mice was indistinguishable from the cytokine response generated in surviving but septic non-diabetic animals. If confirmed in diabetic patients, this may have tangible clinical consequences for monitoring sepsis, which rely on the measurement of circulating cytokines (31). Our data indicate that type 1 diabetic patients may fail to generate a typical cytokine profile during acute sepsis (or other critical illnesses) potentially leading to inappropriate therapeutic choices in this cohort of patients.
The elevated blood glucose level (BGL) in AKITA mice is most comparable to human type 1 diabetes. Hyperglycemia at a corresponding level results in minimal mortality in cases of treatment noncompliance and new-onsettype 1 diabetes (32). It is currently not known whether the hyperglycemic AKITA mice develop diabetic ketoacidosis. Due the minimal volume of blood available for analysis (20μl/mouse), we were unable to test the concentration of ketone bodies prior and during the CLP sepsis. Since our aim was to investigate the effects of pre-existing and uncontrolled hyperglycemia due to diabetes, the experimental design did not include the administration of insulin. Transient acute hyperglycemia is frequent in critically ill patients without a prior history of diabetes and has been considered as a significant risk factor for poor outcome (33). Although both stress-hyperglycemia and diabetes are characterized by elevations of glucose, their pathogenesis is distinctly different. In non-diabetics, a positive linear correlation between mortality and glucose levels was shown (34). In the same study, a similar increasing trend was reported in the diabetic cohort: patients with BGL over 288 mg/dL had 10% mortality compared to 3% in patients with BGL of 160 mg/dL. Although it was not surprising that hyperglycemia in septic AKITA mice led to decreased survival, such an association should not be oversimplified in diabetic subjects. In the large retrospective observational study by Egi et al. (2008), high BGL (> 200 mg/dL) in diabetics was not associated with a higher risk of all-cause ICU/hospital mortality, unlike in hyperglycemic nondiabetics (34). The Leonidou group (35) provided the most relevant insight regarding the correlation between hyperglycemia at admission due to diabetes and other causes and the mortality rate in septic patients. In the cohort of patients with severe sepsis, a BGL of 300 mg/dL in diabetics corresponded to 25% mortality whereas in non-diabetic patients the lower, stress-induced BGL (200 mg/dL) was associated with 43% mortality. Regardless of the origin, however, hyperglycemia at admission always equaled poorer prognosis compared to normoglycemic septic patients (mortality 14%). Overall, the effects of hyperglycemia may be bi-directional, depending on the source and/or duration of glucose elevation. In sepsis (or any other critical illness), lasting hyperglycemia caused by poorly controlled diabetes may be relatively less detrimental when compared to an identical rise of blood glucose triggered by any temporary derangement of glucose metabolism. It should be noted that hypoinsulinemia may have also affected survival and inflammatory response, since AKITAs did not received insulin in our study.
In summary, we demonstrated that untreated pre-existing type 1 diabetes severely exacerbated early mortality in the murine model of abdominal sepsis. Deaths in diabetic animals were not dependent on the pre-lethal activation of cytokines but coincided with a widespread reduction in the inflammatory response. Sepsis syndromes are characterized by distinct inflammatory features and this profile can be modified depending on the source of the infectious challenge and type of comorbidity. Our results clearly indicate that a pre-existing co-morbidity (diabetes) substantially alters the cytokine profile during sepsis.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grant GM67189, GM50401 and DE016933.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Crit Care Med. 2006;34:2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remick DG. Cytokine therapeutics for the treatment of sepsis: why has nothing worked? Curr Pharm Des. 2003;9:75–82. doi: 10.2174/1381612033392567. [DOI] [PubMed] [Google Scholar]
- 5.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 6.Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, Van Dyke TE. Chronic hyerglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol. 2006;177:7250–7256. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- 8.Basu R, Oudit GY, Wang X, Zhang L, Ussher JR, Lopaschuk GD, Kassiri Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am J Physiol Heart Circ Physiol. 2009 Dec;297(6):H2096–108. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt RE, Green KG, Snipes LL, Feng D. Neuritic dystrophy and neuronopathy in Akita (Ins2(Akita)) diabetic mouse sympathetic ganglia. Exp Neuro. 2009;216:207–218. doi: 10.1016/j.expneurol.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber AJ, Antonetti DA, Kern TS, Reiter CE, Soans RS, Krady JK, Levison SW, Gardner TW, Bronson SK. The Ins2Akita mouse as a model of early retinal complications in diabetes. Invest Ophthalmol Vis Sci. 2005;46:2210–2218. doi: 10.1167/iovs.04-1340. [DOI] [PubMed] [Google Scholar]
- 11.Geerlings SE. Urinary tract infections in patients with diabetes mellitus: epidemiology, pathogenesis and treatment. Int J Antimicrob Agents. 2008;31 (Suppl 1):S54–S57. doi: 10.1016/j.ijantimicag.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 12.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 13.Stoeckle M, Kaech C, Trampuz A, Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med Wkly. 2008;138:512–519. doi: 10.4414/smw.2008.12228. [DOI] [PubMed] [Google Scholar]
- 14.Dandona P, Chaudhuri A, Mohanty P, Ghanim H. Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care. 2007;10:511–517. doi: 10.1097/MCO.0b013e3281e38774. [DOI] [PubMed] [Google Scholar]
- 15.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Anti-inflammatory effects of insulin and the pro-inflammatory effects of glucose. Semin Thorac Cardiovasc Surg. 2006;18:293–301. doi: 10.1053/j.semtcvs.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Jain SK, Kannan K, Lim G, Matthews-Greer J, McVie R, Bocchini JA., Jr Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26:2139–2143. doi: 10.2337/diacare.26.7.2139. [DOI] [PubMed] [Google Scholar]
- 17.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 18.Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care. 2009;13:R18. doi: 10.1186/cc7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulmé R, Lepage E, Le Gall R. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–121. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24 (Suppl 1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 21.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 22.Osuchowski MF, Welch K, Yang H, Siddiqui J, Remick DG. Chronic sepsis mortality characterized by an individualized inflammatory response. J Immunol. 2007;179:623–630. doi: 10.4049/jimmunol.179.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight PR, Sreekumar A, Siddiqui J, Laxman B, Copeland S, Chinnaiyan A, Remick DG. Development of a sensitive microarray immunoassay and comparison with standard enzyme-linked immunoassay for cytokine analysis. Shock. 2004;21:26–30. doi: 10.1097/01.shk.0000101668.49265.19. [DOI] [PubMed] [Google Scholar]
- 24.Geerlings SE, Brouwer EC, Van Kessel KC, Gaastra W, Stolk RP, Hoepelman AI. Cytokine secretion is impaired in women with diabetes mellitus. Eur J Clin Invest. 2000;30:995–1001. doi: 10.1046/j.1365-2362.2000.00745.x. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira Martins J, Meyer-Pflug AR, Alba-Loureiro TC, Melbostad H, Costa da Cruz JW, Coimbra R, Curi R, Sannomiya P. Modulation of lipopolysaccharide-induced acute lung inflammation: Role of insulin. Shock. 2006;25:260–266. doi: 10.1097/01.shk.0000194042.18699.b4. [DOI] [PubMed] [Google Scholar]
- 26.Alba-Loureiro TC, Pithon-Curi TC, Curi R. Reduced cytokine production by glycogen-elicited peritoneal cells from diabetic rats. Shock. 2008;30:308–310. doi: 10.1097/SHK.0b013e318164e834. [DOI] [PubMed] [Google Scholar]
- 27.Bouma G, Nikolic T, Coppens JM, van Helden-Meeuwsen CG, Leenen PJ, Drexhage HA, Sozzani S, Versnel MA. NOD mice have a severely impaired ability to recruit leukocytes into sites of inflammation. Eur J Immunol. 2005;35:225–235. doi: 10.1002/eji.200425513. [DOI] [PubMed] [Google Scholar]
- 28.Jacob A, Steinberg ML, Yang J, Dong W, Ji Y, Wang P. Sepsis-induced inflammation is exacerbated in an animal model of type 2 diabetes. Int J Clin Exp Med. 2008;1:22–31. [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks HF, Osabutey CK, Moss RF, Andrews PL, Davies DC. Caecal ligation and puncture in the rat mimics the pathophysiological changes in human sepsis and causes multi-organ dysfunction. Metab Brain Dis. 2007;22:353–373. doi: 10.1007/s11011-007-9058-1. [DOI] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 31.Pape HC, van Griensven M, Rice J, Gänsslen A, Hildebrand F, Zech S, Winny M, Lichtinghagen R, Krettek C. Major secondary surgery in blunt trauma patients and perioperative cytokine liberation: determination of the clinical relevance of biochemical markers. J Trauma. 2001;50:989–1000. doi: 10.1097/00005373-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Lebovitz HE. Diabetic ketoacidosis. Lancet. 1995;345:767–772. doi: 10.1016/s0140-6736(95)90645-2. [DOI] [PubMed] [Google Scholar]
- 33.Cheung NW, Li S, Ma G, Crampton R. The relationship between admission blood glucose levels and hospital mortality. Diabetologia. 2008;51:952–955. doi: 10.1007/s00125-008-1001-4. [DOI] [PubMed] [Google Scholar]
- 34.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Hegarty C, Bailey M. Blood glucose concentration and outcome of critical illness: the impact of diabetes. Crit Care Med. 2008;36:2249–2255. doi: 10.1097/CCM.0b013e318181039a. [DOI] [PubMed] [Google Scholar]
- 35.Leonidou L, Michalaki M, Leonardou A, Polyzogopoulou E, Fouka K, Gerolymos M, Leonardos P, Psirogiannis A, Kyriazopoulou V, Gogos CA. Stress-induced hyperglycemia in patients with severe sepsis: a compromising factor for survival. Am J Med Sci. 2008;336:467–471. doi: 10.1097/MAJ.0b013e318176abb4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.