Summary
Programmed death-1 (PD-1) is a newly characterized negative regulator of immune responses. The interaction of PD-1 with its ligands (PD-L1 and PD-L2) inhibits T cell proliferation and cytokine production in young mice. Increased PD-1 expression has been described during chronic infections, inducing chronic activation of the immune system to control it. As aging is associated with chronic immune activation, PD-1 may contribute to age-associated T cell dysfunction. Our data showed that in aged mice: (i) the number of PD-1-expressing T cells and the level of expression of PD-Ls was increased on dendritic cell subsets and T cells; (ii) PD-1+ T cells were exhausted effector memory T cells, as shown by their lower level of CD127, CD25 and CD28, as well as their limited proliferative and cytokine producing capacity; (iii) the expression of PD-1 was up-regulated after TCR-mediated activation of CD8+ T cells, but not of CD4+ T cells; (iv) blockade of the PD-1/PD-L1 pathway moderately improved the cytokine production of T cells from old mice but did not restore their proliferation; and (v) blockade of the PD-1/PD-L1 pathway did not restore function of PD-1+ T cells; its effect appeared to be exclusively mediated by increased functionality of the PD-1− T cells. Our data thus suggest that blockade of the PD-1/PD-L1 is not likely to be efficient at restoring exhausted T cell responses in aged hosts, although improving the responses of PD-1− T cells may prove to be a helpful strategy in enhancing primary responses.
Keywords: aging, T cells, dendritic cells, PD-1, PD-1 ligands
Introduction
Aging is associated with alterations in the immune responses to various infections and to vaccinations (Gavazzi & Krause 2002). Every component of the immune system is affected by aging, however T cells show the most consistent and largest alterations (Chakravarti & Abraham 1999; Eaton et al. 2004; Linton & Dorshkind 2004; Haynes et al. 2005; Kovaiou & Grubeck-Loebenstein 2006; Effros 2007). Proliferation and cytokine production by T cells are particularly dysregulated (Gardner & Murasko 2002). Declines in T cell function arise from both decreased number of naïve T cells and accumulation of defective naïve and memory T cells (Ernst et al. 1990; Utsuyama et al. 1992; Kurashima et al. 1995; Weyand et al. 1998; Effros 2000; Haynes et al. 2005). Intrinsic defects in T cell signalling in both CD4+ and CD8+ subsets have been described during aging (Kovaiou & Grubeck-Loebenstein 2006).
The importance of negative regulation of immune responses has recently been identified in models of chronic activation (Greenwald et al. 2005). One of these inhibitory molecules is the programmed death-1 (PD-1) receptor, that binds to two ligands, namely, PD-L1 (Dong et al. 1999; Freeman et al. 2000) or PD-L2 (Latchman et al. 2001; Tseng et al. 2001). In young mice, naïve T cells do not express PD-1, which is induced following engagement of the T cell receptor (TCR) (Yamazaki et al. 2002). However, PD-1 remains expressed on the surface of memory T cells (Yamazaki et al. 2002). In young mice, PD-L1 is present on multiple immune cells, including T and B cells, dendritic cells (DC) and macrophages, as well as on non-immune cells, whereas PD-L2 expression is more restricted, i.e. on activated DC and macrophages (Yamazaki et al. 2002). Blockade of PD-1 or PD-L1 results in enhanced T cell responses, either through a direct pathway (Freeman et al. 2006; Petrovas et al. 2006; Trautmann et al. 2006) or by abrogating inhibitory function of regulatory T cells (Treg) (Kitazawa et al. 2007). Since aging is associated with chronic activation of the immune system (Sansoni et al. 1993), PD-1/PD-L interactions could play a very significant role in the age-associated functional defects of T cells.
In this report, we showed that in aged mice: (i) the number of PD-1-expressing cells was increased, likely due to an increased proportion of memory cells; (ii) the expression of PD-Ls on T cell and DC subsets was increased; (iii) PD-1+ T cells were exhausted effector memory T cells, as shown by their lower level of CD127, CD25 and CD28 as well as their limited proliferative and capacity at producing cytokines; (iv) the up-regulated expression of PD-1 was observed in CD8+ T cells and not in CD4+ T cells following activation through the TCR; (v) blockade of the PD-1/PD-L1 pathway moderately improved the cytokine production of T cells but did not restore their proliferation; and vi) blockade of the PD-1/PD-L1 pathway did not restore function of PD-1+ T cells; its effect appeared to be exclusively mediated by increased functionality of the PD-1− T cells.
Results
PD-1 expression on T cells correlates with the increased frequency of memory cells in old mice
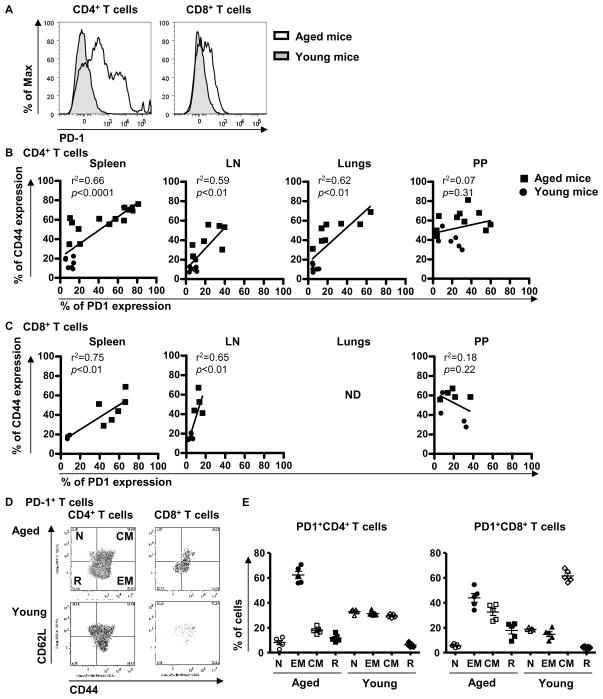
To address the role of the PD-1 pathway in aged mice, we first compared PD-1 expression in multiple tissues of young and aged mice. The percentage of CD4+ T cells expressing PD-1 was increased in the spleen of aged C57Bl/6 mice (≥ 16-month old) compared to young mice (≤ 2-month old) as already described (Channappanavar et al. 2009; Shimada et al. 2009; Shimatani et al. 2009), as well as in lymph nodes (LN) and lungs (Figure 1A and Table I). The same significant increase was observed when intracellular expression of PD-1 was measured in CD4+ T cells from the spleen (32.2 ± 7.2% in aged mice versus 7.5 ± 1.4% in young mice; p<0.05), the LN (11.2 ± 1.0% in aged mice versus 7.1 ± 0.6% in young mice; p=0.01), or the lung (19.2 ± 4.5% in aged mice versus 5.1 ± 0.3% in young mice; p=0.03). Similarly, the frequency of PD-1+CD8+ T cells was increased in the spleen and LN of aged mice (Figure 1A and Table I). In contrast, CD4+ and CD8+ T cells from the Peyer patches (PP) expressed surface PD-1 at the same high frequency in young and old mice (see Table I). Similarly, intracellular expression of PD-1 by PP cells was similar in young and aged mice (24.0 ± 2.8% of PP CD4+ in aged mice versus 27.5 ± 2.0% in young mice, p=0.37; 21.5 ± 5.3% of PP CD8+ in aged mice versus 21.9 ± 2.9% in young mice, p=0.96).
Figure 1. The expression of PD-1 is correlated with the memory and exhausted status of T cells in aged mice.
Single cell suspensions from spleens, LN, lungs and PP were stained for the surface markers CD4, CD8, CD44, CD62L and PD-1. Expression of PD-1 in CD4+ and CD8+ T cells from the spleen of young (3-month old) and aged (16-month old) mice by flow cytometry (A). Expression of PD-1 versus CD44 on CD4+ T cells (B) and on CD8+ T cells (C) from young (≤ 2-month old; circle) and aged (≥ 16-month old; square) mice. r2 indicate the linear regression between CD44 and PD-1. Significance was considered for p values less than 0.05. ND: not determined. Expression of CD62L and CD44 in PD-1+ and PD-1−CD4+ and CD8+ T cells from the spleen of young (3-month old) and aged (16-month old) mice by flow cytometry (D). Proportion of naïve (CD62L+CD44−), effector memory (EM: CD62L−CD44+), central memory (CM: CD62L+CD44+) and revertant (R: CD62L−CD44−) PD-1+CD4+ and CD8+ T cells from the spleen of young (3-month old) and aged (16-month old) mice (E).
Table I.
Expression of PD-1 by T cells from young and aged micea.
| CD4+ | CD8+ | |||||
|---|---|---|---|---|---|---|
| Young | Aged | pb | Young | Aged | pb | |
| Spleen | 10.4 ± 1.4 | 46.5 ± 7.2 | < 0.01 | 7.6 ± 0.8 | 49.7 ± 6.4 | < 0.01 |
| LN | 7.7 ± 1.1 | 23.6 ± 5.2 | 0.01 | 4.6 ± 0.7 | 12.8 ± 1.8 | < 0.01 |
| Lungs | 6.6 ± 1.1 | 27.3 ± 7.5 | 0.04 | NDc | NDc | |
| PP | 19.2 ± 4.0 | 29.7 ± 6.5 | 0.26 | 19.6 ± 6.9 | 18.2 ± 4.2 | 0.86 |
| CD4+CD44+ | CD8+CD44+ | |||||
|---|---|---|---|---|---|---|
| Young | Aged | pb | Young | Aged | pb | |
| Spleen | 28.6 ± 4.7 | 45.7 ± 6.7 | 0.11 | 26.3 ± 2.5 | 35.1 ± 6.8 | 0.45 |
| LN | 24.7 ± 3.4 | 33.3 ± 7.4 | 0.29 | 12.9 ± 1.4 | 18.7 ± 3.2 | 0.10 |
| Lungs | 19.9 ± 4.8 | 28.6 ± 8.1 | 0.42 | NDc | NDc | |
| PP | 33.8 ± 7.6 | 34.2 ± 7.8 | 0.97 | 40.7 ± 18.2 | 19.5 ± 5.0 | 0.21 |
| Treg | |||
|---|---|---|---|
| Young | Aged | pb | |
| Spleen | 20.5 ± 4.1 | 46.7 ± 7.6 | 0.03 |
| LN | 17.5 ± 3.6 | 28.8 ± 6.5 | 0.14 |
| Lungs | 15.9 ± 6.1 | 25.1 ± 5.7 | 0.30 |
| PP | 28.9 ± 7.2 | 26.4 ± 6.8 | 0.81 |
Cells were obtained from young mice (≤2-month-old, N≥ 6) and aged mice (≥16-month-old; N≥ 6) and stained for the surface markers CD4, CD8, CD44 and PD-1, followed by staining for the intracellular marker FoxP3. Treg were defined as CD4+FoxP3+ T cells. Values represent the mean (±SEM) percentage of PD-1 positive cells in the different subsets of T cells.
p values (t-test) compare the percentage of PD-1+ cells in young and aged mice.
ND: not determined
As memory T cells have been shown to express PD-1, we next determined whether the increased percentage of PD-1+ cells in aged mice was a reflection of the increased proportion of memory T cells in these animals. As expected, an increased percentage of CD4+ and CD8+CD44+ T cells was found in all tissues from old mice versus young mice (all p<0.02; data not shown). We thus compared the frequency of PD-1+ cells within the CD44+ memory T cell subsets in young and old mice. Percentages of PD-1+ cells in CD4+ and CD8+CD44+ T cells were identical in young and old mice (Table I). Furthermore, the percentage of PD-1+ T cells was correlated with the proportion of CD4+ and CD8+ T cells expressing CD44 (Figure 1B and C). Again, CD4+ and CD8+ T cells from PP appear different, because PD-1 expression did not correlate with CD44 expression in these tissues.
However, CD44 expression does not allow distinguishing between the two main subsets of memory T cells: the effector memory (EM; CD62L−CD44+) T cells and the central memory (CM; CD62L+CD44+) T cells. Moreover, the proportion of revertant (CD62L−CD44−) T cells increases with aging (Akbar & Fletcher 2005). To better characterized PD-1+ T cells in aged mice, we analyzed the proportion of the different memory subsets within the PD-1+ T cells from the spleen and the LN (Figure 1D, 1E and data not shown). In agreement with recently published data (Channappanavar et al. 2009; Shimada et al. 2009; Shimatani et al. 2009), the majority of the PD-1+CD4+ T cells belonged to the EM subset (>60%) in the spleen of aged mice, the rest being evenly distributed between the other cell subsets. PD-1+CD8+ T cells were mainly EM (~40%) or CM (~35%) cells (Figure 1E). This distribution was different from that observed in young mice, in which PD-1+CD4+ T cells belonged to all subsets, whereas PD-1+CD8+ T cells were mainly CM (Figure 1E). In the LN from aged mice, PD-1+CD4+ and CD8+ T cells were mainly EM and CM, whereas PD-1+ T cells from young mice were mainly CM (data not shown). Moreover, we observed that the expression of PD-1 per cell was increased in all memory CD4+ T cell subsets (EM, CM and revertant) in aged mice compared to young mice (all p<0.05; data not shown). In CD8+ T cells, the same level of PD-1 per cell was found in cells from old and young mice, whatever the subset analyzed (data not shown).
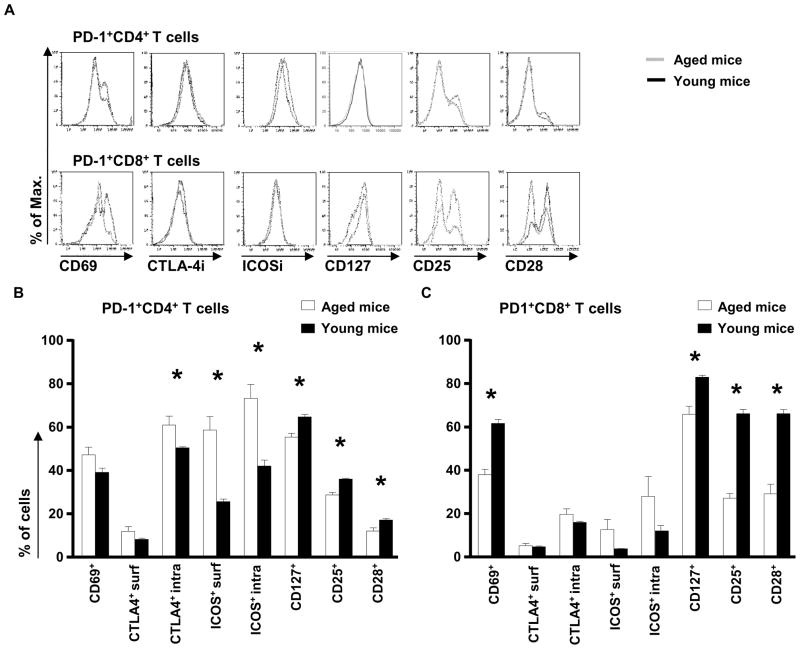
As aged PD-1+ T cells may be chronically-activated T cells, we analyzed the expression of different activation/differentiation markers on PD-1+ T cells from the spleen and LN of aged and young mice (Figure 2 and data not shown). In the spleen, aged PD-1+CD4+ T cells expressed the marker of recent activation CD69 at a similar frequency than young PD-1+CD4+ T cells (Figure 2A and 2B). ICOS, both intracellular and on the surface was expressed at a higher frequency by aged PD1+CD4+ T cells. In contrast, they express less CD25, CD127 and CD28 than young PD-1+CD4+ T cells (Figure 2A and 2B). Contrary to our observation, another study showed that the expression of CD69 and CD25 was increased in PD-1+CD4+ T cells from 23–25 month old mice; however, the majority of PD-1+ T cells (70%) were not recently activated cells (Shimada et al. 2009). In the LN, PD-1+CD4+ T cells expressed higher level of CD69 and ICOS intracellularly and on the surface than their young counterparts (data not shown). Aged PD-1+CD8+ T cells from the spleen also expressed lower levels of CD69, CD127, CD25 and CD28 than young PD-1+CD8+ T cells (Figure 2A and 2C). In the LN, the only difference noted in comparison to the spleen was the fact that PD-1+CD8+ T cells also expressed higher level of ICOS on their surface (data not shown). CTLA-4 expression was low on all PD-1+ T cells, and did not change with age. However, CTLA-4 expression intracellularly was significantly increased in PD-1+CD4+ T cells in the spleen as well as in the LN of aged versus young mice (Figure 2A, 2B and data not shown). Collectively, these data support the hypothesis that PD-1 expression in aged animals is associated with an “exhaustion” phenotype, and not with a phenotype of recent activation.
Figure 2. The expression of activation markers and costimulatory molecules on splenic PD-1+ T cells from aged mice.
Single cell suspensions from spleens were stained for the surface markers CD4, CD8, CD25, CD28, CD69, CD127, CTLA-4, ICOS and PD-1 and intracellularly for CTLA-4, and ICOS. Expression of PD-1 versus CD69 on CD4+ and CD8+ T cells from young (3-month old) and aged (16-month old) mice (A). Percentage of cells in PD-1+CD4+ (B) and PD-1+CD8+ (C) T cells expressing the different listed markers are shown. Significance was considered for p values less than 0.05 and indicated with asterisks.
As Treg have been shown to express PD-1 at a high level (Raimondi et al. 2006; Kitazawa et al. 2007; Polanczyk et al. 2007), we determined if the increased percentage of PD-1+CD4+ T cells in aged mice was a reflection of the increased proportion of Treg in these animals. In agreement with previous findings from our and other studies, Treg proportion was increased in the spleen and LN of aged mice but not in their lungs (Sharma et al. 2006; Thomas et al. 2007; Zhao et al. 2007; Lages et al. 2008) (data not shown). Our present data also showed an increased Treg frequency in PP (16.7%±2.5% in young mice versus 25.1%±2.6% in old mice; p=0.05). Treg appeared to express more frequently PD-1 than non-Treg in young mice (Table I, CD4+ versus Treg in young mice), confirming the trend observed in another study (Channappanavar et al. 2009). However, the level of expression of PD-1 did not differ in Treg from aged and young mice in most of the analyzed tissues, except in the spleen (Table I), as reported by other investigators (Channappanavar et al. 2009).
The expression of PD-Ls is increased on aged DC and T cells
It has been previously shown that PD-1 ligands are expressed by DC (Yamazaki et al. 2002). DC frequency and function is not well characterized in old mice. Therefore, we first characterized the proportion of conventional DC (CD11c+CD8α− or CD8α+ (Diao et al. 2004)) in several tissues. Compared to young mice, the proportion of CD8α− DC was significantly increased in the spleen, PP and lungs, but not LN of aged mice (Table II). The proportion of CD8α+ DC was the same in aged and young mice and CD8α−/CD8α+ ratios were identical in young and old mice (all p>0.05). Increased proportion of myeloid DC (mDC: CD11c+CD11b+Gr1−CD317−) was observed in lungs and LN of aged mice, but not in spleen and PP. The proportion of plasmacytoid DC (pDC: CD11c+CD11b−Gr1+CD317+) was similar between young and aged mice in all tissues (Table II). However, mDC/pDC ratios were identical in young and old mice (all p>0.05).
Table II.
Frequency of different DC subsets in young and aged micea
| Tissue | CD8α− | CD8α+ | ||||
|---|---|---|---|---|---|---|
| Young | Aged | pb | Young | Aged | pb | |
| Spleen | 7.3 ± 1.0 | 20.0 ± 4.5 | 0.03 | 1.1 ± 0.4 | 3.7 ± 1.7 | 0.21 |
| LN | 5.3 ± 1.4 | 11.7 ± 5.6 | 0.25 | 0.6 ± 0.2 | 2.1 ± 1.8 | 0.39 |
| Lungs | 13.1 ± 0.8 | 20.4 ± 2.7 | 0.02 | 0.9 ± 0.3 | 1.4 ± 0.4 | 0.30 |
| PP | 7.0 ± 1.6 | 16.3 ± 2.7 | 0.02 | 1.1 ± 0.4 | 2.3 ± 0.9 | 0.30 |
| Tissue | mDC | pDC | ||||
|---|---|---|---|---|---|---|
| Young | Aged | pb | Young | Aged | pb | |
| Spleen | 1.7 ± 0.3 | 4.9 ± 1.9 | 0.16 | 0.7 ± 0.3 | 0.9 ± 0.6 | 0.80 |
| LN | 0.4 ± 0.0 | 0.7 ± 0.1 | 0.02 | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.81 |
| Lungs | 1.6 ± 0.3 | 4.1 ± 0.9 | 0.02 | 1.5 ± 0.2 | 1.4 ± 0.2 | 0.80 |
| PP | 0.7 ± 0.4 | 1.2 ± 0.5 | 0.51 | 0.6 ± 0.3 | 0.8 ± 0.6 | 0.75 |
Cells were obtained from aged mice (≤2-month-old; N≥ 3) and young mice (≥16-month-old; N≥ 3). CD8α− DC were defined as CD11c+CD8α−, CD8α+ DC as CD11c+CD8α+. mDC are defined as CD11c+CD11b+Gr1−CD317− and pDC as CD11c+CD11b−Gr1+CD317+. Values represent the mean (±SEM) percentage of each subset in the live population defined by 7-AAD exclusion.
p values (t-test) compare the percentage of each subset in young and aged mice.
Thereafter, we characterized the PD-1 ligand expression in the different DC subsets. The proportion of cells expressing PD-L1 was increased in mDC from lungs of aged mice, as well as pDC from spleen of aged mice (Table III). Intensity of PD-L1 expression on a per cell basis was only increased on pDC and mDC subsets from the lungs of aged mice (p<0.03, data not shown). The proportion of DC expressing PD-L2 was increased in CD8α+, CD8α− and mDC from spleen and in CD8α− and mDC from lungs of aged mice compared to young mice (Table III). However, the intensity of PD-L2 expression on a per cell basis was only increased on mDC from spleen of aged mice (p<0.01, data not shown). These data suggest that PD-1 ligands are globally more abundant in older mice, due to either a higher number of DC, a higher proportion of DC expressing these molecules, a higher expression of the ligands per cell, or a combination of these factors.
Table III.
Expression of PD-L1 and PD-L2 by CD8a− and CD8a+ DC, mDC and pDC in young and aged mice
| Tissue | CD8α+ DC PD-L1 | CD8α+ DC PD-L2 | ||||
|---|---|---|---|---|---|---|
| Young | Aged | pb | Young | Aged | pb | |
| Spleen | 45.8 ± 5.6 | 62.9 ± 6.7 | 0.08 | 7.8 ± 2.1 | 18.7 ± 4.2 | 0.03 |
| LN | 41.3 ± 14.5 | 45.3 ± 17.6 | 0.87 | 9.7 ± 3.0 | 12.7 ± 5.5 | 0.62 |
| Lungs | 70.4 ± 5.7 | 58.0 ± 7.3 | 0.68 | 12.9 ± 3.7 | 16.2 ± 2.9 | 0.50 |
| PP | 24.5 ± 10.8 | 32.1 ± 13.4 | 0.67 | 7.3 ± 2.2 | 11.1 ± 7.5 | 0.74 |
| Tissue | CD8α− DC PD-L1 | CD8α−DC PD-L2 | ||||
|---|---|---|---|---|---|---|
| Young | Aged | pb | Young | Aged | pb | |
| Spleen | 42.7 ± 5.1 | 57.0 ± 7.1 | 0.14 | 5.6 ± 1.2 | 18.6 ± 4.8 | 0.05 |
| LN | 42.2 ± 6.6 | 44.8 ± 3.4 | 0.76 | 10.0 ± 3.1 | 11.9 ± 2.6 | 0.67 |
| Lungs | 53.3 ± 2.3 | 55.1 ± 3.2 | 0.23 | 6.6 ± 0.8 | 13.1 ± 1.1 | <0.01 |
| PP | 33.4 ± 12.1 | 39.3 ± 15.1 | 0.77 | 9.7 ± 2.3 | 12.5 ± 7.8 | 0.64 |
| Tissue | mDC PD-L1 | mDC PD-L2 | ||||
|---|---|---|---|---|---|---|
| Young | Aged | pb | Young | Aged | pb | |
| Spleen | 79.2 ± 2.1 | 79.2 ± 5.0 | 1.00 | 3.3 ± 0.7 | 24.1 ± 7.3 | 0.02 |
| LN | 90.5 ± 1.1 | 93.3 ± 2.1 | 0.25 | 31.9 ± 4.6 | 33.5 ± 4.0 | 0.81 |
| Lungs | 28.0 ± 4.4 | 60.5 ± 5.9 | 0.01 | 3.3 ± 0.4 | 10.8 ± 2.6 | 0.02 |
| PP | 80.9 ± 5.1 | 91.6 ± 2.8 | 0.10 | 10.7 ± 5.8 | 22.6 ± 8.1 | 0.27 |
| Tissue | pDC PD-L1 | pDC PD-L2 | ||||
|---|---|---|---|---|---|---|
| Young | Aged | pb | Young | Aged | pb | |
| Spleen | 47.4 ± 8.1 | 78.1 ± 4.8 | 0.01 | 16.9 ± 6.0 | 29.3 ± 7.6 | 0.24 |
| LN | 24.8 ± 5.1 | 25.2 ± 12.9 | 0.97 | 3.1 ± 0.3 | 4.4 ± 2.3 | 0.54 |
| Lungs | 66.8 ± 3.5 | 75.7 ± 4.5 | 0.17 | 7.6 ± 2.6 | 13.2 ± 3.6 | 0.23 |
| PP | 11.1 ± 6.3 | 28.8 ± 15.2 | 0.32 | 8.9 ± 1.2 | 10.9 ± 7.8 | 0.83 |
Cells were obtained from aged mice (≤2-month-old; N≥ 3) and young mice (≤16-month-old; N≥ 3). CD8α− DC were defined as CD11c+CD8α−, CD8α+ DC as CD11c+CD8α+. mDC are defined as CD11c+CD11b+Gr1−CD317− and pDC as CD11c+CD11b−Gr1+CD317+. Values represent the mean (±SEM) percentage of each subset in the live population defined by 7-AAD exclusion.
p values (t-test) compare the percentage of each subset in young and aged mice.
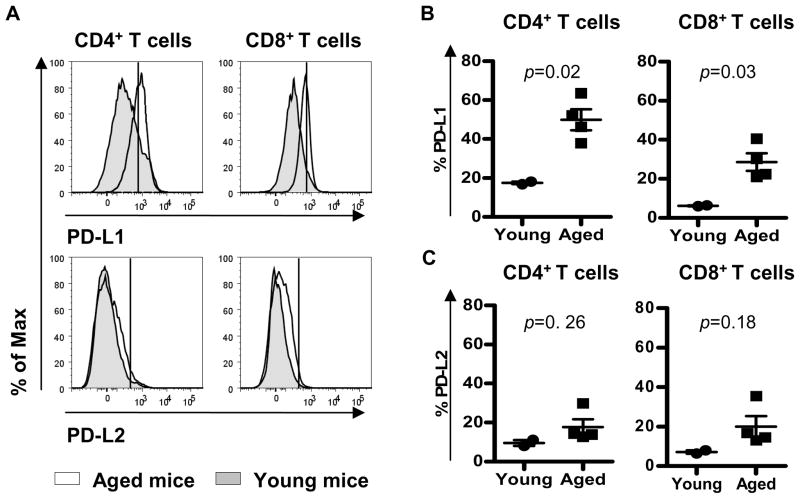
PD-L1 is up-regulated on stimulated T cells while PD-L2 is not expressed on these cells (Yamazaki et al. 2002). We therefore analyzed the expression of both ligands on splenic T cells. The percentage of young T cells expressing PD-L1 and PD-L2 was low (<20%) (Figure 3A, B and C). With this consideration, 30–50% of splenic CD4+ and CD8+ T cells from aged mice expressed PD-L1. PD-L1 expression was significantly increased on CD4+ and CD8+ T cells from older mice (Figure 3B). .In contrast, PD-L2 expression showed a minor non-significant, increase in older mice (Figure 3C).
Figure 3. The expression of PD-L1 and PD-L2 is increased on splenic T cells from aged mice.
Single cell suspensions from spleens were stained for the surface markers CD4, CD8, PD-L1 and PD-L2. Expression of PD-L1 and PD-L2 in CD4+ and CD8+ T cells from young (≤ 2-month old) and aged (≥ 16-month old) mice was analyzed by flow cytometry (A). PD-L1 and PD-L2 gates were setup on the highly positive population observed in young mice. Percentage of PD-L1+ (B) and PD-L2+ (C) cells in CD4+ and CD8+ T cells from young (circle) and aged (square) mice is shown. Horizontal lines represent the mean percentage of PD1-Ls + in CD4+ or CD8+ splenic T cells from young and old mice. p values (t-test) compare this percentage between young and old mice.
PD-1 expression can be induced in vitro on T cells from old mice
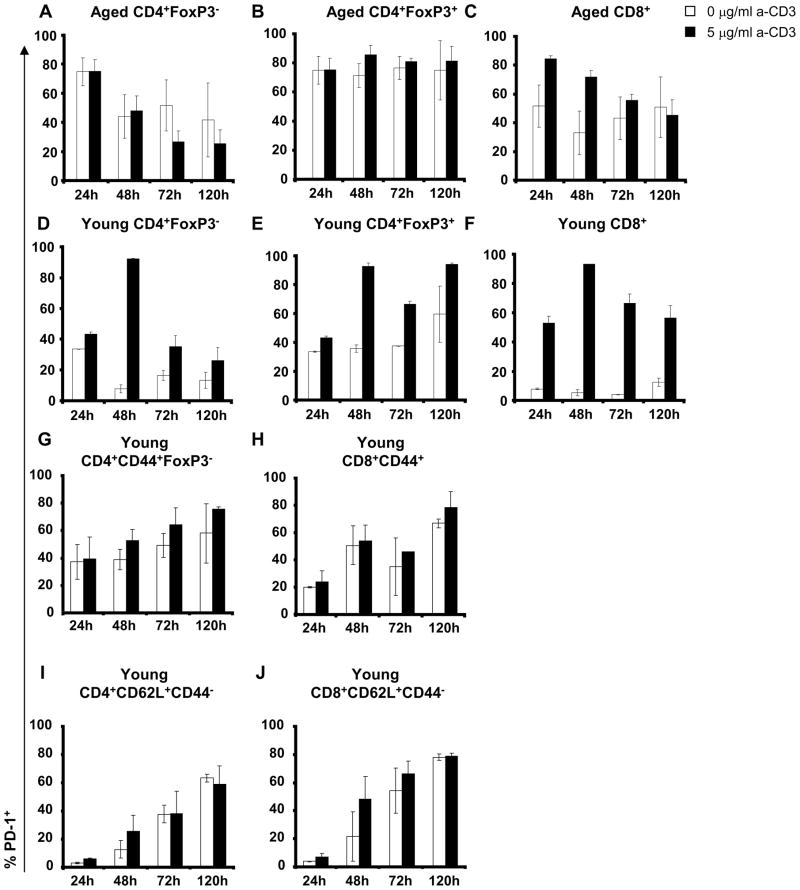
PD-1 expression is transiently up-regulated following T cell activation in young mice (Yamazaki et al. 2002). We therefore compared PD-1 expression by anti-CD3-stimulated young and old CD4+ and CD8+ T cells from the spleen and the lungs. We separately analyzed PD-1 induction on activated non-Treg (defined as CD4+FoxP3− T cells, Figure 4A and D) and Treg (defined as CD4+FoxP3+ T cells, Figure 4B and E), because Treg constitutively express a higher PD-1 level than total CD4+ (see Table I). PD-1 expression by old CD4+ T cells, both Treg and non-Treg was high in absence of stimulation (>60%) and did not change following stimulation (Figure 4A and B), as reported for total CD4+ T cells (Shimada et al. 2009). Decreased expression by non-Treg was observed 48 h after culture initiation, in stimulated and non stimulated cells (Figure 4A). In contrast, the PD-1 expression remained stable and high in Treg (Figure 4B). This pattern was different from that observed for young T cells. Most of the young CD4+ T cells, both non-Treg and Treg, expressed PD-1 after 48 h (Figure 4D and E). PD-1 expression remained high on young Treg after 120 h (Figure 4E).
Figure 4. PD-1 expression is modulated in old spleens after CD3-stimulation.
Percentage of PD-1+ T cells in spleens from young and aged mice. Single cell suspension from spleens was stimulated without anti-CD3 (white bar) or with 5 μg/ml anti-CD3 (black bar) from 24 h to 120 h. Recovered cells were first stained for the surface markers CD4, CD8 and PD-1, followed by staining for the intracellular marker FoxP3 to analyze the expression of PD-1 in CD4+ non-Treg (A), Treg (B) and CD8+ T cells (C) from aged (≥ 16-month old; top; N=3) mice and in CD4+ non-Treg (D), Treg (E) and CD8+ T cells (F) from young (≤ 2-month old; bottom; N=3) mice. The mean of two independent experiments is shown as well as the standard deviation. T cells isolated from spleens of young mice were sorted for CD44 expression and CD44+ T cells were stimulated without anti-CD3 (white bar) or with 5 μg/ml anti-CD3 (black bar) from 24 h to 120 h. Recovered cells were first stained for the surface markers CD4, CD8 and PD-1, followed by staining for the intracellular marker FoxP3 to analyze the percentage of PD-1+ T cells in CD44+CD4+ non-Treg (G), CD44+CD8+ T cells (H), CD62L+CD44−CD4+ T cells (I) and CD62L+CD44−CD8+ T cells (J) from young mice (≤ 3-month old; N=8).
PD-1 expression was up-regulated on old CD8+ T cells after anti-CD3 stimulation. In older mice, most of the CD8+ T cells expressed PD-1 after 24 h, its expression returning to baseline level after 120 h (Figure 4C). In young mice, PD-1 was strongly up-regulated on CD8+ T cells, and expressed on most of the CD8+ T cells 48 h after anti-CD3 stimulation. The down-regulation of PD-1 expression was slower in young mice than that in old mice, with 60% of the CD8+ cells still expressing PD-1 120 h after stimulation (Figure 4F).
We also determined the effect of different doses of anti-CD3 since the PD-1/PD-L pathway appears to play a particularly important role when T cells receive low levels of TCR stimulation (Cai et al. 2004). Intermediate dose of anti-CD3 (2.5 μg/ml) induced similar upregulation of PD-1 expression than the higher dose of anti-CD3 (5 μg/ml) (data not shown). Upon stimulation with low anti-CD3 dose (0.1 μg/ml), PD-1 expression was not induced neither in old nor in young mice (data not shown). Similar results were observed with activated T cells purified from the lungs (data not shown).
To ascertain if the lower up-regulation of PD-1 expression observed in old mice was linked with the higher proportion of memory T cells, which already express a high level of PD-1, we isolated memory CD44+ T cells from young mice and analyzed the expression of PD-1 after TCR stimulation. Basal PD-1 expression was higher in CD44+ T cells than in total T cells or naïve CD62L+CD44− T cells from young mice (Figure 4G and 4H versus 4D and 4F), confirming our previous results. Anti-CD3 stimulation moderately increased PD-1 expression in all subsets (Figure 4G, H, I and J).
The PD-1/PD-L1 pathway inhibits cytokine production by PD-1− T cells from aged mice
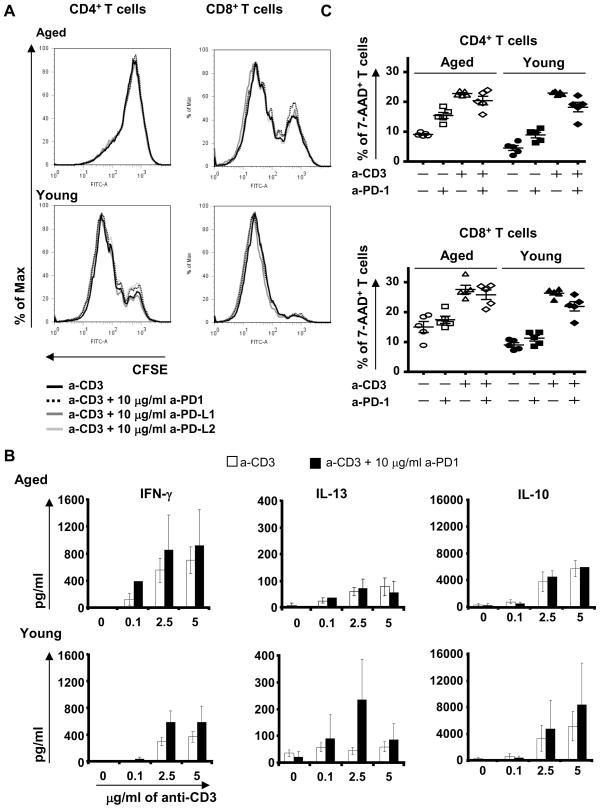
Proliferation of aged CD4+ and CD8+ T cells after TCR stimulation is dramatically impaired (Song et al. 1993), and our results are in agreement with that finding (Figure 5A). To determine if the high expression of PD-1 and PD-Ls in aged mice may be responsible for such decreased reactivity of old T cells, we analyzed the effect of blocking anti-PD-1, PD-L1 and PD-L2 antibodies on the proliferation of splenic T cells from old mice. Blockade of the PD-1 pathway did not improve the proliferation of old CD4+ and CD8+ T cells, whatever the concentration of blocking antibody and of anti-CD3 antibody used (Figure 5A and data not shown). T cells from old mice already showed elevated levels of IFN-γ production compared to T cells from young mice, and PD-1/PD-L1 blockade further enhanced this difference, albeit not significantly (Figure 5B for anti-PD-1 antibody, anti-PD-L1 antibody not shown). IL-13 production was poorly induced by anti-CD3 stimulation, and anti-PD-1 antibody did not improve it (Figure 5B), nor did this treatment improve IL-10 production (Figure 5B). In young mice, PD-1/PD-L1 blockade trend to increased IFN-γ IL-13 and IL-10 production but had no impact on T cell proliferation (Figure 5A and 5B). In contrast, anti-PD-L2 antibody had no effect on the proliferation or cytokine production of T cells from either young or old mice (data not shown).
Figure 5. Blockade of PD-1 pathway did not improve T cell survival and proliferation, but improved IFN-γ and IL-10 productions by old splenic cells.
Splenic cells from young (≤ 2-month old; N=4) and aged (≥ 16-month old; N=6) mice were stain with CFSE and stimulated for 72h with anti-CD3 alone or with Ab blocking PD-1/PD-Ls pathway. The proliferation of CD4+ and CD8+ T cells in young (bottom) and aged (top) mice was analyzed by flow cytometry (A). 5 μg/ml of anti-CD3 was used alone (black line) or in combination with 10 μg/ml of anti-PD-1 (dotted black line), anti-PD-L1 (dark gray line) or anti-PD-L2 (light gray line). One representative mouse is shown for each age group. The production of IFN-γ, IL-13 and IL-10 was analyzed by ELISA in 5 day-supernatants (B). Anti-CD3 at 0, 0.1, 2.5 and 5 μg/ml was used alone (white column) or in combination with 10 μg/ml (black column) of anti-PD-1. This experiment is representative of two independent experiments. Splenic and LN cells from young (3-month old; N=4) and aged (15-month old; N=4) mice were stimulated for 5 days with or without anti-CD3 alone (5 μg/ml), in presence or not of anti-PD-1 antibody (10 μg/ml). The percentage of 7-AAD+ CD4+ and CD8+ T cells in young and aged mice was analyzed after 5 days of culture by flow cytometry (C).
The PD-1 pathway was initially identified by its role in cell survival (Ishida et al. 1992). Increased production of cytokines following its blockade may thus simply reflect enhanced cell survival. To determine if this hypothesis was correct, we analyzed the percentage of 7-AAD+ cells in all culture conditions (Figure 5C). Blocking the PD-1 pathway did not significantly change the percentage of 7-AAD+ CD4+ or CD8+T cells 5 days after stimulation, and that in both aged and young mice (Figure 5C). In absence of activation, the blocking PD-1 antibody slightly increased CD4+ T cell death, but its effect was the same in aged and young mice (Figure 5C).
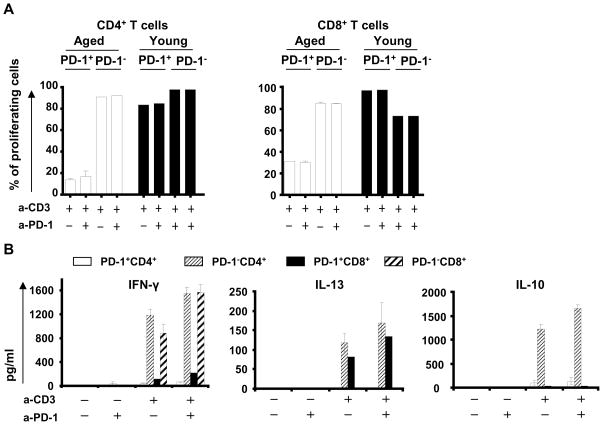
Next, to better characterize the functionality of PD-1 pathway in T cells, we isolated PD-1+ and PD-1− CD4+ or CD8+ T cells from old spleen and LN, and stimulated them by anti-CD3 antibody in presence of young DC. This strategy was chosen to specifically study the contribution of high PD-1 expression in the T cell functional defects in aged animals. PD-1+CD4+ or CD8+ T cells from old mice proliferated poorly after stimulation, as recently reported (Shimada et al. 2009; Shimatani et al. 2009), and blockade of the PD-1 pathway did not improve this defect (Figure 6A). In contrast, PD-1+ T cells from young mice proliferated as much as PD-1− cells from the same animals. Interestingly, PD-1− cells from aged mice exhibited normal levels of proliferation after stimulation, which were not further enhanced by anti-PD-1 antibody (Figure 6A and data not shown).
Figure 6. Blockade of PD-1 pathway in T cells from old mice did not improve T cell proliferation or survival, but improved cytokine production by PD-1− CD4+ and CD8+ T cells.
Splenic cells from young (3-month old; N=6) and aged (12-month old; N=6) mice were stained with CFSE and stimulated for 5 days with or without 5 μg/ml of anti-CD3 alone or with 10 μg/ml of antibody blocking PD-1/PD-Ls pathway. The proliferation of CD4+ and CD8+ T cells in young (bottom) and aged (top) mice was analyzed by flow cytometry and the percentage of cells which divided at least once is summarized (A). The production of IFN-γ, IL-13 and IL-10 was analyzed by ELISA (B). The percentage of 7-AAD+ T cells in PD-1+CD4+ or CD8+ and PD-1−CD4+ or CD8+ was analyzed by flow cytometry (C).
We also analyzed the effect of PD-1 blockade on cytokine production by PD-1+ and PD-1− CD4+ and CD8+ T cells from aged mice. Old PD-1+ CD4+ or CD8+ T cells did not produce IFN-γ or IL-10 after TCR stimulation, suggesting that PD-1 expression on T cells from aged mice characterized a subset of exhausted T cells. However a small production of IL-13 was observed in PD-1+CD8+ T cells. Blocking the inhibitory PD-1 pathway did not restore the functionality of those PD-1+ T cells, as evidenced by the lack of improvement in their proliferation or cytokine production (Figure 6A and 6B). In contrast, PD-1 blockade significantly increased IFN-γ production by both PD-1−CD4+ and PD-1−CD8+ T cells from aged mice, and elicited significantly higher levels of IL-10 production by PD-1−CD4+ T cells (Figure 6B). IL-13 production in PD-1−CD4+ T cells exhibited a trend toward increase; but remained low. Anti-PD-1 antibody did not significantly promote cell survival of any of the isolated T cell subsets (data not shown), again suggesting that the increased production of cytokines by PD-1− T cells was likely not due to their better survival.
Discussion
Mechanisms underlying immune suppression in aging are not well characterized. Altered balance between positive and negative regulators of the immune response could represent an underlying mechanism in such decreased responsiveness. In this study, we focused on a recently identified inhibitory pathway, the interaction between PD-1 and it ligands. The PD-1 pathway is exploited by many viruses to evade immune responses and establish persistent infection (Jun et al. 2005; Barber et al. 2006; Day et al. 2006; Petrovas et al. 2006; Urbani et al. 2006; Golden-Mason et al. 2007; Shimauchi et al. 2007; Zhang et al. 2007; Peng et al. 2008).
Higher percentages of CD4+ and CD8+ T cells expressing PD-1 were observed in most tissues from old C57Bl/6 mice compared to young animals, as recently reported for CD4+ T cells from the spleen (Channappanavar et al. 2009; Shimada et al. 2009; Shimatani et al. 2009), but not in T cells from the spleen of Balb/c mice (Mirza et al. 2010) suggesting that PD-1 expression is differently regulated in different mouse strains. This increased PD-1 expression correlated with the increased proportion of memory T cells in most of the analyzed tissues from old mice, confirming the increased proportion of PD-1+CD44+CD4+ T cells observed in the spleen during aging (Shimatani et al. 2009). However, when PD-1+ T cells were further characterized in aged mice, our data show that they exhibit a profile typical of exhausted cells and not of recently activated cells; they have predominantly an EM phenotype, as recently reported (Channappanavar et al. 2009; Shimada et al. 2009; Shimatani et al. 2009); they lost the expression of CD25 and CD28 and expressed low levels of CD127. In contrast, PD-1+ cells from young mice expressed higher levels of activation markers than in old mice. Altogether, PD-1+ T cells from old mice exhibit a profile reminiscent of the PD-1+ T cells that accumulate during chronic viral infection (Wherry et al. 2007). Of note, PD-1+ CD4+ and CD8+ cells did not distribute similarly between memory subsets, nor did they express similar levels of activation/maturation markers, a difference that may reflect the fact that different mechanisms regulate survival of memory CD4+ and memory CD8+ T cells (Wojciechowski et al. 2007).
Interestingly, the only tissues in which we did not observe differences in the expression of PD-1 between young and aged mice were the PP, in which the proportion of memory T cells is twice the proportion observed in other tissues in young mice, as described earlier (Carril et al. 2002). The chronic stimulation of the gut by food intake and bacterial microenvironment may be responsible for these observations and suggest that an important function of the PD-1 pathway could be to maintain peripheral tolerance in the gut associated lymphoid tissue.
PD-1 ligands are expressed on DC (Yamazaki et al. 2002). Increased expression of both PD-1 ligands by DC was observed in aged mice, as a result of either the increased expression of ligand per DC, or increased percentage of PD-L-expressing DC such as CD8α− DC and mDC. The same trend was observed in macrophages and CD11c+ DC from the spleen of aged Balb/c mice (Mirza et al. 2010). Moreover, old CD4+ and CD8+ T cells expressed higher levels of PD-L1 than T cells from young C57BL/6 mice. As a consequence, global PD-L levels were significantly increased in old C57BL/6 animals. However, this increase was only observed in CD8+ T cells from Balb/c mice (Mirza et al. 2010). The discrepancy could be due to the differences in the mouse strain studied and/or in the gating strategy used to define PD-L1 expression. Indeed, our threshold of positivity for PD-L1 appears higher than the one used by Mirza and collaborators, who also reported a high basal expression of PD-L1 on T cells from young mice (Mirza et al. 2010). The combined increase of both PD-1 expression and PD-L expression suggests that the PD-1/PD-L pathway could play an important role in the immune suppression associated with aging. However, we did not see any age-related improvement in the capacity of T cells to proliferate after TCR engagement by blocking PD-1, PD-L1, or PD-L2. This is in contrast to the increased T cell proliferation reported in PD-1 knock-out C57BL/6 mice (Freeman et al. 2000; Wang et al. 2007). Blocking antibodies, such as those used in our study, would be expected to be less efficient than a genetic knock-out and this difference could explain why our in vitro model was not able to reveal an increased proliferation after PD-1/PD-L1 blockade. Alternatively, an acute model of activation such as in vitro stimulation by anti-CD3 antibody may not be optimal to reveal a role of the PD-1 pathway in the lack of T cell responses in aged mice. Indeed, the selective expansion of exhausted CD8+ T cells by anti-PD-L1 blockade has been shown during chronic infection, but not acute infection (Blackburn et al. 2008), suggesting a role of the PD-1 pathway mainly during chronic infection. Another explanation could be the type of activation used. We used DC and anti-CD3 antibody, whereas Mirza and collaborators used beads coated by both anti-CD3 and anti-CD28 (Mirza et al. 2010). DC express a vast array of molecules involved in T cell activation, and the concomitant presence of these molecules may have modulated the effect of anti-PD-L1 antibody in vitro. In contrast to its lack of effect on T cell proliferation, blockade of PD-1/PD-L1 improved cytokine production, a data in agreement with a previous study performed in young mice (Barber et al. 2006). However, PD-1 blockade was less efficient to improve functionality of T cells in old mice, as it only trend to improve IFN-γ production in these mice.
In our study, anti-PD-L2 antibody had no effect, as reported by other studies in humans (Cai et al. 2004). Contrary to PD-L2, PD-L1 can also interact with CD80 to inhibit T cell responses in mice and humans (Butte et al. 2007; Butte et al. 2008). Since CD80 expression is also increased on old splenic DC ((Sun et al. 2004) and data not shown), the fact that anti-PD-L1 but not anti-PD-L2 increased IFN-γ production could result from CD80/PD-L1 interaction. However, we did not observe any differences in the effect of anti-PD-1 and anti-PD-L1, which supports the hypothesis that blocking PD-1/PD-L1 interaction, and not CD80/PD-L1, was mainly responsible of the increased cytokine production.
Interestingly, PD-1+ T cells, both CD4+ and CD8+ T cells, from old mice showed more profound defects than their young counterparts. In particular, their capacity to proliferate in response to TCR stimulation was severely abolished (Figure 6A, (Shimada et al. 2009; Shimatani et al. 2009)). This defect could be associated with their higher level of PD-1 expression per cell, likely due to the cumulative pressure on the immune system exerted by a lifespan of chronic stimulation. Alternatively, the changed balance between positive and negative signals may be responsible for the defects exhibited by PD-1+ T cells from aged mice. Indeed, CD28 expression is significantly reduced in PD-1+ T cells from aged mice compared to their young counterparts (Figure 2). However, the level of ICOS on CD4+ T cells from the spleen and LN of aged mice and on CD8+ T cells from LN of aged mice was increased. The positive signals given through CD28 engagement will likely be defective in aged PD-1+ T cells but not the positive signals given through ICOS engagement. Of note, similar low levels of CTLA-4 expression were found on T cells from young and old mice, a result that does not suggest a major role for CTLA-4-mediated inhibition of T cell proliferation in old mice.
Separated PD-1− T cells from aged mice proliferated normally after stimulation by young DC, suggesting that PD-1+ T cells actively inhibited the activation of PD-1− cells. Several underlying mechanisms could be involved. PD-1+ T cells may directly inhibit PD-1− T cell activation by interacting with PD-L1, which is induced on the surface of these cells by TCR engagement (Yamazaki et al. 2002), thus inhibiting their proliferation. Alternatively, PD-1+ T cells may indirectly control PD-1− T cell responses, by controlling DC activation. Indeed, interactions of PD-1+ T cells with DC induce a state of incomplete activation in these DC, which will decrease the signal given to PD-1− cells (Fife et al. 2009). Of note, PD-1+CD4+ T cells, but not PD-1−CD4+ T cells, were reported to produce IL-10 in young mice (Hatachi et al. 2003; Meng et al. 2006), and this mechanism could also participate into the control exerted by PD-1+ T cells. However, our results do not favour this hypothesis, because production of IL-10 by old PD-1+ T cells was very low, even after stimulation (Figure 6B).
Blocking the PD-1 pathway increased both IFN-γ and IL-10 production by PD-1−CD4+ T cells, but not PD-1+ T cells, from aged mice. It also increased IFN-γ production by old PD-1−CD8+ T cells. Those data suggest the hypothesis that TCR stimulation of PD-1− T cells induces PD-1 expression, which can interact with PD-L1 expressed by DC or other activated T cells, thus triggering inhibitory pathways. Although PD-1 was originally described as regulating death (Ishida et al. 1992), our data do not support the hypothesis that blocking PD-1-mediated death is important in this improved functionality, as the percentage of dead CD4+ or CD8+ T cells was not decreased by the blocking anti-PD-1 antibody (Fig. 6C). Blocking PD-1 pathway did not restore the functionality of aged exhausted PD-1+ T cells but increased that of PD-1− T cells. Improving the responses of these PD-1− T cells in aged mice may enhance primary responses (i. e. vaccines, tumor therapies…) in these animals. Of note, anti-PD-L1 antibody increased the primary response against a tumor, restoring survival of treated mice to levels similar to those achieved in young animals (Mirza et al. 2010).
In summary, although the expression of all the molecules involved in the PD-1/PD-L pathway is increased in aged mice, its involvement in the immune dysfunction associated with age appears minor, and is limited to PD-1− cells.
Experimental procedures
Mice
C57BL/6 mice were purchased from the Taconic Farms, Jackson Laboratories, and the National Institutes of Aging Colony. All mice were used for the experiments at 6–12 weeks (young mice) and more than 16 month-old (aged mice). Mice were housed under specific pathogen free (SPF) conditions in the Cincinnati Children’s Hospital vivarium. All animal protocols were reviewed and approved by our Institutional Animal Care and Use Committee (IACUC).
Cell isolation
Lungs, spleen, lymph nodes (LN), and Peyer patches (PP) were recovered and incubated with 0.5 mg/ml Liberase CI (Roche Diagnostics, Indianapolis, IN) and 0.5 mg/ml DNase I (Sigma, St. Louis, MO) at 37°C for 45 minutes. The tissue was forced through a 70 μm cell strainer, and red blood cells were lysed with ACK lysis buffer (Invitrogen, Carlsbad, CA).
Splenic low-density (LOD) cells were obtained by centrifugation of cell suspension on 30% BSA. To purify splenic DC, splenic LOD cell suspensions were incubated with pan-DC biotinylated antibodies followed by anti-biotin microbeads according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). Pan-T Cell Isolation kits were used to purify T cells from the pellet of the BSA-gradient. To purify CD44+ and CD44+CD62L+ T cells, cells were sorted on their expression of CD62L and CD44 with a FACSAria after staining with anti-CD62L-PE (MEL-14) and CD44-Pacific Blue (IM7) antibodies. To purify PD-1+CD4+ or CD8+ and PD-1−CD4+ or CD8+ T cells, T cells were sorted on their expression of PD-1, CD4 and CD8 with a FACSAria after staining with anti-PD-1-PE (J43), CD4–PE-Cy7 (RM4-5) and CD8–AF700 (53-6.7) antibodies.
Flow cytometry
Staining was performed at 4°C following incubation with Fc-Block (mAb 2.4G2) for 30 minutes. CD8α− DC (CD11c+CD8α−), CD8α+ DC (CD11c+CD8α+), mDC (CD11c+CD11b+Gr1−CD317−) and pDC (CD11c+CD11b−Gr1+CD317+) were quantified using anti-CD11c-APC (HL3), anti-CD8α-AF700 (53-6.7), anti-CD11b-PE-Cy7 (M1/70), anti-Gr1-APC-Cy7 (RB6-8C5) and anti-CD317-AF488 (120g8). Co-stimulatory molecule expression was examined using PE-conjugated mAbs to PD-L1 (MIH5) and PD-L2 (TY25). Dead cells were excluded using 7-AAD. All antibodies were purchased from eBioscience (eBioscience Inc., San Diego, CA).
PD-1 expression in T cells was quantified using anti-CD4-PE-Cy7 (RM4-5) or -AF700 (RM4-5 or L3T4), anti-CD8α-AF700, -APC-Cy7 or -APC-AF750 (53-6.7), anti-CD25-FITC (7D4), anti-CD28-FITC (37.51.1), anti-CD44-FITC or -Pacific Blue (IM7), anti-CD62L-FITC, anti-CD62L-APC-AF750 or anti-CD62L-PE-Cy7 (MEL-14), anti-CD69-PerCP-Cy5.5 (H1.2F3), anti-CD127-FITC (A7R34), anti-PD-1-FITC or -PE (J43), anti-CTLA-4-PE (UC10-4B9), anti-ICOS-PE (C398.4A), anti-PD-L1-PE or anti-PD-L2-PE. All antibodies were purchased from eBioscience (eBioscience Inc., San Diego, CA), except anti-CD25 (BD Biosciences (San Jose, CA) and anti-CD28 (AbDSerotec; Raleigh, NC). Cells were then fixed and stained intracellularly with anti-FoxP3-AF647 (FJK-16s), PD-1-PE, CTLA-4-PE and ICOS-PE according to eBioscience protocol.
Cell acquisition was performed on a LSRII flow cytometer (BD Biosciences). Spectral overlap was compensated using the FACSDiva software (BD Biosciences) and analyzed using FlowJo software (Treestar Inc., Ashland, OR).
In vitro stimulation
250,000 cells were cultured in 300 μl of RPMI supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 292 μg/ml L-glutamine (Invitrogen) and 10% FCS in a 96 well plate with or without anti-CD3ε (145-2C11), anti-PD-1 (RMP1-14), anti-PD-L1 (MIH5) or anti-PD-L2 (TY25) at the indicated concentrations. All antibodies were purchased from eBioscience.
Proliferation assay
Cells were labelled for 5 min at room temperature with 0.625 μM of carboxyfluorescein diacetate succinimidyl ester, CFSE (Molecular Probes, Eugene, OR). CFSE-labelled T cells were cultured with unlabeled DC at a ratio of 10:1 and stimulated or not for 24h, 48h, 72h and 120h at 37ºC in 5% CO2. Cells were harvested, incubated with Fc-Block for 30 min at 4°C and surface stained with anti-CD4-PE-Cy7, anti-CD8-AF700, anti-CD44-PB and anti-CD62L-APC-AF750.
Cytokine assays
Cytokine levels in culture supernatants were measured by an enzyme linked immunosorbent assay (ELISA) using matched antibody pairs for IFN-γ (BD Pharmingen, San Jose, CA), IL-13 and IL-10 (both from R&D Systems, Minneapolis, MN). Culture supernatants were frozen at −80°C and thawed for assay.
Statistical analysis
Experimental data are expressed as mean ± SD. The statistical significance of differences in mean values was determined using Student’s t tests. p<0.05 is considered as significant.
Acknowledgments
The authors would like to acknowledge Simon Hogan for Peyer Patches recovery and Julio Aliberti for help with DC isolation. We also thank David Hildeman, Kris Orsborn and Anjali Mishra for their comments during the preparation of this manuscript.
Grant NIH R01 AG033057 to CC
Footnotes
Author contributions
Celine S. Lages and Ian Lewkowich, Alyssa Sproles performed the experiments and analyzed the data. CSL, IL, MWK and CC designed experiments and wrote the manuscript.
References
- Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8(+) T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2008 doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Molecular immunology. 2008;45:3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G, Karni A, Oliveira EM, Weiner HL, Hafler DA, Freeman GJ. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cellular immunology. 2004;230:89–98. doi: 10.1016/j.cellimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Carril MS, Aragon JP, Gonzalez Fernandez A. Age-related accumulation of memory cells in mouse Peyer’s patches. Immunol Lett. 2002;83:39–45. doi: 10.1016/s0165-2478(02)00072-x. [DOI] [PubMed] [Google Scholar]
- Chakravarti B, Abraham GN. Aging and T-cell-mediated immunity. Mech Ageing Dev. 1999;108:183–206. doi: 10.1016/s0047-6374(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Channappanavar R, Twardy BS, Krishna P, Suvas S. Advancing age leads to predominance of inhibitory receptor expressing CD4 T cells. Mech Ageing Dev. 2009;130:709–712. doi: 10.1016/j.mad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Diao J, Winter E, Chen W, Cantin C, Cattral MS. Characterization of distinct conventional and plasmacytoid dendritic cell-committed precursors in murine bone marrow. J Immunol. 2004;173:1826–1833. doi: 10.4049/jimmunol.173.3.1826. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature medicine. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. The Journal of experimental medicine. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Long-term immunological memory against viruses. Mech Ageing Dev. 2000;121:161–171. doi: 10.1016/s0047-6374(00)00207-4. [DOI] [PubMed] [Google Scholar]
- Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine. 2007;25:599–604. doi: 10.1016/j.vaccine.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Ernst DN, Hobbs MV, Torbett BE, Glasebrook AL, Rehse MA, Bottomly K, Hayakawa K, Hardy RR, Weigle WO. Differences in the expression profiles of CD45RB, Pgp-1, and 3G11 membrane antigens and in the patterns of lymphokine secretion by splenic CD4+ T cells from young and aged mice. J Immunol. 1990;145:1295–1302. [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nature immunology. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. The Journal of experimental medicine. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. The Journal of experimental medicine. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, Murasko DM. Age-related changes in Type 1 and Type 2 cytokine production in humans. Biogerontology. 2002;3:271–290. doi: 10.1023/a:1020151401826. [DOI] [PubMed] [Google Scholar]
- Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis. 2002;2:659–666. doi: 10.1016/s1473-3099(02)00437-1. [DOI] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Hatachi S, Iwai Y, Kawano S, Morinobu S, Kobayashi M, Koshiba M, Saura R, Kurosaka M, Honjo T, Kumagai S. CD4+ PD-1+ T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol. 2003;30:1410–1419. [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. The Journal of experimental medicine. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun H, Seo SK, Jeong HY, Seo HM, Zhu G, Chen L, Choi IH. B7-H1 (CD274) inhibits the development of herpetic stromal keratitis (HSK) FEBS Lett. 2005;579:6259–6264. doi: 10.1016/j.febslet.2005.09.098. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y, Fujino M, Wang Q, Kimura H, Azuma M, Kubo M, Abe R, Li XK. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation. 2007;83:774–782. doi: 10.1097/01.tp.0000256293.90270.e8. [DOI] [PubMed] [Google Scholar]
- Kovaiou RD, Grubeck-Loebenstein B. Age-associated changes within CD4+ T cells. Immunol Lett. 2006;107:8–14. doi: 10.1016/j.imlet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Kurashima C, Utsuyama M, Kasai M, Ishijima SA, Konno A, Hirokawa K. The role of thymus in the aging of Th cell subpopulations and age-associated alteration of cytokine production by these cells. International immunology. 1995;7:97–104. doi: 10.1093/intimm/7.1.97. [DOI] [PubMed] [Google Scholar]
- Lages CS, Suffia I, Velilla PA, Huang B, Warshaw G, Hildeman DA, Belkaid Y, Chougnet C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J Immunol. 2008;181:1835–1848. doi: 10.4049/jimmunol.181.3.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature immunology. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature immunology. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Meng Q, Yang P, Li B, Zhou H, Huang X, Zhu L, Ren Y, Kijlstra A. CD4+PD-1+ T cells acting as regulatory cells during the induction of anterior chamber-associated immune deviation. Invest Ophthalmol Vis Sci. 2006;47:4444–4452. doi: 10.1167/iovs.06-0201. [DOI] [PubMed] [Google Scholar]
- Mirza N, Duque MA, Dominguez AL, Schrum AG, Dong H, Lustgarten J. B7-H1 Expression on Old CD8+ T Cells Negatively Regulates the Activation of Immune Responses in Aged Animals. J Immunol. 2010;184:5466–5474. doi: 10.4049/jimmunol.0903561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Molecular immunology. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. The Journal of experimental medicine. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) International immunology. 2007;19:337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- Raimondi G, Shufesky WJ, Tokita D, Morelli AE, Thomson AW. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol. 2006;176:2808–2816. doi: 10.4049/jimmunol.176.5.2808. [DOI] [PubMed] [Google Scholar]
- Sansoni P, Cossarizza A, Brianti V, Fagnoni F, Snelli G, Monti D, Marcato A, Passeri G, Ortolani C, Forti E, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–2773. [PubMed] [Google Scholar]
- Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol. 2006;177:8348–8355. doi: 10.4049/jimmunol.177.12.8348. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Hayashi M, Nagasaka Y, Ohno-Iwashita Y, Inomata M. Age-associated up-regulation of a negative co-stimulatory receptor PD-1 in mouse CD4+ T cells. Exp Gerontol. 2009;44:517–522. doi: 10.1016/j.exger.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Shimatani K, Nakashima Y, Hattori M, Hamazaki Y, Minato N. PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc Natl Acad Sci U S A. 2009;106:15807–15812. doi: 10.1073/pnas.0908805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimauchi T, Kabashima K, Nakashima D, Sugita K, Yamada Y, Hino R, Tokura Y. Augmented expression of programmed death-1 in both neoplastic and non-neoplastic CD4+ T-cells in adult T-cell leukemia/lymphoma. Int J Cancer. 2007;121:2585–2590. doi: 10.1002/ijc.23042. [DOI] [PubMed] [Google Scholar]
- Song L, Kim YH, Chopra RK, Proust JJ, Nagel JE, Nordin AA, Adler WH. Age-related effects in T cell activation and proliferation. Exp Gerontol. 1993;28:313–321. doi: 10.1016/0531-5565(93)90058-l. [DOI] [PubMed] [Google Scholar]
- Sun Y, Li H, Langnas AN, Zhao Y. Altered allogeneic immune responses in middle-aged mice. Cellular & molecular immunology. 2004;1:440–446. [PubMed] [Google Scholar]
- Thomas DC, Mellanby RJ, Phillips JM, Cooke A. An early age-related increase in the frequency of CD4+ Foxp3+ cells in BDC2.5NOD mice. Immunology. 2007;121:565–576. doi: 10.1111/j.1365-2567.2007.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature medicine. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. The Journal of experimental medicine. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsuyama M, Hirokawa K, Kurashima C, Fukayama M, Inamatsu T, Suzuki K, Hashimoto W, Sato K. Differential age-change in the numbers of CD4+CD45RA+ and CD4+CD29+ T cell subsets in human peripheral blood. Mech Ageing Dev. 1992;63:57–68. doi: 10.1016/0047-6374(92)90016-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Han R, Hancock WW. Programmed cell death 1 (PD-1) and its ligand PD-L1 are required for allograft tolerance. Eur J Immunol. 2007;37:2983–2990. doi: 10.1002/eji.200737583. [DOI] [PubMed] [Google Scholar]
- Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4+ CD28- T cells in the aging immune system. Mech Ageing Dev. 1998;102:131–147. doi: 10.1016/s0047-6374(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. The Journal of experimental medicine. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, Azuma M, Yagita H. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, Shi M, Gao GF, Wu H, Wang FS. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. Journal of leukocyte biology. 2007;81:1386–1394. doi: 10.1189/jlb.0506364. [DOI] [PubMed] [Google Scholar]