Summary
The human pathogen Staphylococcus aureus has a plethora of virulence factors that promote its colonization and survival in the host. Among such immune modulators are staphylococcal superantigen-like (SSL) proteins, comprising a family of 14 small, secreted molecules that seem to interfere with the host innate immune system. SSL7 has been described to bind immunoglobulin A (IgA) and complement C5, thereby inhibiting IgA-FcαRI binding and serum killing of E. coli. As C5a generation, in contrast to C5b-9-mediated lysis, is crucial for immune defense against staphylococci, we investigated the impact of SSL7 on staphylococcal-induced C5a-mediated effects. Here, we show that SSL7 inhibits C5a generation induced by staphylococcal opsonization, slightly enhanced by its IgA-binding capacity. Moreover, we demonstrate a strong protective activity of SSL7 against staphylococcal clearance in human whole blood. SSL7 strongly inhibited the C5a-induced phagocytosis of S. aureus and oxidative burst in an in vitro whole blood inflammation model. Furthermore, we found that SSL7 affects all three pathways of complement activation and inhibits the cleavage of C5 by interference of its binding to C5 convertases. Finally, SSL7 effects were also demonstrated in vivo. In a murine model of immune complex peritonitis, SSL7 abrogated the C5a-driven influx of neutrophils in mouse peritoneum.
Keywords: Staphylococcus aureus, SSL7, complement evasion, C5a, IgA
Introduction
Cellular and humoral immune responses are important defense mechanisms that protect the host from invading pathogens and promote their clearance (Beutler, 2004). Complement is a major defense system that is initiated via three different pathways. The classical pathway is triggered by antibody-bound bacteria, while the lectin pathway specifically recognizes microbial sugar moieties. The alternative pathway is activated spontaneously or amplifies complement on bacteria previously opsonized via the classical and lectin pathways (Gasque, 2004; Walport, 2001). All pathways converge at the level of C3 by the formation of C3 convertases. The C3 convertases are responsible for the generation of C3b and iC3b, two components that mediate bacterial uptake by phagocytes via complement receptors. Incorporation of another C3b molecule into existing C3 convertases generates C5 convertases that cleave C5, resulting in the release of C5a and the sequential deposition of C5b-C9, leading to the formation of the so-called membrane attack complex (Pangburn and Rawal, 2002). This complex is responsible for direct killing of Gram-negative bacteria through osmotic lysis but is ineffective against Gram-positive bacteria that resist this response due to their thick peptidoglycan cell wall (Muller-Eberhard, 1986). The C5-cleavage product C5a is a proinflammatory molecule that attracts phagocytes to the site of infection, and primes them for bacterial uptake. Thus, generation of C5a is important for the clearance of Gram-positive bacteria (Mullaly and Kubes, 2006; Bohnsack et al., 1997; Easmon and Glynn, 1976).
Staphylococcus aureus is an important human pathogen that causes community- as well as hospital-acquired infections. It is well-known for its suppurative diseases such as skin-limited abscesses and boils, but also causes endocarditis, pneumonia, sepsis, and toxic shock syndrome. Its pathology is linked to its arsenal of immune evasive molecules (Chavakis et al., 2007). In addition to molecules targeting effector cells, S. aureus also secretes several small proteins that specifically interfere with the activity of the complement system (Rooijakkers and van Strijp, 2007). Staphylococcal complement inhibitor (SCIN) targets C3 convertases (Rooijakkers et al., 2005), while extracellular fibrinogen binding (Efb) and extracelullar complement binding (Ecb, also known as Ehp) proteins interact with C3b-containing convertases (Jongerius et al., 2007; Hammel et al., 2007). Thereby, these proteins effectively inhibit complement-mediated innate immune responses. The importance of interference with C5a-mediated responses was not only demonstrated for Ecb and Efb, but also for chemotaxis inhibitory protein of S. aureus (CHIPS) (de Haas et al., 2004). CHIPS not only targets the C5a receptor (C5aR), but also binds the formyl peptide receptor (FPR). Thereby, CHIPS effectively blocks neutrophil chemotaxis towards C5a and formylated peptides. The staphylococcal superantigen-like (SSL) 7 binds immunoglobulin A (IgA) and complement component C5 (Langley et al., 2005). The two-domain structure of SSL7 is homologous to superantigens, but it does not show superantigen activity (Al-Shangiti et al., 2005). The N-terminal domain of SSL7 binds IgA and blocks IgA binding to the FcαR (Ramsland et al., 2007).
The binding of SSL7 to C5 has been shown to inhibit complement-mediated cell lysis of erythrocytes and E. coli, presumably by blocking the C5b-9 formation (Langley et al., 2005). As SSL7 is produced by staphylococci that resist C5b-9, this study focused on the effects of SSL7 generation of C5a during opsonization of S. aureus and on complement-mediated clearance of staphylococci in vitro. We show a strong protective activity of SSL7 against staphylococcal clearance in human whole blood, independent of its IgA binding capacity. Finally, SSL7 also showed strong activity in vivo in a murine model of immune complex peritonitis.
Results
SSL7 inhibits C5a generation independent of IgA binding
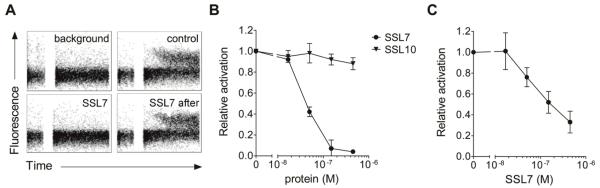
Langley et al. (Langley et al., 2005) have described that SSL7 binds C5 and inhibits serum lysis of E. coli and erythrocytes, most likely by preventing the cleavage of C5 and therefore the subsequent C5b-9-mediated cell lysis. However, C5b-9-mediated lysis does not play a role in the innate immune response to S. aureus as the thick peptidoglycan layer of the bacterium protects it from lysis. On the other hand, the generation of C5a is important in the innate immune defense during staphylococcal infections. To investigate whether SSL7 production by S. aureus inhibits generation of C5a, we incubated 10% human serum with S. aureus for 30 min. This leads to the opsonization of the bacterium with C3b and generation of C5 convertases on the bacterial surface that cleave C5 into C5a and C5b. After pelleting of bacteria, the supernate was tested for its C5a content using a calcium mobilization assay with U937 cells expressing the C5aR. Serum alone did not induce a calcium mobilization, whereas a clear response was observed when bacteria were added to the serum (Fig. 1A). Addition of SSL7 during bacterial opsonization completely inhibited this C5a-mediated response. In contrast, SSL7 could not inhibit the C5a-mediated response in U937-C5aR cells when it was added to the supernate after bacterial opsonization (Fig. 1A). This indicates that SSL7 acts during bacterial opsonization by binding C5 and does not bind and inhibit C5a itself nor its cellular C5aR. Figure 1B shows the same results with a concentration range of SSL7. Already 150 nM (equals 3 μg/ml) of SSL7, added during bacterial opsonization, could completely inhibit the C5a-mediated response. To investigate the specificity of SSL7 in this response, we also tested SSL10 in this assay. This staphylococcal protein is highly homologous to SSL7 and binds the chemokine receptor CXCR4 (Walenkamp et al., 2009). In contrast to SSL7, SSL10 did not affect the C5a generation (Fig. 1B). Thus, SSL7 prevents cleavage of C5 and thereby abrogates the generation of C5a during staphylococcal opsonization by complement.
Fig. 1.
SSL7 inhibits generation of C5a during staphylococcal opsonisation. (A,B) Staphylococci were treated with 10% human serum for 30 minutes at 37°C in the presence of 0 – 450 nM SSL7 or SSL10. In one case (“SSL7 after”), 450 nM SSL7 was added to serum supernate after opsonisation. C5a generation was measured by using supernates as stimuli for calcium mobilization in U937-C5aR cells. Results depict in (A) representative calcium mobilization plots and in (B) mean values ± SEM of three independent experiments and are expressed relative to cells treated with supernate without staphylococcal proteins. In (A), “background” and “control” represent cells stimulated with untreated serum and serum after opsonisation without SSL7, respectively. (C) Cell-bound C5 convertases were constructed on staphylococci by initial deposition of C3b and subsequent addition of soluble fD, fB, and properdin. C5 was subsequently added in the presence of 0 – 450 nM SSL7, and C5a generation in the absence of IgA was analysed by using supernates as stimuli for calcium mobilization in U937-C5aR cells. Results represent mean values ± SEM of three independent experiments and are expressed relative to cells treated with supernate without SSL7.
In addition to C5 binding, SSL7 is described to bind IgA (Langley et al., 2005). The interaction with IgA may be of importance in its ability to prevent the cleavage of C5. To examine whether IgA contributes to the C5-inhibitory activity of SSL7, we determined the generation of C5a in an environment lacking IgA. For this purpose, S. aureus was first incubated with human serum for full opsonization. Subsequently, the bacteria were washed extensively and surface-bound C2a and Bb of the generated C3 and C5 convertases were allowed to dissociate to obtain C3b-covered bacteria. After washing, soluble purified C3, factor B, factor D, and properdin were added. Factor D cleaves factor B into activate Bb that interacts with deposited C3b to generate cell-bound C5 convertases, which are stabilized by properdin. After addition of C5 in the presence or absence of SSL7, the generation of C5a by the C5 convertases was evaluated by testing the supernates using the C5a-mediated calcium mobilization in U937-C5aR cells. Under these conditions, in which IgA was not present, SSL7 still effectively inhibited the generation of C5a (Fig. 1C), although its effect was somewhat reduced as compared to Fig. 1B.
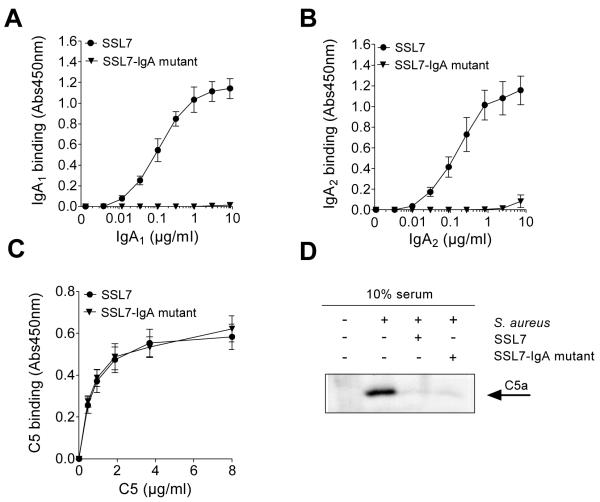
To further evaluate the requirement of IgA for SSL7-induced C5-inhibition, an SSL7 variant lacking IgA-binding capacity was constructed. SSL7 mutants with the single point mutations L79A, N38T, and P82A are described to have 91-, 35-, and 35-fold reduced IgA affinities, respectively (Ramsland et al., 2007). Here, we made a mutant of SSL7 with all three single mutations (SSL7-IgA mutant). This SSL7-IgA mutant did not bind IgA1 or IgA2 (Fig. 2A,B), but retained full C5-binding capacity (Fig. 2C), as compared to the wild type SSL7 protein. The effect of the SSL7-IgA mutant was tested in the S. aureus serum opsonization assay. In this case, C5a generated in the supernate after opsonization of the bacteria was examined by Western blotting. As depicted in Figure 2D, C5a could clearly be detected in the supernate after opsonization of S. aureus with serum, in contrast to serum alone. Addition of SSL7 as well as SSL7-IgA mutant to serum during opsonization resulted in a clear inhibition of C5a generation. Therefore, these results demonstrate that SSL7 does not require IgA binding for its C5-inhibitory effect.
Fig. 2.
SSL7 effects are not dependent on IgA binding. (A-C) ELISA experiments showing binding of IgA1 (A), IgA2 (B), or C5 (C) to wells coated with 450 nM SSL7 and SSL7-IgA mutant. Results represent mean values ± SEM of two independent experiments. (D) Staphylococci were opsonized with 10% human serum in the presence of 450 nM SSL7 or SSL7-IgA mutant. Supernates were examined for generated C5a by Western blotting. Representative of three experiments.
SSL7 inhibits the C5a-dependent clearance of S. aureus in an in vitro whole blood inflammation model
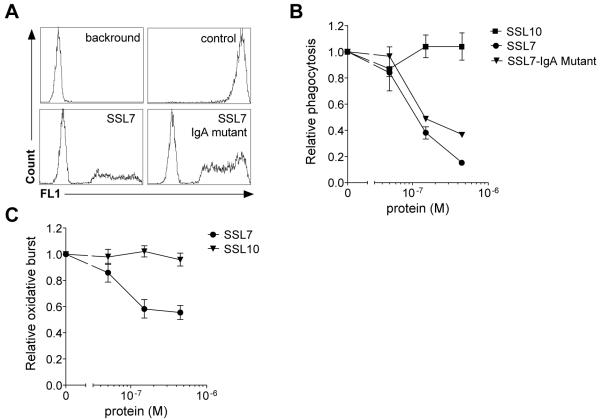
The generation of C5a after staphylococcal opsonization is crucial for a proper innate immune response to the bacteria. First, C5a induces a chemotactic gradient for attraction of neutrophils to the site of infection. Next, it activates neutrophils for enhanced phagocytosis and oxidative burst leading to proper clearance of the bacteria. Using a C5a-dependent whole blood inflammation model (Rooijakkers et al., 2006; Mollnes et al., 2002), we tested the effect of SSL7 on C5a-mediated phagocytosis of FITC-labeled S. aureus. In the absence of SSL7, we observed strong phagocytosis of staphylococci by neutrophils (Fig. 3A). When phagocytosis was measured with SSL7 or SSL7-IgA mutant added to whole blood, both proteins dose-dependently inhibited phagocytosis of staphylococci by neutrophils (Fig. 3 A,B). Wild type SSL7, still able to bind IgA, showed a higher activity than the SSL7-IgA mutant. Subsequently, the oxidative burst was determined upon addition of bacteria to whole blood by measuring the reactive oxygen species generated in response to bacterial recognition. SSL7 also clearly inhibited the oxidative burst induced by S. aureus (Fig. 3C). Neither the S. aureus-induced oxidative burst nor the phagocytosis was affected by SSL10 under the same conditions (Fig. 3A,C), demonstrating SSL7-specific effects. These experiments indicate a strong protective activity of SSL7 against staphylococcal clearance in human whole blood.
Fig. 3.
SSL7 inhibits phagocytosis and generation of oxidative burst in whole human blood. (A,B) Phagocytosis of FITC-labeled staphylococci was measured in whole human blood in the presence of 0 – 450 nM SSL7, SSL7-IgA mutant, or SSL10. (A) Representative histograms depicting background neutrophil fluorescence, neutrophil phagocytosis of bacteria without (control) and with 450 nM SSL7 or SSL7-IgA mutant. (B) Results express phagocytosis of FITC-labeled staphylococci in whole human blood treated with staphylococcal proteins relative to control-treated blood and represent mean values ± SEM of three independent experiments. (C) After addition of 0 – 450 nM SSL7 or SSL10 and staphylococci to whole human blood, oxidative burst was measured over time. Results are expressed relative to control-treated cells and represent mean values ± SEM of three independent experiments.
SSL7 prevents binding of C5 to C5 convertases
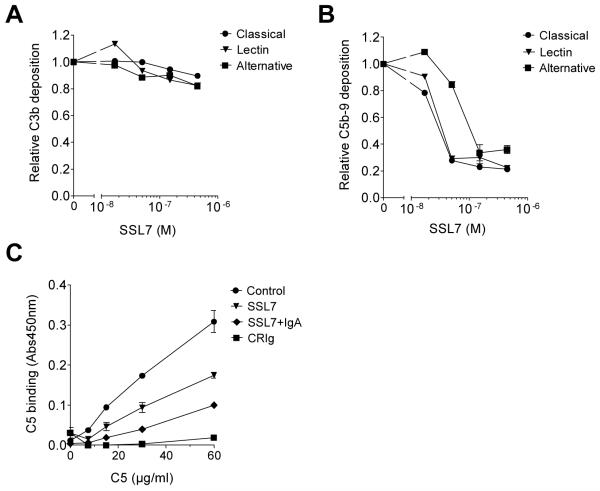
By binding C5, SSL7 could block the generation of C5a through two different mechanisms. SSL7 may prevent the recognition of C5 by C5 convertases, or it may allow for the recognition and binding by C5 convertases but block its subsequent cleavage. Two different C5 convertases exist. C4b2aC3b is the C5 convertase produced upon activation of the classical and lectin pathway of complement, whereas (C3b)2Bb is the C5 convertase of the alternative pathway. Thusfar, the effect of SSL7 on these separate complement pathways, which are all activated upon staphylococcal opsonization, is not known. To gain more insight into the inhibitory mechanism of SSL7, we examined its effect on all three separate complement pathways. Therefore, ELISA-based experiments were performed that examine all three pathways of complement activation individually at the C3 and C5 level by detection with antibodies directed against C3c and C5b-9, respectively. As expected, SSL7 did not affect complement activation when measured at the C3 level (Fig. 4A), but it effectively inhibited all complement pathways when the effects were measured at the C5 level of complement activation (Fig. 4B). This suggests that SSL7 activity is not dependent on one of the two C5 convertases.
Fig. 4.
SSL7 affects binding of C5 to C5 convertases. (A,B) Complement ELISA experiments showing C3b (A) and C5b-9 (B) deposition after complement activation of the classical (5% serum), lectin (5% serum), or alternative (25% serum) pathway in the presence of 0 – 450 nM SSL7. Results are expressed relative to control-treated serum and represent mean values ± SEM of three independent experiments. (C) Binding of C5 to (C3b)2 in the presence of 10 μg/ml CRIg (Complement Receptor of the Immunoglobulin superfamily recognizes C3b molecules), SSL7 (450 nM), or SSL7 (450 nM) and IgA (60 μg/ml). Results represent mean values ± SEM of two independent experiments.
To further address the mechanism of action of SSL7, we determined whether SSL7 could interfere with C5 binding to C5 convertases. For this purpose an ELISA-based experiment was set up using the alternative pathway of complement activation to generate a (C3b)2 surface. For that reason, wells were coated with LPS, and the alternative complement pathway was activated with 30% serum in the presence of EGTA and MgCl2, resulting in the deposition of (C3b)2Bb complexes. The covalently-bound C3b subunits of these C5 convertases are responsible for C5 binding, whereas the non-covalently bound Bb molecules mediate the cleavage of C5. As C5 convertases are very unstable due to the dissociation of the Bb molecule, a sole (C3b)2 surface was generated by allowing Bb molecules to dissociate for 30 min. The created surface enables the analysis of binding the substrate C5 without its cleavage by Bb (Wiesmann et al., 2006). Figure 4C shows that C5 binding to C5 convertases is successful and specific as CRIg, which recognizes C3b molecules, abrogated C5 binding. SSL7 alone inhibited the binding of C5 to the C5 convertases for about 50%, whereas addition of IgA increased the SSL7 activity. Thus, the basis for SSL7-mediated inhibition of C5a generation seems to reside in its effect on C5 binding to C5 convertases. In this effect on C5 binding, IgA increases the SSL7 activity.
SSL7 inhibits complement activation in vivo in a murine inflammatory model
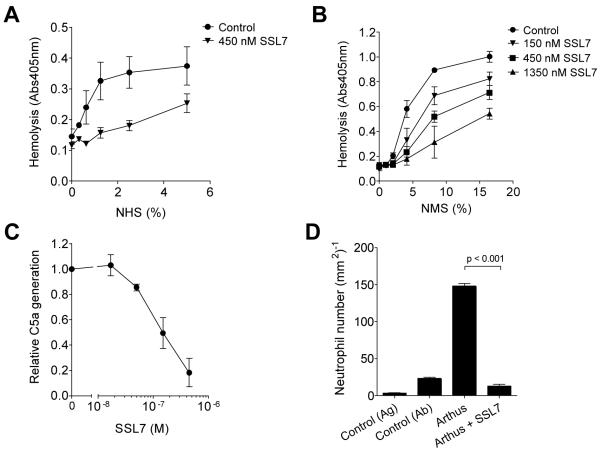
To determine SSL7 effects in vivo, we explored SSL7 activity in a relevant mouse disease model. First, the interaction of SSL7 with the mouse complement system was investigated. Previous studies already demonstrated that SSL7 binding to C5 was not human specific (Langley et al., 2005); SSL7 is reported to bind C5 from human, chimpanzee, baboon, sheep, pig, rabbit, and goat serum, while binding to mouse, cow, and horse C5 could not be observed. Nevertheless, we assessed the effects of SSL7 on complement activation in mouse serum through a functional assay that examines the classical pathway-mediated hemolysis. Herein, opsonization of erythrocytes with antibodies prior to incubation with serum leads to complement-mediated lysis. In human serum, SSL7 inhibited the complement-mediated hemolysis (Fig. 5A). SSL7 also effectively blocked hemolytic complement activation in mouse serum (Fig. 5B). To further characterize the effect of SSL7 on mouse C5 cleavage, C5a generation after bacterial opsonization was measured. Addition of SSL7 during bacterial opsonization in mouse serum effectively blocked the generation of C5a (Fig. 5C). SSL7 thus interacts with mouse complement C5, and its functional activity cannot only be measured in human immune assays but also in mouse models.
Fig. 5.
SSL7 inhibits C5a generation in vivo. (A and B) Classical complement pathway-mediated hemolysis of sheep (A) or rabbit (B) red blood cells in human (A) or mouse (B) serum preincubated with SSL7. Results represent mean values ± SEM of three independent experiments. NHS and NMS stand for normal human serum and normal mouse serum, respectively. (C) Staphylococci were treated with 30% mouse serum for 30 minutes at 37°C in the presence of 0 – 450 nM SSL7. C5a generation was measured by using supernates as stimuli for calcium mobilization in U937-C5aR cells. Results depict mean values ± SEM of four independent experiments and are expressed relative to cells treated with supernate without staphylococcal proteins. (D) In the reverse passive Arthus reaction peritonitis model, peritoneal cavity lavage was performed six hours after challenge of mice with immune complexes. SSL7 was injected 30 minutes before challenge with immune complexes or was used as a challenge itself. Collected neutrophils were subsequently quantified. P < 0.001 by analysis of variance (n = 10 mice per group).
Then, to assess the in vivo activity of SSL7, we used the well established model of immune complex-mediated peritonitis (Heller et al., 1999a). In this model, formation of immune complexes in the peritoneum initiates the activation of the classical and alternative pathway of complement. This not only leads to edema and hemorrhage, but also to infiltration of neutrophils to the site of injection. Six hours after in vivo challenge with immune complexes in the peritoneal cavity, neutrophils infiltrated the mouse peritoneum (Fig. 5D). When mice were injected with SSL7 prior to challenge with immune complexes, we observed a complete inhibition of neutrophil accumulation in the peritoneum. Administration of SSL7 did not induce any neutrophil influx into the peritoneum. In conclusion, SSL7 inhibits complement-induced neutrophil influx in vivo.
Discussion
Staphylococcus aureus secretes several virulence factors to modulate immune responses and thereby escapes recognition and clearance by the host (Chavakis et al., 2007). In this paper we demonstrate that the C5-binding protein SSL7 inhibits complement-mediated processes important for staphylococcal clearance. In an ex vivo whole human blood inflammatory model, SSL7 not only inhibited the generation of reactive oxygen species by phagocytes in response to staphylococci but also affected staphylococcal uptake by phagocytes. Previous experiments with this whole blood model demonstrated that the complement-mediated S. aureus- and E. coli-induced oxidative burst heavily depends on the generation of C5a and the C5a-C5aR interaction (Rooijakkers et al., 2006; Mollnes et al., 2002). Furthermore, this study is the first to provide evidence for the anti-inflammatory activity of SSLs in an animal model.
Human C5 is a glycoprotein of 196 kDa that is secreted by the liver into plasma (Fredslund et al., 2008; Fernandez and Hugli, 1978). It comprises an α-chain and a β-chain that are linked by a disulfide bridge. C5 convertases cleave C5 in the α-chain at Arg751-Leu752 to generate the small C5a of 8.2 kDa and the large C5b, which consists of the β-chain and a large part of the α-chain. SSL7 binds C5 and was described to prevent the C5b-9-mediated lysis of E. coli and human erythrocytes (Langley et al., 2005). SSL7 not only targets C5 but concomitantly binds IgA. Both binding of serum and mucosal IgA is described, and SSL7 thereby prevents the interaction of IgA with its receptor FcαRI (Langley et al., 2005). Co-crystallization of SSL7 and IgA demonstrated that SSL7 targets the C2α/C3α interface of IgA, which is also recognized by the FcαRI (Ramsland et al., 2007). Since IgA is found abundantly in the mucosa, SSL7 may be beneficial during colonization by S. aureus. Here, we demonstrate that SSL7 clearly prevents the generation of C5a. Although binding of IgA per se is not needed for the C5 inhibition by SSL7, it clearly adds to its activity. In both the C5a generation assay without IgA as in the phagocytosis assay using the SSL7-IgA mutant, less C5-inhibiting activity was found than in situations where IgA was present or wild type SSL7 was used. The same was true in the C5-C5 convertase binding assay, in which IgA increased the activity of SSL7. Therefore, we conclude that SSL7, with additional help of IgA, prevents C5 activation by inhibiting the binding of C5 to C5 convertases. We have tried to investigate the effect of SSL7 on C5 binding to the C5 convertase of the classical and alternative pathway (C4b2aC3b) as well but have failed by lack of specificity in this assay. During the reviewing process a publication by Laursen et al. of the crystal structure of the SSL7-C5 complex appeared showing that SSL7 binds to C5 far from the C5a binding site (Laursen et al., 2010). They observed a slightly diminished activity of their SSL7-IgA mutant on C5a generation, comparable to our results. Therefore, they proposed that IgA most likely adds to C5-cleavage inhibition through steric hindrance. Here, we show that SSL7 does not seem to inhibit C5 cleavage but rather inhibits C5 binding to C5 convertases. Indeed, as suggested by Laursen et al., IgA binding possibly aids in the inhibition by steric hindrance, as binding of IgA makes the C5-SSL7 complex more than twice as large.
Previous experiments pertaining to species specificity of SSL7 demonstrated that this protein was not human specific but also binds C5 of several other species (Langley et al., 2005). Binding to murine C5, however, was not observed. In our in vitro and in vivo mouse models, SSL7 did impede the C5-driven processes. It is well-documented that mice often show genetic deficiency of C5. In fact, up to 39% of common inbred murine strains carry this deficiency and lack detectable C5 (Cinader et al., 1964). The initial study describing the inability of SSL7 to interact with murine C5 did not document the mouse strain used for serum isolation. In our experiments, we made use of BALB/c serum and BALB/c mice, which express functional C5 (Cinader et al., 1964).
To test the in vivo activity of SSL7 we selected an inflammatory model that is highly C5a-dependent. In the Arthus reaction, the formation of immune complexes propagates an immune reaction by complement activation. Hereby, C5a and the C5aR are essential effectors in the observed complement-mediated inflammatory reactions (Godau et al., 2004; Heller et al., 1999b). Administration of SSL7 fully blocked neutrophil influx into the mouse peritoneum in this model. SSL7 is therefore highly anti-inflammatory in this model of immune complex disease. Although C5a acts as a proinflammatory signal, aberrant expression has been implicated in several chronic and acute diseases such as stroke, reperfusion/ischemia, transplant rejection, rheumatoid arthritis, and tumor progression (Ricklin and Lambris, 2007). SSL7 thus may have a therapeutic potential as an anti-inflammatory compound in a variety of diseases and disease states. It must be noted however that, because of its bacterial origin, SSL7 is immunogenic, and identification of mimics that are less immunogenic but retain the same specificity and activity would make it more suitable as therapeutic agent.
Since C5 plays a role in bacterial clearance once the bacterium reaches the host tissue, C5-binding SSL7 probably plays an important role during the colonization and infection of S. aureus. The presence of antibodies directed against SSL7 in human serum (Arcus et al., 2002) indicates production of SSL7 by the bacterium when it gets into contact with the host. SaPI2 encoding the main ssl cluster is present in all clinical S. aureus isolates, but its individual gene composition is variable (Fitzgerald et al., 2003). Analysis of many S. aureus isolates from sheep, cows, and poultry demonstrated that this cluster is also present in all the animal isolates tested so far. For SSL7 specifically, it is described that the gene encoding for this protein is found in 90% of clinical isolates (Monecke et al., 2008) and is present in all of the tested animal isolates from chicken, sheep, goat, rabbit, and camel (Shuiep et al., 2008; Smyth et al., 2007). The high prevalence of ssl7 in S. aureus strains and the production of SSL7 following host contact strongly suggest a beneficial role for SSL7 in bacterial survival within the host environment.
In addition to SSL7, S. aureus secretes several other complement inhibitors (Jongerius et al., 2007; Rooijakkers and van Strijp, 2007). SCIN binds C3 convertases and inhibits the cleavage of C3 and all downstream effector functions (Rooijakkers et al., 2005). We have recently described that extracellular complement-binding protein (Ecb) blocks C3b-containing convertases and also effectively inhibits C5a-mediated responses in the mouse model used in this study (Jongerius et al., 2007). Moreover, S. aureus produces CHIPS, a potent C5aR inhibitor (de Haas et al., 2004). Thus, staphylococci have generated a diverse, synergistic system of immune inhibitors that specifically target the complement system. The fact that so many staphylococcal proteins prevent C5a-mediated responses highlights the critical role for C5a in S. aureus killing. The importance of C5a in S. aureus infections has been demonstrated by Mullaly et al. (Mullaly and Kubes, 2006), showing that challenging of mice with live S. aureus in the presence of anti-C5aR antibodies results in significant increase in morbidity as compared to untreated mice. In addition, relevance of C5/C5a during S. aureus infection has been demonstrated in several other mouse models (von Köckritz-Blickwede et al., 2009; Hopken et al., 1996; Cerquetti et al., 1983). The production of many complement inhibitors though complicates the use of an infection model. Therefore, we have chosen to test SSL7 activity in a mouse model that is fully dependent on the cleavage of C5a from C5. Currently, several S. aureus knock-out strains are constructed for future infection studies. These will provide us with a better understanding of the individual or joint contributions of the distinct staphylococcal C5a inhibitors.
Experimental procedures
Cloning, expression and purification of SSL7
The genes encoding for SSL7 and SSL10 were cloned from S. aureus strain NCTC8325. SSL10 was cloned and expressed as described previously for SSL5 (Bestebroer et al., 2007). For the expression of SSL7 lacking a histidine tag, ssl7 was cloned into the expression vector pRSETB (Invitrogen) using the NdeI and BglII restriction sites. SSL7 with L79A, N38T, and P82A point mutations (SSL7-IgA mutant) was generated using overlap extension PCR and cloned in the same way as SSL7. Amplified products were verified by sequencing. SSL7 and SSL7-IgA mutant were expressed in Rosetta-Gami(DE3)pLysS E. coli according to the manufacturer’s protocol (Novagen, Dormstadt, Germany). The expressed SSL7 proteins were purified from inclusion bodies using CelLytic B Cell Lysis Reagent (Sigma), according to the manufacturer’s description. Shortly, bacterial cells were lysed in CelLytic B Cell Lysis Reagent containing EDTA-free protease inhibitor cocktail tablets (Roche Diagnostics), 200 μg/ml lysozym, 20 μg/ml DNase and RNase (Roche), and 1.25 mM EDTA. After lysis, the inclusion bodies were pelleted down and washed twice with 0.5% LDAO (lauryl dimethyldodecylamine N-oxide; Sigma). Then, the inclusion bodies were dissolved in 50 mM Tris, 6 M Guanidine pH 10. The isolated SSL7 proteins were properly refolded by dialysis in 50 mM Tris pH 10 and pH 9 for 24 h and 16 h, respectively. Finally, the pH of the dialysate was lowered in 0.2 steps, of each 2 h, to reach pH 8. Then SSL7 was rebuffered to 20 mM sodiumphosphate pH 7 for further purification using ion exchange chromatography on a HiTrap-SP XL column. Finally, all isolated proteins were rebuffered to PBS and estimated to be more than 95% pure when assayed on SDS-polyacrylamide gel electrophoresis.
C5a generation
Generation of C5a during opsonization was performed with laboratory S. aureus strain Wood. Bacteria were grown in Luria Bertani (LB) medium to an OD660nm of 0.5 and heat killed for 30 minutes at 70°C. Bacteria (1 × 107) were incubated with 10 % normal human serum (NHS)or fresh 30% normal mouse serum from CD-1 mice (NMS) in presence or absence of SSL7 or SSL7-IgA mutant for 30 minutes at 37°C (total volume: 100 μl). The bacteria were spun down, and the supernates were tested for presence of C5a by supernate-induced calcium-mobilization of U937-C5aR cells (a generous gift from Dr. E. Prossnitz, University of New Mexico, Albuquerque, NM). When indicated, SSL7 was added to the spun-down supernates after opsonization. For calcium mobilization, U937-C5aR cells were labeled with 2 μM Fluo-3-AM, a fluorescent probe for free intracellular calcium. Calcium mobilization was measured with a flow cytometer (FACSCalibur; Becton Dickinson, Franklin Lanes, NJ) for 10 seconds before and 40 seconds after stimulation with diluted supernates. Stimulation was determined at 5 seconds after stimulation and is expressed as the relative calcium increase of SSL7-treated cells compared to untreated cells.
Supernates were also tested for presence of C5a by analyzing the samples by SDS-PAGE and Western blotting. C5a was detected by staining the blots with polyclonal anti-C5a (Calbiochem, San Diego, CA) and goat anti-rabbit-HRP (Southern Biotechnology, Birmingham, AL).
To assess the importance of IgA binding to SSL7, C5a generation via bacterial opsonization was also tested in an environment deficient of IgA. Therefore, cleavage of purified C5 was assessed by bacterium-bound C5 convertases. To prepare C3b-covered bacteria, S. aureus was incubated with 20% human sera for 30 min in HBS2+ (Hepes-buffered saline, 20 mM Hepes, 140 mM NaCl, 5 mM CaCl2, and 2.5 mM MgCl2). After extensive washing, surface-bound C2a/Bb were allowed to dissociate for 30 min in PBS. To create C5 convertases, C3b-covered bacteria were incubated with 100 μg/ml fB, 0.75 μg/ml fD, and 4 μg/ml properdin (Calbiochem) in HBS2+ for 30 min at 37°C. C5 cleavage was assessed by adding 25 μg/ml C5 (Calbiochem) in the presence or absence of SSL7. Formation of C5a was measured by analyzing supernates in a calcium mobilization assay as described above.
ELISAs
Complement ELISAs were performed as previously described (Jongerius et al., 2007). Briefly, the lectin pathway was assessed by using immobilized mannan (Saccharomyces cerevisiae, Sigma) as a ligand, while LPS (Salmonella enteriditis, Sigma) and human IgM (Calbiochem) were used for the classical and alternative pathways, respectively. Plates were blocked with 4% BSA/0.05% Tween-20/PBS. Then, serum samples were added for 1h at 37°C. Serum samples were diluted in a buffer containing 20 mM Hepes, 140 mM NaCl, 0.1% gelatin (pH 7.4) to which 5 mM CaCl2 and 2.5 mM MgCl2 was added to assess the lectin and classical pathway, while 5 mM MgCl2 and 10 mM EGTA were added for the alternative pathway. To evaluate SSL7 effect, serum samples were preincubated with SSL7 for 15 minutes at room temperature before adding to the plates. Finally, deposited C3b and C5b-9 were detected using anti-C3c WM1 (ATCC) and anti–C5b-9 (Abcam) antibodies, respectively, followed by peroxidase (PO)-conjugated goat anti–mouse IgG (Southern Biotechnology Associates).
For the analysis of C5 binding to C5 convertases, LPS (20 μg/ml, Salmonella enteriditis, Sigma) was adsorbed to a microtitre plate overnight at 4°C. After blocking with 4% BSA/PBS for 1 h at 37°C, 30% human serum in 20 mM Hepes buffer containing 10 mM EGTA and 5 mM MgCl2 was added for one hour at 37°C for alternative pathway complement deposition. This was repeated with freshly prepared 30% human serum under the same conditions for an additional hour. Surface-bound Bb molecules were allowed to dissociate for 30 min in PBS. A dilution series of C5 was added in the presence or absence of 10 μg/ml SSL7 (430 nM), SSL7 (430 nM) and IgA (60μg/ml) or CRIg (Complement Receptor of the Immunoglobulin superfamily, CRIg-S, Genentech) for 1 h at 37°C. Bound C5 was detected using anti-C5/C5a (Hycult biotechnology, The Netherlands) and peroxidase (PO)-conjugated goat anti–mouse IgG.
To study the binding of SSL7 to C5, IgA1, and IgA2, wells were coated with 430 nM SSL7 or SSL7-IgA mutant and incubated with a dilution series of C5, IgA1, or IgA2 (human anti-sheep erythrocyte immunoglobulin A (Boel et al., 2000). Binding of C5 was detected with anti-C5a (Calbiochem) and goat anti-rabbit IgG-PO (Southern Biotechnologies) and IgA1 and IgA2 with sheep anti-human IgA-PO (ICN Biomedicals).
Phagocytosis
S. aureus was first labeled with FITC by incubating an exponential growth culture with 100 μg/ml FITC for 1 hour at 37°C in 0.1 M carbonate buffer (pH 9.6). Freshly isolated human blood was anticoagulated using 50 μg/ml lepirudin (Refludan, Schering, Kenilworth, NJ). Phagocytosis was performed by incubating FITC-labeled staphylococci (1 × 108) with 50% blood for 25 minutes at 37°C (total volume: 200 μl) under vigorous shaking. The cells were fixed and erythrocytes lysed by addition of BD FACS lysing solution for 15 minutes at room temperature. After washing, phagocytosis was measured with a flow cytometer. Neutrophils were selected through forward and side scatter gating. Phagocytosis is expressed as the mean fluorescence of neutrophils.
Oxidative burst
Freshly isolated human blood was anticoagulated using 50 μg/ml lepirudin. Oxidative burst was initiated upon addition of 100 μl luminol and heat-killed staphylococci (1.5 × 108) to 10% blood in the presence or absence of SSL7 (total volume of 225 μl). The oxidative burst was measured every 30 seconds for 60 minutes at 37°C by chemiluminescence using a luminometer (Berthold Technologies, Bad Wildbag, Germany). Chemiluminescence is expressed as the area under the curve.
Hemolysis assay
In the classical pathway hemolytic assay, NHS and BALB/c mouse serum (NMS, Innovative Research, Novi, MI) were first incubated with sheep or rabbit erythrocytes, respectively, to preclear serum of antibodies directed against erythrocytes. Concurrently, sheep and rabbit erythrocytes were opsonized with anti-erythrocyte IgM. The opsonized rabbit or sheep erythrocytes (2 × 107) were then incubated with 10% precleared NHS or NMS, respectively, in the presence of 0 – 1350 nM SSL7 in HBS2+. After 1 hour at 37 °C, the samples were centrifuged, and the absorbance of the supernatants at 405 nm was measured.
Peritoneal Arthus reaction
The Arthus reaction was initiated upon i.v. injection of BALB/c mice with 100 μl of OVA (20 mg/kg of body weight; Sigma-Aldrich), immediately followed by an i.p. injection of 800 μg of rabbit anti-OVA IgG (MP Biomedicals). In inhibition experiments, 60 μg SSL7 was i.v. and i.p. administered 30 minutes prior to initiation of the Arthus reaction. In control experiments, 60 μg SSL7 was administered i.v. and i.p. in the absence of the Arthus reaction. Mice of different treatment groups were killed 6 hours after the onset of the peritoneal Arthus reaction, and the peritoneal cavity was lavaged with 6 ml of ice-cold 0.1% BSA/PBS. Collected peritoneal cells were washed with PBS, and the cell number was adjusted to 5 × 105 cells/ml. 50 μl of this cell suspension was used to prepare cytospin slides, which were stained with DiffQuick. Neutrophil numbers per square millimeter were calculated from ≥20 different microscopic fields. Animal care was provided in accordance with National Institutes of Health guidelines. Animal studies were approved by either the Bezirksregierung Hannover or the Cincinnati Children’s Hospital Medical Center institutional animal care and use committee.
Acknowledgements
The authors declare no competing financial interests. This work was supported by the Technology Foundation STW (UKG.6609), the Netherlands Organisation for Scientific Research NWO-VENI (916-76-037), the European community (FP6-512093), and the National Institutes of Health (AI059305 to J. Köhl).
Footnotes
Disclosures
The authors declare no competing financial interests.
References
- Al-Shangiti AM, Nair SP, Chain BM. The interaction between staphylococcal superantigen-like proteins and human dendritic cells. Clin Exp Immunol. 2005;140:461–469. doi: 10.1111/j.1365-2249.2005.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcus VL, Langley R, Proft T, Fraser JD, Baker EN. The Three-dimensional structure of a superantigen-like protein, SET3, from a pathogenicity island of the Staphylococcus aureus genome. J Biol Chem. 2002;277:32274–32281. doi: 10.1074/jbc.M203914200. [DOI] [PubMed] [Google Scholar]
- Bestebroer J, Poppelier MJ, Ulfman LH, Lenting PJ, Denis CV, van Kessel KP. Staphylococcal superantigen-like 5 binds PSGL-1 and inhibits P-selectin-mediated neutrophil rolling. Blood. 2007;109:2936–2943. doi: 10.1182/blood-2006-06-015461. el al. [DOI] [PubMed] [Google Scholar]
- Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Boel E, Verlaan S, Poppelier MJ, Westerdaal NA, van Strijp JA, Logtenberg T. Functional human monoclonal antibodies of all isotypes constructed from phage display library-derived single-chain Fv antibody fragments. J Immunol Methods. 2000;239:153–166. doi: 10.1016/s0022-1759(00)00170-8. [DOI] [PubMed] [Google Scholar]
- Bohnsack JF, Widjaja K, Ghazizadeh S, Rubens CE, Hillyard DR, Parker CJ. A role for C5 and C5a-ase in the acute neutrophil response to group B streptococcal infections. J Infect Dis. 1997;175:847–855. doi: 10.1086/513981. el al. [DOI] [PubMed] [Google Scholar]
- Cerquetti MC, Sordelli DO, Ortegon RA, Bellanti JA. Impaired lung defenses against Staphylococcus aureus in mice with hereditary deficiency of the fifth component of complement. Infect Immun. 1983;41:1071–1076. doi: 10.1128/iai.41.3.1071-1076.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis T, Preissner KT, Herrmann M. The anti-inflammatory activities of Staphylococcus aureus. Trends Immunol. 2007;28:408–418. doi: 10.1016/j.it.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Cinader B, DUBISKI S, WARDLAW AC. Distribution, inheritance, and properties of an antigen, MuB1, and its relation to hemolytic complement. J Exp Med. 1964;120:897–924. doi: 10.1084/jem.120.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, van Wamel WJ, Heezius EC. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199:687–695. doi: 10.1084/jem.20031636. el al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easmon CS, Glynn AA. Comparison of subcutaneous and intraperitoneal staphylococcal infections in normal and complement-deficient mice. Infect Immun. 1976;13:399–406. doi: 10.1128/iai.13.2.399-406.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez HN, Hugli TE. Primary structural analysis of the polypeptide portion of human C5a anaphylatoxin. Polypeptide sequence determination and assignment of the oligosaccharide attachment site in C5a. J Biol Chem. 1978;253:6955–6964. [PubMed] [Google Scholar]
- Fitzgerald JR, Reid SD, Ruotsalainen E, Tripp TJ, Liu M, Cole R. Genome diversification in Staphylococcus aureus: Molecular evolution of a highly variable chromosomal region encoding the Staphylococcal exotoxin-like family of proteins. Infect Immun. 2003;71:2827–2838. doi: 10.1128/IAI.71.5.2827-2838.2003. el al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredslund F, Laursen NS, Roversi P, Jenner L, Oliveira CL, Pedersen JS. Structure of and influence of a tick complement inhibitor on human complement component 5. Nat Immunol. 2008;9:753–760. doi: 10.1038/ni.1625. el al. [DOI] [PubMed] [Google Scholar]
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Godau J, Heller T, Hawlisch H, Trappe M, Howells E, Best J. C5a initiates the inflammatory cascade in immune complex peritonitis. J Immunol. 2004;173:3437–3445. doi: 10.4049/jimmunol.173.5.3437. el al. [DOI] [PubMed] [Google Scholar]
- Hammel M, Sfyroera G, Pyrpassopoulos S, Ricklin D, Ramyar KX, Pop M. Characterization of Ehp, a secreted complement inhibitory protein from Staphylococcus aureus. J Biol Chem. 2007;282:30051–30061. doi: 10.1074/jbc.M704247200. el al. [DOI] [PubMed] [Google Scholar]
- Heller T, Gessner JE, Schmidt RE, Klos A, Bautsch W, Kohl J. Cutting edge: Fc receptor type I for IgG on macrophages and complement mediate the inflammatory response in immune complex peritonitis. J Immunol. 1999a;162:5657–5661. [PubMed] [Google Scholar]
- Heller T, Hennecke M, Baumann U, Gessner JE, zu Vilsendorf AM, Baensch M. Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J Immunol. 1999b;163:985–994. el al. [PubMed] [Google Scholar]
- Hopken UE, Lu B, Gerard NP, Gerard C. The C5a chemoattractant receptor mediates mucosal defence to infection. Nature. 1996;383:86–89. doi: 10.1038/383086a0. [DOI] [PubMed] [Google Scholar]
- Jongerius I, Kohl J, Pandey MK, Ruyken M, van Kessel KP, van Strijp JA, Rooijakkers SH. Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med. 2007;204:2461–2471. doi: 10.1084/jem.20070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley R, Wines B, Willoughby N, Basu I, Proft T, Fraser JD. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc alpha RI binding and serum killing of bacteria. J Immunol. 2005;174:2926–2933. doi: 10.4049/jimmunol.174.5.2926. [DOI] [PubMed] [Google Scholar]
- Laursen NS, Gordon N, Hermans S, Lorenz N, Jackson N, Wines B. Structural basis for inhibition of complement C5 by the SSL7 protein from Staphylococcus aureus. Proc Natl Acad Sci U S A. 2010;107:3681–3686. doi: 10.1073/pnas.0910565107. el al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollnes TE, Brekke OL, Fung M, Fure H, Christiansen D, Bergseth G. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. el al. [PubMed] [Google Scholar]
- Monecke S, Slickers P, Ehricht R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol Med Microbiol. 2008;53:237–251. doi: 10.1111/j.1574-695X.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- Mullaly SC, Kubes P. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J Immunol. 2006;177:8154–8163. doi: 10.4049/jimmunol.177.11.8154. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard HJ. The membrane attack complex of complement. Annu Rev Immunol. 1986;4:503–528. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- Pangburn MK, Rawal N. Structure and function of complement C5 convertase enzymes. Biochem Soc Trans. 2002;30:1006–1010. doi: 10.1042/bst0301006. [DOI] [PubMed] [Google Scholar]
- Ramsland PA, Willoughby N, Trist HM, Farrugia W, Hogarth PM, Fraser JD, Wines BD. Structural basis for evasion of IgA immunity by Staphylococcus aureus revealed in the complex of SSL7 with Fc of human IgA1. Proc Natl Acad Sci U S A. 2007;104:15051–15056. doi: 10.1073/pnas.0706028104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6:920–927. doi: 10.1038/ni1235. el al. [DOI] [PubMed] [Google Scholar]
- Rooijakkers SH, Ruyken M, van RJ, van Kessel KP, van Strijp JA, van Wamel WJ. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol. 2006;8:1282–1293. doi: 10.1111/j.1462-5822.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- Rooijakkers SH, van Strijp JA. Bacterial complement evasion. Mol Immunol. 2007;44:23–32. doi: 10.1016/j.molimm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Shuiep ES, Kanbar T, Eissa N, Alber J, Lammler C, Zschock M. Phenotypic and genotypic characterization of Staphylococcus aureus isolated from raw camel milk samples. Res Vet Sci. 2008 doi: 10.1016/j.rvsc.2008.07.011. el al. [DOI] [PubMed] [Google Scholar]
- Smyth DS, Meaney WJ, Hartigan PJ, Smyth CJ. Occurrence of ssl genes in isolates of Staphylococcus aureus from animal infection. J Med Microbiol. 2007;56:418–425. doi: 10.1099/jmm.0.46878-0. [DOI] [PubMed] [Google Scholar]
- von Köckritz-Blickwede M, Konrad S, Foster S, Gessner JE, Milder FJ. Protective Role of Complement C5a in an Experimental Model of Staphylococcus aureus Bacteremia. J Innante Immun. 2009 doi: 10.1159/000247157. 10.1159/000247157. [DOI] [PubMed] [Google Scholar]
- Walenkamp AM, Boer IG, Bestebroer J, Rozeveld D, Timmer-Bosscha H, Hemrika W. Staphylococcal superantigen-like 10 inhibits CXCL12-induced human tumor cell migration. Neoplasia. 2009;11:333–344. doi: 10.1593/neo.81508. el al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- Wiesmann C, Katschke KJ, Yin J, Helmy KY, Steffek M, Fairbrother WJ. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature. 2006;444:217–220. doi: 10.1038/nature05263. el al. [DOI] [PubMed] [Google Scholar]