Abstract
The present study determined whether the muscle atrophy produced by colitis is associated with altered rates of muscle protein synthesis or degradation, as well as the potential role of the local (e.g., muscle) insulin-like growth factor (IGF) system and muscle-specific ubiquitin E3 ligases atrogin-1 and MuRF1 in mediating altered muscle protein balance. Colitis was induced in C57BL/6 mice by intra-rectal administration of trinitrobenzne sulfonic acid (TNBS), and blood and tissues collected on day 10. Mice with inflammatory bowel disease (IBD) demonstrated reduced skeletal muscle mass and protein content, whereas colonic segment weight and gross damage score were both increased in mice with colitis, compared to time-matched control values. There was no change in muscle protein synthesis in mice with IBD, but there was an increased protein breakdown (45%), proteasome activity (85%), and mRNA expression for atrogin-1 and MuRF1 (200–300%) in muscle. These changes were associated with a reduction in liver (but not muscle) IGF-I mRNA as well as a reduction in both total and free IGF-I in the blood. Colitis decreased the hepatic content of IGF binding protein (IGFBP)-3 mRNA by 40% and increased IGFBP-1 mRNA by 100%. In contrast, colitis did alter IGFBP mRNAs in muscle. The TNFα, IL-6 and NOS2 mRNA content of both liver and skeletal muscle was increased in TNBS-treated mice, and plasma TNFα and IL-6 concentrations were also elevated. These data suggest TNBS-induced colitis is independent of a change in muscle protein synthesis but dependent on stimulation of protein degradation via increased expression of muscle-specific atrogenes, which may be mediated in part by the reduction in circulating concentration of IGF-I and the concomitant increase in inflammatory mediators observed in the blood and muscle per se.
Keywords: IGF-I, MuRF1, atrogin-1, inflammatory cytokines, atrophy
1. Introduction
Impaired linear growth, osteopenia, and reduction in lean body mass (LBM) are commonly seen in children with inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis [1, 2]. IBD-related growth failure and decreased muscle mass have been attributed to a variety of mechanisms including decreased nutrient intake, malabsorption of ingested nutrients, increased metabolic rate, and the inhibitory effects of inflammation on the growth hormone (GH)/insulin-like growth factor (IGF)-I axis [3–5]. Normally, circulating GH binds to trans-membrane GH receptors and activates the transcription of IGF-I in liver and other tissues [6]. The synthesis of circulating IGF-I by liver and its local production by bone and muscle stimulates the proliferation of chondrocytes and osteoblasts in the epiphyseal growth plate of long bones resulting in linear growth [7]. IGF-I also regulates muscle mass by enhancing protein synthesis and slowing the rate of proteolysis [8, 9]. Conversely, both plasma and muscle IGF-I are decreased in response to diverse inflammatory insults which accelerate the loss of muscle protein [9]. The inflammatory cytokines tumor necrosis factor (TNF)-α and interleukin-6 (IL-6) in particular are elevated during IBD [10–12]. Such increases produce hepatic GH resistance and decrease circulating IGF-I which ultimately impairs growth and accretion of LBM [13]. However, tissue IGF-I availability is complex and regulated not only by the concentration of IGF-I per se but also by changes in either the amount or type(s) of various IGF binding proteins (IGFBPs) produced by the liver or target organ (e.g., muscle) [14]. For example, elevations in IGFBP-1 effectively inhibit the ability of IGF-I to stimulate muscle protein synthesis [15, 16]. While changes in the circulating concentration of several IGFBPs have been reported in patients with IBD [17, 18], no comparable information is available in rodent models of IBD and whether the synthesis of the various IGFBPs differs between liver and muscle in this inflammatory condition is unknown.
Muscle catabolism is multifactorial being caused by both reduced protein synthesis and increased protein degradation. Previous studies which have directly assessed muscle protein synthesis have yielded conflicting results, with muscle protein synthesis being decreased in rats receiving dextran sulfate sodium (DSS) [19] and increased in HLA-B27 rats which develop spontaneous IBD [20]. Furthermore, there are essentially no data on muscle protein degradation in IBD and colitis. Current evidence indicates that the ubiquitin (Ub)-proteasome dependent pathway is the most prevalent mechanism regulating muscle proteolysis during systemic inflammation [21]. This pathway identifies specific proteins for degradation through a series of enzymatic reactions which attach a polyubiquitin chain to the target peptide, resulting in its proteasomal degradation [22]. The targeting process involves the coordinating activity of E1 (Ub-activating), E2 (Ub-conjugating), and E3 (Ub-ligating) enzymes. The Ub-ligating enzymes, muscle RING finger 1 (MuRF1) and muscle atrophy F-box (atrogin-1), are muscle-specific enzymes which appear to be causally related to muscle protein breakdown observed in a number of catabolic conditions including denervation, sepsis, diabetes, fasting and excessive production of glucocorticoids and inflammatory cytokines [12, 23–25]. To further emphasize the significance of these enzymes, MuRF1 and atrogin-1 are often referred to as atrophy-related or “atrogenes.” The interaction of IGF-I and atrogenes in controlling muscle integrity is evidenced by the ability of this anabolic hormone to decrease muscle atrogene expression and thereby limit protein degradation by inhibiting proteolytic pathways [24, 26–28]. Therefore, the purpose of the present study was to determine whether the muscle atrophy produced by trinitrobenzne sulfonic acid (TNBS)-induced colitis is associated with altered rates of muscle protein synthesis or degradation, as well as the potential role of the local (e.g., muscle) insulin-like growth factor (IGF) system and muscle-specific Ub E3 ligases atrogin-1 and MuRF1 in mediating altered muscle protein balance.
2. Materials and Methods
2.1 Animal model of colitis and experimental protocol
Male C57Bl/6 mice (approximately 50 days of age; Charles River Breeding Laboratories, Cambridge, MA) were used in all studies and were housed in a light-controlled room under constant environmental conditions. Standard rodent chow was provided ad libitum. All experiments were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine and adhere to the National Institutes of Health (NIH) guidelines for the use of experimental animals. Mice were anesthetized with an intraperitoneal injection of ketamine (136 mg/kg) and xylazine (9.6 mg/kg) followed by the intra-rectal administration of 2, 4, 6-TNBS (40 μg/g in 50% ethanol; 100 μl volume) or an equal volume of 50% ethanol on day 0 [29]. The mice underwent a second administration of TNBS or ethanol on day 7 of the study to re-activate the hapten-mediated colitis. Animals were followed for a total of 10 days, weighed every day, and were provided rodent chow ad libitum. Our preliminary studies indicated that two injections of TNBS were necessary to develop a sustained inflammatory condition capable of producing a detectable decrease in muscle mass. Specifically, mice were killed 3 days after the second TNBS injection (e.g., 10 days from first injection) because this time point represented the peak of the inflammatory state. This animal model is characterized by transmural and chronic colonic inflammation similar to that observed in patients with Crohn’s disease [4]. While we recognize that ketamine anesthesia can alter muscle protein metabolism [30], control animals received the same dose of anesthetic as those administered TNBS. Body weight was determined every other day and the overall length measured from the tip of the nose to the tail on study days 0 and 10. On day 10 the mice were anesthetized, and then blood and tissues were collected. An intracardiac puncture was performed and blood collected in heparinized tubes. The plasma was removed and stored at −20° C for later analyses. The gastrocnemius-plantaris complex was removed together with a portion of liver; tissues were frozen to the temperature of liquid nitrogen, weighed and then stored at −80° C. The colon from each mouse was weighed and graded on degree of inflammation. The colonic damage was assessed on a scale of 0–10 using the colonic macroscopic scoring system described in Table 1.
Table 1.
Colonic macroscopic scoring system
| Score | Parameter |
|---|---|
| 0 | No damage |
| 1 | Hyperemia without ulcers |
| 2 | Hyperemia & bowel thickening, without ulcers |
| 3 | One site of ulceration without bowel thickening |
| 4 | Two or more sites of ulceration or inflammation |
| 5 | > 2 sites of damage or 1 site 0.5 cm |
| 6–10 | Damage of at least 1 cm, with score increasing by 1 for each additional 0.5 cm of damage |
Each colon was weighed and graded on degree of inflammation with colonic damage assessed on a scale of 0–10.
2.2 Skeletal muscle protein metabolism
Protein synthesis was determined using the flooding-dose technique as originally described by Garlick et al [31] and modified by our laboratory [16, 32, 33]. Mice were fasted for 4 h and then injected intraperitoneally with [3H]-L-phenylalanine (Phe; 150 mM, 30 μCi/ml; 1 ml/100 g body weight [BW]). An arterial blood sample was collected 15 min later for the determination of phenylalanine concentration and radioactivity, and the rate of protein synthesis was calculated using the specific radioactivity of the plasma Phe as the precursor pool. Immediately following the removal of the blood, the gastrocnemius was excised, frozen between liquid nitrogen-cooled aluminum blocks, and stored at −80 °C. Tissue protein concentration was determined using the bicinchoninic acid procedure (Pierce Chemical, Rockford, IL) with crystalline BSA as a standard.
As tyrosine is neither synthesized nor degraded in muscle, we determined the rate of protein degradation in plantaris muscle by assessing the tyrosine released into the incubation medium. The plantaris muscle was secured to a plastic support to maintain resting length during incubation, and the incubation composition and conditions were exactly as previously described [34]. Tyrosine in the medium was assayed fluorometrically [35].
2.3 Proteasome activity
in vitro proteasome activity was assessed by quantifying the chymotryptic-like peptidase activity in gastrocnemius muscle. Muscle was homogenized in buffer containing 50 μM Tris-HCl (pH 7.4), 5 mM MgCl2, 250 mM sucrose, 2 mM ATP, and 1 mM DTT. The homogenate was first centrifuged at 400 × g (5 min) and clarified by sequential centrifugation steps (10,000 × g for 20 min followed by 100,000 × g for 5 h) to isolate the 20S and 26S proteasomes, as previously described [36, 37]. After resuspension, proteasome chymotryptic-like activity was determined using a fluorometer (Molecular Devices SpectraMax Gemini EM) to measure the release of 7-amino-4-methylcoumarin (AMC) from the fluorogenic peptide substrate LLVY-AMC (Chemicon International, Temecula, CA).
2.4 Ribonuclease protection assays (RPAs)
Primer selection for mouse genes of interest was determined with the help of Genefisher software and template production performed as previously described [24]. Primers were synthesized (IDT, Coralville, IA) with restriction sites for EcoRI or KpnI at the 5′ end and with three extra bases at the extreme 5′ end. Primer sequences for one template contained IGF-I, atrogin-1, MuRF1, and E3a1 which have been previously published by our laboratory [25]. Riboprobes for cytokine studies were synthesized from custom multi-probe mouse template sets containing probes for IL-6, TNFα, IL-1α and nitric oxide synthase (NOS)-2 as previously described [38, 39]. Quantitation of the mRNA content for IGF binding protein (IGFBP)-1, -2, -3, -4, -5 and -6 as well as the acid-labile subunit (ALS) was as described [40]. Each template contained actin, L32 and/or GAPDH as loading controls. In general, PCR was conducted using HotStarTaq DNA Polymerase (Qiagen, Valencia, CA) and total RNA reverse-transcribed with Superscript™ First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). PCR products were phenol:chloroform extracted, ethanol precipitated, and sequentially digested with KpnI and EcoRI (Promega, Madison, WI). Digested products were gel-purified, re-extracted, and cloned into KpnI/EcoRI-digested pBluescript II SK+ (Stratagene, La Jolla, CA). Plasmid DNA was isolated with both QIAprepR Spin Miniprep and Plasmid Maxi Kits (Qiagen). Plasmids with inserts were verified by sequencing in the Pennsylvania State College of Medicine Core Facility. Final constructs were linearized with EcoRI, gel-purified, and quantitated spectrophotometrically.
Total RNA was extracted from cells using TRI Reagent (Molecular Research Center, Cincinnati, OH). Tissue mRNA content was determined by ribonuclease protection assay (RPA) as described [15, 25, 39]. Briefly, a 2 μl aliquot of template was prepared using T7 Polymerase with buffer (Fermentas, Hanover, MD), NTPs and tRNA (Sigma-Aldrich), RNaisin and DNase (Promega), and 32P-UTP (Amersham Biosciences, Piscatawy, NJ). Unless otherwise noted, the RPA procedure including labeling conditions, component concentrations, sample preparation, and gel electrophoresis was as published (BD Pharminigen, San Diego, CA). Hybridization buffer was 80% formamide and 20% stock buffer (200 mM Pipes pH 6.4, 2 M NaCl, and 5 mM EDTA). Hybridization proceeded overnight at 56° C in a dry bath incubator (Fisher Scientific, Pittsburgh, PA). Samples were treated with RNase A+T1 (Sigma) in 1× RNase buffer (10 mM Tris-HCl pH 7.5, 5 mM EDTA, and 300 mM NaCl) followed by Proteinase K in1× Proteinase K buffer (50 mM Tris pH 8.0, 1 mM EDTA, 1% Tween-20). Following ethanol precipitation, samples were resuspended in loading buffer (98% formamide (v/v), 0.05% xylene cyanol (w/v), 0.05% bromphenol blue (w/v), and 10 mM EDTA). Polyacrylamide gels were run in an S3S Sequencing System (Owl Separation Systems, Portsmouth, NH), transferred to chromatography paper, and dried (FB GD 45 Gel Dryer, Fisher Scientific). Gels were exposed to a PhosphorImager screen (Molecular Dynamics Inc., Sunnyvale, CA). Data were visualized and analyzed using ImageQuant software (Version 5.2, Molecular Dynamics). Signal densities within the linear range were normalized to densities for actin, L32 and/or GAPDH mRNA.
2.5 Western blot analysis
The tissue preparation was the same as described by our laboratories [41–45]. Muscle was homogenized in ice-cold homogenization buffer (pH 7.4) composed of (in mM): 20 mM HEPES, 2 EGTA, 50 NaF, 100 KCl, 0.2 EDTA, 50 $-glycerophosphate, 1 DTT, 0.1 PMSF, 1 benzamidine, 0.5 sodium vanadate, plus one protease inhibitor cocktail tablet from Roche, and clarified by centrifugation. The samples were subjected to SDS-PAGE and the proteins electrophoretically transferred to PVDF membranes. The blots were incubated with either primary antibodies to total and phosphorylated (Thr37/46) eukaryotic initiation factor 4E binding protein-1 (4E-BP1; Bethyl Laboratories, Montgomery, TX), or total and phosphorylated (Thr389; Cell Signaling, Beverly, MA) ribosomal protein S6 kinase-1 (S6K1). Blots were washed with TBS-T (1X TBS including 0.1% Tween-20) and incubated with secondary antibody (horseradish peroxidase conjugated goat anti-mouse or goat anti-rabbit) IgG. Blots were developed with enhanced chemiluminescence Western blotting reagents (Amersham, Piscataway, NJ) and exposed to X-ray film in a cassette equipped with a DuPont Lightning Plus intensifying screen. After development, the film was scanned (Microtek ScanMaker IV) and analyzed using NIH Image 1.6 software.
2.6 Plasma IGF-I, insulin and cytokine determinations
Total plasma IGF-I was measured using a mouse/rat IGF-I radioimmunoassay (RIA) kit (Diagnostic Systems Laboratories, Webster, TX). The plasma concentration of free (or readily dissociable) IGF-I was determined by centrifugal ultrafiltration, exactly as previously described [15]. The plasma insulin concentration was determined by RIA (Linco Research, St. Louis, MO). TNFα and IL-6 in plasma were quantified by commercially available ELISAs (R&D Systems, Minneapolis, MN, USA).
2.7 Statistical analysis
Data are presented as mean ± SE and the exact sample size is indicated in the figure and table legends. Statistical evaluation of the data was performed by 2-tailed t-test using Instat GraphPad 5.02 (San Diego, CA). Differences among groups were considered significant at P < 0.05. Differences between groups with a P-value > 0.05 but < 0.01 are referred to as trends, while between group differences with a P > 0.10 were considered not statistically significant.
3. Results
3.1 Food intake, body weight, muscle weight, linear growth, and severity of colitis
Food consumption was reduced for 1–2 days after each injection of TNBS, compared with time-matched vehicle-treated control values (day 1 - control = 3.7 ± 0.2, TNBS = 2.1 ± 0.4 g/day; day 2 - control = 4.3 ± 0.3, TNBS = 3.4 ± 0.3 g/day; and day 7 - control = 4.1 ± 0.3, TNBS = 2.0 ± 0.4 g/day; P < 0.05). However, food intake did not differ between control and TNBS-treated mice on days 3–6 (control = 3.9 ± 0.3 vs TNBS = 3.6 ± 0.4 g/day; P > 0.10) or on days 8–10 (control = 4.3 ± 0.3, TNBS = 4.1 ± 0.3 g/day; P > 0.10). The final body weight (control = 23.1 ± 0.3 vs TNBS = 22.6 ± 0.3 g) and rate of body weight gain (control = 0.50 ± 0.04 g/day vs TNBS = 0.42 ± 0.04 g/day) did not differ between TNBS-treated and control mice. The increment in linear growth of mice with colitis tended to be reduced compared with control values (controls = 3.7 ± 0.4 mm/10 days vs TNBS = 2.4 ± 0.04 mm/10 days; P = 0.07). However, the wet weight of the gastrocnemius-plantaris complex was significantly reduced by 13% in mice with colitis, compared with control values (control = 219 ± 8 mg vs 188 ± 8 mg; P < 0.05). In contrast, the wet weight of the heart did not differ between groups (control = 95 ± 4 mg vs TNBS = 92 ± 3 mg). Colonic weight of TNBS-treated mice was increased compared to control values (control = 107 ± 4 mg/cm3 vs 136 ± 7 mg/cm3; P < 0.05). Likewise, the severity of disease, based on the gross damage score, was greater in the mice with colitis (control = 0.7 ± 0.2 vs 3.3 ± 0.4; P < 0.05).
3.2 Muscle protein concentration, synthesis and degradation
Skeletal muscle protein concentration was reduced by 11% in mice with colitis, compared with time-matched control values (control = 167. 4 ± 6.6 vs TNBS = 148.2 ± 7.1 mg protein/g wet weight; P < 0.05; n = 8 per group). However, in vivo-determined muscle protein synthesis did not differ between control and TNBS-treated mice (0.85 ± 0.05 vs 0.83 ± 0.04 nmoles Phe incorporated/h/mg protein, respectively; n = 8 per group). The mammalian target of rapamycin (mTOR) is a Ser/Thr kinase which regulates muscle protein synthesis by integrating various nutrient and growth factor signals [46], and this regulation is largely achieved by the phosphorylation of two downstream targets, 4E-BP1 and S6K1. Western blot analysis of muscle from TNBS-treated mice and control animals indicated no significant difference (P > 0.10) in either the extent of phosphorylation (e.g., activation) or total amount of 4E-BP1 or S6K1 (data not shown).
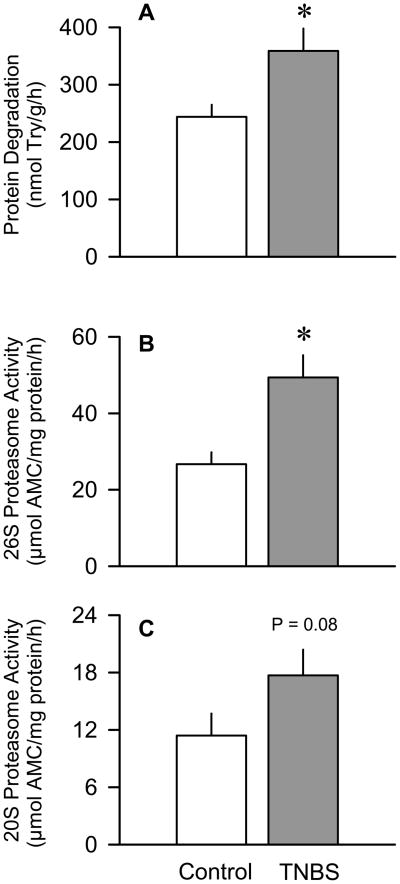
The rate of protein degradation in the plantaris muscle was determined in vitro and shown to be increased 45% (P < 0.05) in mice with colitis, compared with time-matched control values (Figure 1A). Additionally, in vitro-determined 26S proteasome activity of gastrocnemius was increased 85% (P < 0.05) in muscle from TNBS-treated mice (Figure 1B). The 20S proteasome activity also tended to be increased (P = 0.08) in TNBS-treated mice (Figure 1C).
Figure 1.
Effect of TNBS-induced colitis on in vitro-determined rates of muscle protein breakdown and proteasome activity. Panel A: Protein degradation in plantaris determined by tyrosine (Try) release into medium. Panels B and C: Activity of the 26S and 20S proteasome, respectively. Values are means ± SEM; n = 8–10 per group for protein degradation and 6 per group for proteasome activity. *P < 0.05 compared to time-matched control values.
3.3 Muscle mRNA expression of MuRF1, atrogin-1 and E3α1
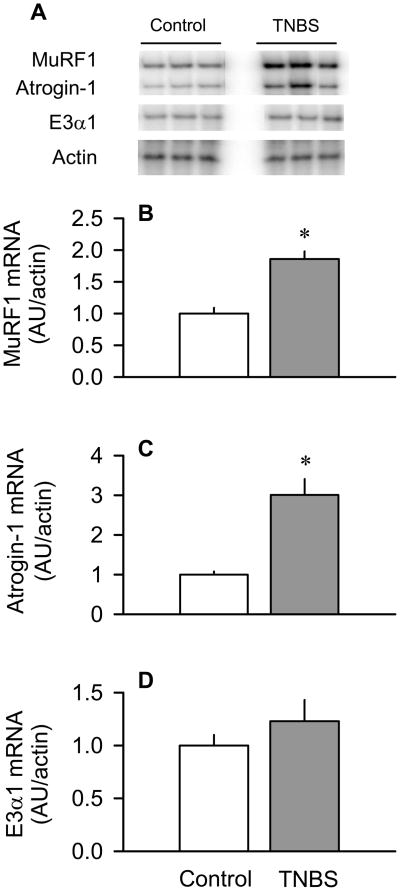
The E3 ligases MuRF1 and atrogin-1 are important regulators of protein degradation in muscle and, therefore, we examined the mRNA levels of these enzymes. The MuRF1 mRNA content of skeletal muscle was increased –100% (P < 0.05) in TNBS-treated mice, compared with control animals (Figure 2A and 2B). There was a similar increase (P < 0.05) in atrogin-1 mRNA content of muscle from mice with colitis (Figure 2A and 2C). However, the E3α1 mRNA content did not differ between TNBS-treated and control mice (Figure 2A and 2D).
Figure 2.
Effect of TNBS-induced colitis on MuRF1, atrogin-1 and E3αI mRNA content in skeletal muscle. Panel A: representative autoradiographs of E3 ligases and actin (loading control). Panels B, C and D: bar graphs represent means ± SEM of n = 9–11; data were normalized to actin and expressed as arbitrary densitometry units (AU)/actin. Data from control mice were arbitrarily set at 1.0 AU. *P < 0.05 compared to time-matched control values.
3.4 IGF-I system
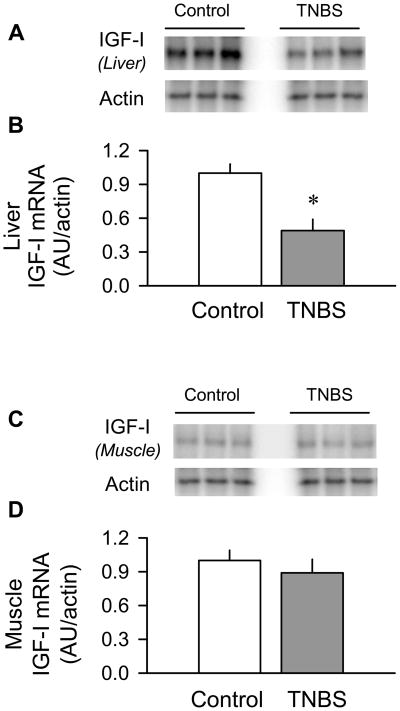
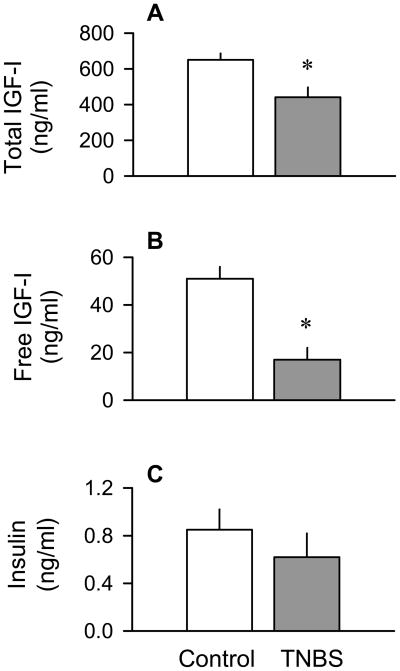
We next systematically examined the level of IGF-I in the plasma and IGF-I mRNA content in liver and skeletal muscle. We found the liver IGF-I mRNA content was decreased 55% (P < 0.05) in the TNBS-treated mice, compared to control values (Figure 3A and 3B). In contrast, the muscle IGF-I mRNA content did not differ between groups (Figure 3C and 3D). The colitis-induced reduction in the liver IGF-I mRNA was consistent with the 40% reduction (P < 0.05) in the plasma total IGF-I concentration (Figure 4A). The “free” or easily dissociable IGF-I in the circulation demonstrated a 70% reduction (P < 0.05) in mice with colitis (Figure 4B). The plasma insulin concentration did not differ between TNBS-treated and control mice (Figure 4C).
Figure 3.
Effect of TNBS-induced colitis on the IGF-I mRNA content of liver and skeletal muscle. Panels A and C: representative autoradiographs of IGF-I mRNA in liver and muscle (gastrocnemius/plantaris), respectively. Panels B and D: bar graphs represent means ± SEM of n = 9–11, data were normalized to actin and data from control mice were arbitrarily set at 1.0 AU. *P < 0.05 compared to time-matched control values.
Figure 4.
Effect of TNBS-induced colitis on the plasma concentration of total and free IGF-I, and insulin (panels A–C, respectively). Bar graphs represent means ± SEM of n = 9–11. *P < 0.05 compared to time-matched control values.
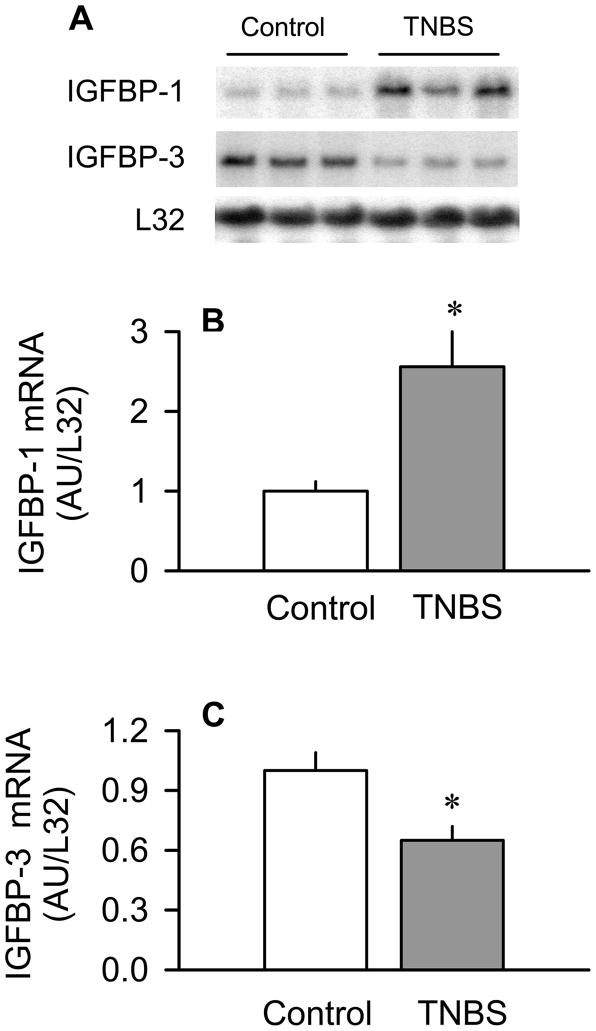
TNBS-induced colitis also altered the mRNA content for several of the IGF binding proteins. IGFBP-3 binds most of the plasma IGF-I (3), whereas elevations in plasma IGFBP-1 can decrease muscle protein synthesis (32). Therefore, the levels of IGFBP-1 and IGFBP-3 mRNA in liver – their primary site of synthesis – as well as in skeletal muscle were determined because they might affect local IGF-I bioactivity. In liver, TNBS increased IGFBP-1 by 100% (P < 0.05) and decreased IGFBP-3 mRNA by 40% (P < 0.05), compared to control values (Figure 5). In contrast, the mRNA content of the other detectable IGF-I binding proteins in liver (e.g., IGFBP-2, -4, -5, -6 and ALS) and in skeletal muscle (e.g., IGFBP-3, -4, and -5) did not differ between groups (Table 2).
Figure 5.
Effect of TNBS-induced colitis on IGFBP-1 and IGFBP-3 mRNA content in liver. Panel A: representative autoradiographs of IGFBP-1 and IGFBP-3 mRNA in liver. Panels B and C: bar graphs represent means ± SEM of n = 9–11, where data were normalized to mouse ribosomal protein L32 mRNA signal and expressed as AU/L32. Data from control mice were arbitrarily set at 1.0 AU. *P < 0.05 compared to time-matched control values.
Table 2.
IGF binding protein (IGFBP) mRNA in liver and muscle from control and TNBS-treated mice
| Control | TNBS | ||
|---|---|---|---|
| Liver | IGFBP-2 | 1.00 ± 0.05 | 1.11 ± 0.12 |
| IGFBP-4 | 1.00 ± 0.08 | 0.89 ± 0.14 | |
| IGFBP-5 | 1.00 ± 0.09 | 0.99 ± 0.07 | |
| IGFBP-6 | 1.00 ± 0.08 | 1.17 ± 0.13 | |
| ALS | 1.00 ± 0.09 | 0.87 ± 0.15 | |
| Muscle | IGFBP-4 | 1.00 ± 0.11 | 0.98 ± 0.08 |
| IGFBP-5 | 1.00 ± 0.08 | 1.07 ± 0.11 | |
| IGFBP-6 | 1.00 ± 0.07 | 1.03 ± 0.08 |
Values are means ± SEM of n = 8. ALS = acid labile subunit. Data were normalized to mouse ribosomal protein L32 mRNA signal and data from control mice were arbitrarily set at 1.0 AU. There are no statistically significant (P > 0.1) differences between TNBS-treated and time-matched control values for any IGFBP. The mRNAs for IGFBP-1 and -2 were not detectable in skeletal muscle by the RPA methods used in this study.
3.5 Blood and tissue content of inflammatory cytokines
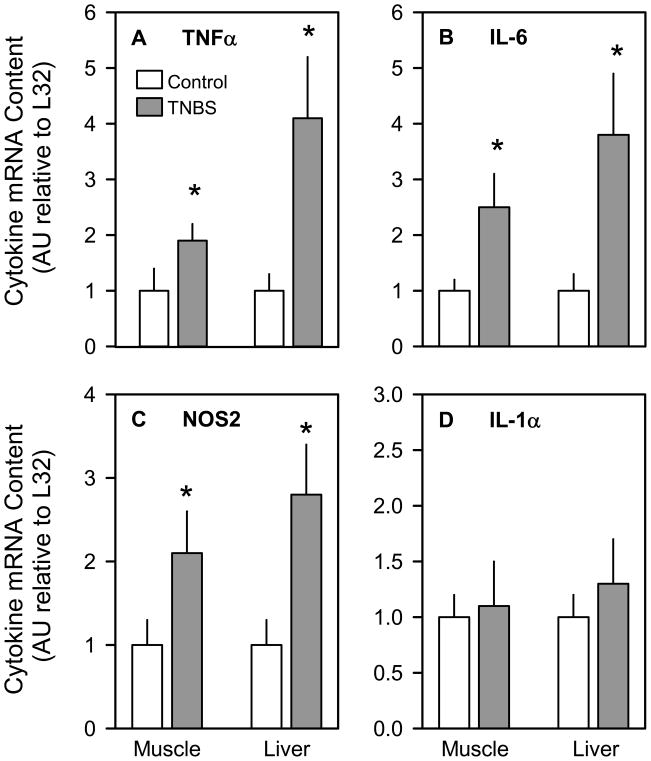
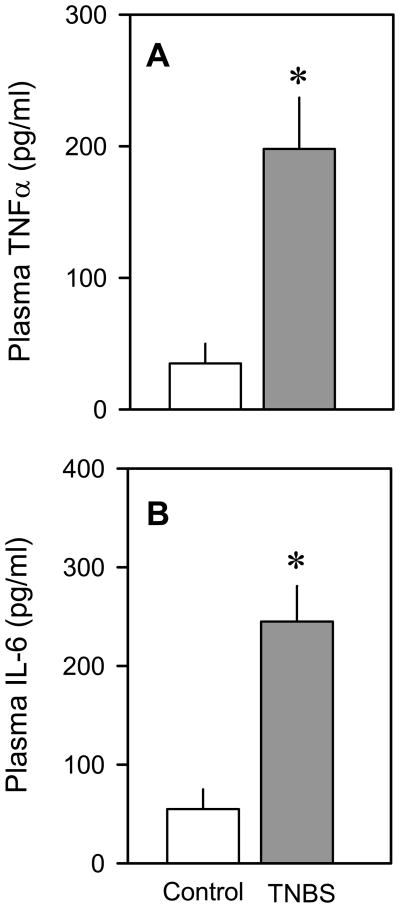
The TNFα mRNA content was increased –100% (P < 0.05) in muscle and almost 400% (P < 0.05) in liver (Figure 6A), while IL-6 mRNA levels were increased –250% (P < 0.05) in muscle and nearly 400% (P < 0.05) in liver (Figure 6B). Likewise, NOS2 mRNA levels were elevated –200–250% (P < 0.05) in both muscle and liver of mice with colitis (Figure 6C). In contrast, the IL-1α mRNA content of both muscle and liver did not differ between groups (Figure 6D). Finally, the plasma concentrations of TNFα (Figure 7A) and IL-6 (Figure 7B) were also elevated (P < 0.05) in mice with colitis, compared to vehicle-treated control mice.
Figure 6.
Effect of TNBS-induced colitis on muscle and liver mRNA content for TNFα, IL-6, NOS2, and IL-1α (panels A–D, respectively). Bar graphs represent means ± SEM of n = 9–11, where data were normalized to mouse ribosomal protein L32 mRNA signal and data from control mice were arbitrarily set at 1.0 AU. *P < 0.05 compared to time-matched control values for the same tissue.
Figure 7.
Effect of TNBS-induced colitis on the plasma concentration of TNFα and IL-6. Bar graphs in panel A (TNFα) and panel B (IL-6) represent means ± SEM of n = 9–11. *P < 0.05 compared to time-matched control values.
4. Discussion
TNBS-induced colitis decreased both skeletal muscle mass and protein concentration over the duration of the 10-day experimental protocol. Muscle protein content represents the balance between protein synthesis and degradation, and not all catabolic insults affect both sides of the protein balance equation in the same manner. Hence, it was important to characterize both rates of synthesis and breakdown in the murine TNBS model of colitis. No significant difference in the in vivo-determined rate of protein synthesis was detected in muscle of control and TNBS-treated mice, and this finding was supported by the comparable phosphorylation status of both 4E-BP1 and S6K1 which are important regulators of mRNA translation. Although our data on protein synthesis and mTOR activity are internally consistent, they contrast with previous reports where muscle protein synthesis was either reduced [19] or even increased [20] in response to intestinal inflammation. The reason for these highly divergent results is unclear but may be related to use of different experimental models of IBD. Alternatively, because synthesis was only determined at a single time point, we cannot exclude the possibility that a TNBS-induced alteration in muscle protein synthesis occurred at an earlier time point but was transient in nature. Finally, the comparable rates of muscle protein synthesis between groups suggest the transient reduction in food intake seen in the TNBS-treated mice did not have a sustained effect on this component of the protein balance equation.
An increased rate of muscle protein breakdown is often observed in catabolic states and the activation of the Ub-proteasome pathway appears largely responsible [21]. In the current study, protein breakdown in incubated plantaris was increased in TNBS-treated mice. Likewise, in vitro-determined proteasome activity was increased in muscle from mice colitis. Finally, the mRNA content for two muscle-specific E3 ligases, atrogin-1 and MuRF1 (but not the more ubiquitously expressed E3α1), were shown to be increased in TNBS-treated mice. While atrogin-1 and MuRF1 protein content were not determined in our study, the TNBS-induced increase in atrogene mRNA expression is consistent with the up-regulation of muscle atrogin-1 and MuRF1 mRNA in response to other catabolic disease states with muscle wasting, including sepsis, burn, diabetes and denervation [23–26, 28]. Moreover, the atrophic response to denervation was attenuated in either atrogin-1 or MuRF1 null mice [23], clearly demonstrating the central role of these proteins in muscle wasting. Collectively, our data indicate that the skeletal muscle atrophy detected in TNBS-treated mice appears to be predominantly due to an accelerated rate of protein breakdown mediated by the up-regulation of the Ub-proteasome proteolytic pathway.
IGF-I, via its ability to stimulate protein synthesis and inhibit proteolysis in skeletal muscle, has a central role in maintaining muscle mass [8], and its effects on muscle metabolism are mediated by not only endocrine but paracrine/autocrine mechanisms [6]. Other catabolic conditions are associated with a reduction in both the circulating concentration of total IGF-I, derived primarily from the liver, and IGF-I synthesized within skeletal muscle per se [32, 33, 47]. Previous studies in various rodent models of colitis have reported a reduction in the circulating and/or hepatic synthesis of IGF-I [4, 12], a response comparable to that seen in IBD patients [1, 17, 18] This IBD-induced fall in IGF-I results from the development of GH resistance [48] resulting from the over production of various inflammatory cytokines, particularly TNFα and IL-6 [4, 11, 12, 18, 49] – a mechanism which is also present in several other catabolic conditions [13]. The GH resistance and decreased plasma IGF-I in children with IBD appear largely responsible for the observed growth failure. The current study confirms the previously reported colitis-induced reduction in blood-borne IGF-I [4, 11, 12, 18]. However, our data also extend these observations by demonstrating colitis does not decrease IGF-I mRNA content in skeletal muscle. This finding suggests that colitis may be somewhat unique compared to other catabolic conditions in that it produces muscle wasting primarily via the loss of the classical endocrine actions of IGF-I as opposed to the local muscle-specific production of the hormone.
In addition to determining total IGF-I, we also assessed the concentration of “free” IGF-I, that is the amount of IGF-I not bound to one of the many IGFBPs and which is believed to represent the physiologically active pool of the hormone [50]. The concentration of free IGF-I is also reduced in other catabolic conditions and this reduction is proportional to the reduction in total IGF-I [51–53]. However, in the current study, colitis produced a disproportionate fall in free IGF-I which may have resulted from concomitant changes in the various IGFBPs, as these proteins function to alter IGF-I half-life and bioavailability. Of the six high-affinity binding proteins, IGFBP-3 is the most predominant and binds the majority of the IGF-I in blood [14]. IGF-I and IGFBP-3 forms a ternary complex with ALS which slows the clearance of IGF-I from the circulation. Many cell types, including hepatocytes, produce IGFBP-3, whereas ALS is produced almost exclusively in the liver under the control of GH [54]. TNBS-induced colitis decreased the hepatic mRNA content of IGFBP-3 and ALS, although the later change did not achieve statistical significance. Although not directly determined in our study, such changes in IGFBP mRNA would be expected to lower the circulating concentration of both proteins. In addition, catabolic conditions are often associated with extensive proteolytic cleavage of IGFBP-3 [55] which would be expected to further exacerbate the decreased hepatic synthesis of this binding protein. In contrast, IGFBP-1 is acutely up-regulated by infection or catabolic insults [9], and elevated IGFBP-1 decreases both the plasma concentration of free IGF-I and muscle protein synthesis [15, 16]. However, because only a small fraction of total IGF-I is bound to IGFBP-1, the colitis-induced decrease in IGFBP-3 should more than compensate for the elevation in IGFBP-1 and is likely responsible for the disproportionate reduction in free IGF-I. The down-regulation of IGFBP-3 and up-regulation of IGFBP-1 can be coordinately controlled by the same inflammatory cytokines which decrease hepatic IGF-I synthesis and reduce muscle mass [56–60]. One caveat of our study is that only the mRNA content for the various IGFBPs was determined in muscle because of the limited amount of available muscle. Therefore, we cannot exclude the possibility that the TNBS-induced change in the tissue mRNA and protein expression for IGFBP-1 and -3 are not coordinately regulated.
The current study also quantifies the mRNA content of the other IGFBPs in the liver and muscle, which have not been previously reported in colitis. Serum levels of IGFBP-2 have been reported to be higher in human patients experiencing a relapse of Crohn’s disease compared to healthy controls [18]. However, we did not detect a significant change in IGFBP-2 mRNA content in liver of mice administered TNBS. Similarly, we also failed to detect significant changes in either IGFBP-4, -5 or -6 in either liver or skeletal muscle. The lack of a change in muscle IGFBP-5 was unexpected given that muscle atrophy resulting from disuse or denervation is associated with a marked increase in this IGFBP [61]. Moreover, the lack of change in muscle IGFBP-4 was not anticipated because bacterial infection, which also increases proinflammatory cytokines, has been reported to decrease muscle IGFBP-4 [62]. Our data suggest that compared to other catabolic conditions, colitis produces a somewhat unique pattern of IGFBP mRNA expression in muscle and the lack of change in muscle IGF-I, IGFBP-4 and IGFBP-5 in this condition suggests a different mechanism of action.
The growth failure of inflammatory bowel disease of various etiologies is associated with elevated blood concentrations of TNFα and IL-6, and at least part of this elevation may be attributable to up-regulated cytokine production by the inflamed section of gut [4, 11, 12]. Both of these cytokines decrease IGF-I and have the ability to impair linear growth and decrease LBM [63, 64], whereas neutralization of either TNFα or IL-6 in part restores IGF-I and growth [4, 11, 12, 18]. We extended these observations by showing the mRNA content for both TNFα and IL-6 (but not IL-1α) was increased in liver and skeletal muscle in TNBS-treated mice. Elevation in TNFα and IL-6 was associated with reduced circulating IGF-I, elevated MuRF1 and atrogin-1 in skeletal muscle, and the erosion of LBM in mice with colitis. However, we cannot differentiate between an endocrine or paracrine/autocrine mechanism for these cytokines because TNFα and IL-6 were increased in liver (the primarily source for the elevation in the plasma) as well as in muscle. Finally, gut inflammation is also associated with overproduction of NO via activation of NOS2 in intestinal epithelial cells [67], and the sustained elevation of NO is likely to be partially responsible for the increased gut permeability and pathology in IBD [68]. However, no data are available on colitis-induced NO production in skeletal muscle, and this is important because enhanced NO production can lead to muscle atrophy [39]. In the present study, colitis increased NOS2 mRNA in both liver and muscle. Regardless of their source, it is noteworthy that the elevated levels of TNFα, IL-6 and NO failed to decrease muscle protein synthesis in TNBS-treated mice. This lack of response differs from the decreased muscle protein synthesis seen in other catabolic states characterized elevated inflammatory cytokines (e.g., sepsis) or which results from elevated cytokine levels per se [46, 65, 66]. The mechanism for this discordant response is not known.
In conclusion, the present study demonstrates that TNBS-induced colitis produces muscle atrophy most likely by increasing protein breakdown as opposed to changing protein synthesis. Moreover, the stimulation of protein degradation appears mediated via activation of the Ub-proteasome pathway and enhanced expression of atrogin-1 and MuRF1. The increased protein degradation is associated with a reduction in circulating (but not muscle) IGF-I as well as increased muscle and circulating TNFα, IL-6 and NO. Hence, interfering with the activation of the Ub-proteasome pathway may prove to be beneficial in ameliorating the debilitating effects of growth retardation and the impairment in muscle protein accretion in patients with IBD.
Acknowledgments
Support: National Institute of Health Grants GM-55639 (RNC), GM-38032 (CHL), T32-GM64332 (FP), and The Crohn’s and Colitis Foundation of America from the Litwin Foundation (RNC)
We thank Jay Nystrom and Anne Pruznak for their expert technical assistance.
Footnotes
Conflict of Interest: All authors declare that they have no conflict-of-interest or financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Griffiths AM, Nguyen P, Smith C, et al. Growth and clinical course of children with Crohn’s disease. Gut. 1993;34:939–943. doi: 10.1136/gut.34.7.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motil KJ, Grand RJ, vis-Kraft L, et al. Growth failure in children with inflammatory bowel disease: a prospective study. Gastroenterology. 1993;105:681–691. doi: 10.1016/0016-5085(93)90883-e. [DOI] [PubMed] [Google Scholar]
- 3.Ballinger A. Fundamental mechanisms of growth failure in inflammatory bowel disease. Horm Res. 2002;58 (Suppl 1):7–10. doi: 10.1159/000064756. [DOI] [PubMed] [Google Scholar]
- 4.Ballinger AB, Azooz O, El-Haj T, et al. Growth failure occurs through a decrease in insulin-like growth factor 1 which is independent of undernutrition in a rat model of colitis. Gut. 2000;46:694–700. doi: 10.1136/gut.46.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shamir R, Phillip M, Levine A. Growth retardation in pediatric Crohn’s disease: pathogenesis and interventions. Inflamm Bowel Dis. 2007;13:620–628. doi: 10.1002/ibd.20115. [DOI] [PubMed] [Google Scholar]
- 6.Le RD, Bondy C, Yakar S, et al. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 7.Olney RC. Regulation of bone mass by growth hormone. Med Pediatr Oncol. 2003;41:228–234. doi: 10.1002/mpo.10342. [DOI] [PubMed] [Google Scholar]
- 8.Clemmons DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. 2009;20:349–356. doi: 10.1016/j.tem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Frost RA, Lang CH. Alteration of somatotropic function by proinflammatory cytokines. J Anim Sci. 2004;82(E-Suppl):E100–E109. doi: 10.2527/2004.8213_supplE100x. [DOI] [PubMed] [Google Scholar]
- 10.Denson LA, Held MA, Menon RK, et al. Interleukin-6 inhibits hepatic growth hormone signaling via upregulation of Cis and Socs-3. Am J Physiol Gastrointest Liver Physiol. 2003;284:G646–G654. doi: 10.1152/ajpgi.00178.2002. [DOI] [PubMed] [Google Scholar]
- 11.DiFedele LM, He J, Bonkowski EL, et al. Tumor necrosis factor alpha blockade restores growth hormone signaling in murine colitis. Gastroenterology. 2005;128:1278–1291. doi: 10.1053/j.gastro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Sawczenko A, Azooz O, Paraszczuk J, et al. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the -174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci U S A. 2005;102:13260–13265. doi: 10.1073/pnas.0503589102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang CH, Hong-Brown L, Frost RA. Cytokine inhibition of JAK-STAT signaling: a new mechanism of growth hormone resistance. Pediatr Nephrol. 2005;20:306–312. doi: 10.1007/s00467-004-1607-9. [DOI] [PubMed] [Google Scholar]
- 14.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 15.Frost RA, Lang CH. Differential effects of insulin-like growth factor I (IGF-I) and IGF-binding protein-1 on protein metabolism in human skeletal muscle cells. Endocrinology. 1999;140:3962–3970. doi: 10.1210/endo.140.9.6998. [DOI] [PubMed] [Google Scholar]
- 16.Lang CH, Vary TC, Frost RA. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology. 2003;144:3922–3933. doi: 10.1210/en.2002-0192. [DOI] [PubMed] [Google Scholar]
- 17.Eivindson M, Gronbaek H, Flyvbjerg A, et al. The insulin-like growth factor (IGF)-system in active ulcerative colitis and Crohn’s disease: relations to disease activity and corticosteroid treatment. Growth Horm IGF Res. 2007;17:33–40. doi: 10.1016/j.ghir.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Eivindson M, Gronbaek H, Skogstrand K, et al. The insulin-like growth factor (IGF) system and its relation to infliximab treatment in adult patients with Crohn’s disease. Scand J Gastroenterol. 2007;42:464–470. doi: 10.1080/00365520601010115. [DOI] [PubMed] [Google Scholar]
- 19.Mercier S, Breuille D, Mosoni L, et al. Chronic inflammation alters protein metabolism in several organs of adult rats. J Nutr. 2002;132:1921–1928. doi: 10.1093/jn/132.7.1921. [DOI] [PubMed] [Google Scholar]
- 20.El YM, Breuille D, Papet I, et al. Increased tissue protein synthesis during spontaneous inflammatory bowel disease in HLA-B27 rats. Clin Sci (Lond) 2003;105:437–446. doi: 10.1042/CS20020313. [DOI] [PubMed] [Google Scholar]
- 21.Lecker SH, Solomon V, Mitch WE, et al. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- 22.Roos-Mattjus P, Sistonen L. The ubiquitin-proteasome pathway. Ann Med. 2004;36:285–295. doi: 10.1080/07853890310016324. [DOI] [PubMed] [Google Scholar]
- 23.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 24.Frost RA, Nystrom GJ, Jefferson LS, et al. Hormone, cytokine, and nutritional regulation of sepsis-induced increases in atrogin-1 and MuRF1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E501–E512. doi: 10.1152/ajpendo.00359.2006. [DOI] [PubMed] [Google Scholar]
- 25.Krawiec BJ, Nystrom GJ, Frost RA, et al. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab. 2007;292:E1555–E1567. doi: 10.1152/ajpendo.00622.2006. [DOI] [PubMed] [Google Scholar]
- 26.Li HH, Kedar V, Zhang C, et al. Atrogin-1/muscle atrophy F-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an SCF ubiquitin ligase complex. J Clin Invest. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nystrom G, Pruznak A, Huber D, et al. Local insulin-like growth factor I prevents sepsis-induced muscle atrophy. Metabolism. 2009;58:787–797. doi: 10.1016/j.metabol.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacheck JM, Ohtsuka A, McLary SC, et al. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab. 2004;287:E591–E601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- 29.Morris GP, Beck PL, Herridge MS, et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 30.Zhang XJ, Cortiella J, Doyle D, et al. Ketamine anesthesia causes greater muscle catabolism in rabbits than does propofol. J Nutr Biochem. 1997;8:133–139. [Google Scholar]
- 31.Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980;192:719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang CH, Frost RA. Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab. 2004;287:E721–E730. doi: 10.1152/ajpendo.00132.2004. [DOI] [PubMed] [Google Scholar]
- 33.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol. 2005;203:144–155. doi: 10.1002/jcp.20207. [DOI] [PubMed] [Google Scholar]
- 34.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1777–R1789. doi: 10.1152/ajpregu.00056.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waalkes TP, Udenfriend S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med. 1957;50:733–736. [PubMed] [Google Scholar]
- 36.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem. 1996;271:26690–26697. doi: 10.1074/jbc.271.43.26690. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Hu Z, Hu J, et al. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 38.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;283:R698–R709. doi: 10.1152/ajpregu.00039.2002. [DOI] [PubMed] [Google Scholar]
- 39.Frost RA, Nystrom GJ, Lang CH. Lipopolysaccharide stimulates nitric oxide synthase-2 expression in murine skeletal muscle and C(2)C(12) myoblasts via Toll-like receptor-4 and c-Jun NH(2)-terminal kinase pathways. Am J Physiol Cell Physiol. 2004;287:C1605–C1615. doi: 10.1152/ajpcell.00010.2004. [DOI] [PubMed] [Google Scholar]
- 40.Lang CH, Pruznak AM, Nystrom GJ, et al. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: Comparable effects in young and mature rats. Nutr Metab (Lond) 2009;6:4. doi: 10.1186/1743-7075-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lang CH, Frost RA, Nairn AC, et al. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336–E347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 42.Lang CH, Frost RA. Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab. 2004;287:E721–E730. doi: 10.1152/ajpendo.00132.2004. [DOI] [PubMed] [Google Scholar]
- 43.Lang CH, Frost RA. Endotoxin disrupts the leucine-signaling pathway involving phosphorylation of mTOR, 4E-BP1, and S6K1 in skeletal muscle. J Cell Physiol. 2005;203:144–155. doi: 10.1002/jcp.20207. [DOI] [PubMed] [Google Scholar]
- 44.Lang CH, Frost RA. Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med. 2006;12:291–299. doi: 10.2119/2006-00071.Lang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang CH, Frost RA. Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism. 2007;56:49–57. doi: 10.1016/j.metabol.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 47.Pruznak AM, Hong-Brown L, Lantry R, et al. Skeletal and cardiac myopathy in HIV-1 transgenic rats. Am J Physiol Endocrinol Metab. 2008;295:E964–E973. doi: 10.1152/ajpendo.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theiss AL, Fruchtman S, Lund PK. Growth factors in inflammatory bowel disease: the actions and interactions of growth hormone and insulin-like growth factor-I. Inflamm Bowel Dis. 2004;10:871–880. doi: 10.1097/00054725-200411000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Mitsuyama K, Sata M, Rose-John S. Interleukin-6 trans-signaling in inflammatory bowel disease. Cytokine Growth Factor Rev. 2006;17:451–461. doi: 10.1016/j.cytogfr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Frystyk J. Utility of free IGF-I measurements. Pituitary. 2007;10:181–187. doi: 10.1007/s11102-007-0025-y. [DOI] [PubMed] [Google Scholar]
- 51.Bereket A, Lang CH, Blethen SL, et al. Growth hormone treatment in growth retarded children with end stage renal failure: effect on free/dissociable IGF-I levels. J Pediatr Endocrinol Metab. 1997;10:197–202. doi: 10.1515/jpem.1997.10.2.197. [DOI] [PubMed] [Google Scholar]
- 52.Co Ng LL, Lang CH, Bereket A, et al. Effect of hyperthyroidism on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in adolescent children. J Pediatr Endocrinol Metab. 2000;13:1073–1080. doi: 10.1515/jpem.2000.13.8.1073. [DOI] [PubMed] [Google Scholar]
- 53.Lang CH, Liu X, Nystrom G, et al. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol. 2000;35:148–158. doi: 10.1093/alcalc/35.2.148. [DOI] [PubMed] [Google Scholar]
- 54.Laursen T, Flyvbjerg A, Jorgensen JO, et al. Stimulation of the 150-kilodalton insulin-like growth factor-binding protein-3 ternary complex by continuous and pulsatile patterns of growth hormone (GH) administration in GH-deficient patients. J Clin Endocrinol Metab. 2000;85:4310–4314. doi: 10.1210/jcem.85.11.6957. [DOI] [PubMed] [Google Scholar]
- 55.Belizon A, Kirman I, Balik E, et al. Major surgical trauma induces proteolysis of insulin-like growth factor binding protein-3 in transgenic mice and is associated with a rapid increase in circulating levels of matrix metalloproteinase-9. Surg Endosc. 2007;21:653–658. doi: 10.1007/s00464-006-9103-x. [DOI] [PubMed] [Google Scholar]
- 56.Barreca A, Ketelslegers JM, Arvigo M, et al. Decreased acid-labile subunit (ALS) levels by endotoxin in vivo and by interleukin-1beta in vitro. Growth Horm IGF Res. 1998;8:217–223. doi: 10.1016/s1096-6374(98)80114-7. [DOI] [PubMed] [Google Scholar]
- 57.Benbassat CA, Lazarus DD, Cichy SB, et al. Interleukin-1 alpha (IL-1 alpha) and tumor necrosis factor alpha (TNF alpha) regulate insulin-like growth factor binding protein-1 (IGFBP-1) levels and mRNA abundance in vivo and in vitro. Horm Metab Res. 1999;31:209–215. doi: 10.1055/s-2007-978721. [DOI] [PubMed] [Google Scholar]
- 58.Delhanty PJ. Interleukin-1 beta suppresses growth hormone-induced acid-labile subunit mRNA levels and secretion in primary hepatocytes. Biochem Biophys Res Commun. 1998;243:269–272. doi: 10.1006/bbrc.1998.8089. [DOI] [PubMed] [Google Scholar]
- 59.Fan J, Char D, Bagby GJ, et al. Regulation of insulin-like growth factor-I (IGF-I) and IGF-binding proteins by tumor necrosis factor. Am J Physiol. 1995;269:R1204–R1212. doi: 10.1152/ajpregu.1995.269.5.R1204. [DOI] [PubMed] [Google Scholar]
- 60.Fan J, Wojnar MM, Theodorakis M, et al. Regulation of insulin-like growth factor (IGF)-I mRNA and peptide and IGF-binding proteins by interleukin-1. Am J Physiol. 1996;270:R621–R629. doi: 10.1152/ajpregu.1996.270.3.R621. [DOI] [PubMed] [Google Scholar]
- 61.Bayol S, Loughna PT, Brownson C. Phenotypic expression of IGF binding protein transcripts in muscle, in vitro and in vivo. Biochem Biophys Res Commun. 2000;273:282–286. doi: 10.1006/bbrc.2000.2667. [DOI] [PubMed] [Google Scholar]
- 62.Lang CH, Krawiec BJ, Huber D, et al. Sepsis and inflammatory insults downregulate IGFBP-5, but not IGFBP-4, in skeletal muscle via a TNF-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;290:R963–R972. doi: 10.1152/ajpregu.00684.2005. [DOI] [PubMed] [Google Scholar]
- 63.De BF, Alonzi T, Moretta A, et al. Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin-like growth factor-I. A model for stunted growth in children with chronic inflammation. J Clin Invest. 1997;99:643–650. doi: 10.1172/JCI119207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martensson K, Chrysis D, Savendahl L. Interleukin-1beta and TNF-alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J Bone Miner Res. 2004;19:1805–1812. doi: 10.1359/JBMR.040805. [DOI] [PubMed] [Google Scholar]
- 65.Janssen SP, Gayan-Ramirez G, Van den BA, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 66.Lang CH, Frost RA, Nairn AC, et al. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am J Physiol Endocrinol Metab. 2002;282:E336–E347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 67.Wu F, Chakravarti S. Differential expression of inflammatory and fibrogenic genes and their regulation by NF-kappaB inhibition in a mouse model of chronic colitis. J Immunol. 2007;179:6988–7000. doi: 10.4049/jimmunol.179.10.6988. [DOI] [PubMed] [Google Scholar]
- 68.Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]