Abstract
Cells adhere to one another and/or to matrices that surround them. Regulation of cell-cell (intercellular) and cell-matrix adhesion is tightly controlled in normal cells, however, defects in cell adhesion are common in the majority of humancancers. Multilateral communication among tumor cells with the extracellular matrix (ECM) and neighbor cells is accomplished through adhesion molecules, ECM components, proteolytic enzymes and their endogenous inhibitors. There is sufficient evidence to suggest that reduced adherence is a tumor cell propertyengaged during tumor progression. Tumor cells acquire the ability to change shape, detach and easily move through spaces disorganizing the normal tissue architecture. This property is due to changes in expression levels of adhesion molecules and/or due to elevated levels of secreted proteolytic enzymes, including matrix metalloproteinases (MMPs). Among other roles, MMPsdegrade the ECMand, therefore, prepare the path for tumor cells to migrate, invade and spread to distant secondary areas, where they form metastasis. Tissue Inhibitors of Metalloproteinases or TIMPs control MMP activities and, therefore, minimize matrix degradation. Both MMPs and TIMPs are involved in tissue remodeling and decisively regulate tumor cell progression including tumor angiogenesis. In this review, we describe and discuss data that support the important role of MMPs and TIMPs in cancer cell adhesion and tumor progression.
Keywords: Cell adhesion molecules, Extracellular matrix, Matrix Metalloproteinases, Tissue Inhibitors of Metalloproteinases
1. Introduction
In tissues, cells tightly adhered to one another and to their surrounding matrix, thus, preserving tissue integrity and three-dimensional architecture[1,2]. These cellular interactions fortify perception of the extracellular topography and adaptation to environmental changes. Maintainingnormal tissue architecture is paramount for proper tissue function and physiology, yet, other important cellular functions are also linked to cell adhesion including proliferation, motility, migration and apoptosis. Cell adhesion is deregulated in a number of pathologies including cancer progression [3]. While primary tumors can stay localized at their original site and may be easily accessible to surgical resection, during tumor progression tumor cells become less adhesive and more migratory, behaviors that contribute to invasion and metastasis. In cancer, where the cellular repair mechanisms are compromised, local injuries and disruption of tumor cell adhesion causes extensive localized tissue damage. Genetic alterations, epigenetic signals, augmented proliferation, tumor inflammation and angiogenesis can elicit modified adhesive tumor cell behavior [4].
Cell adhesion molecules includereceptors expressed on the cell surface thatphysically interact with specific molecules found on the surface of the neighboring cells or in association with their matrix. They also interact with non-receptor and receptor tyrosine kinases (RTK), members from the Rho family small GTPases and from the Wnt signaling pathway [5, 6]. Compromised function could have dramatic consequences during developmental organogenesis, immunity, inflammation, angiogenesis and cancer. Activatedreceptors initiate a sequence of signals transmittedvia cytoskeletal proteins that are propagatedall the way to the cell nucleus. Alterations in this multistage process affect not only the ability of adhesion molecules to interact with their ligands but also the activation of downstream intracellular signaling, a phenomenon commonly seen in cancer [7].
A number of cell adhesion molecules act as tumor suppressors. Loss of E-cadherin (epithelial cadherin) from the cell surface, commonly occursin epithelial tumors, leads to disruption of cell contacts, tumor cell detachment, shape change and local invasion, events that initiate a program named epithelial to mesenchymal transition (EMT)[6, 8, 9]. Tumor invasion is supported by the increased enzymatic activity of tumor or stroma cell secreted active MMPs. Extensive stroma degradation and damage facilitates tumor cell release and spread, therefore, MMPs are positive regulators of tumor invasion and growth [10, 11]. Solid tumors have developed mechanisms that allow enhanced ability of tumor cells to invade the extracellular matrix facilitating the formation of distant metastatic foci. Tumor invasion does not always lead to metastasis formation; onlyabout 0.01 % of escaped tumor cells initiate a more complex distant disease process of metastasis [12]. Metastasis is a multistep process: primary tumor cells have reduced adhesion ability, detach easily from their matrix, secrete proteolytic enzymes that degrade the matrix, invade the neighbor tissues and blood vessels and become free to move from the primary tumor site to a secondary site, either by direct invasion, hematogenous or lymphatic spread. Therefore, understanding how cell adhesion is regulated is critical in identifying novel ways to inhibit tumor cell dissemination.
MMPs are endopeptidases and their primary function is tissue remodeling by selective proteolytic degradation[13, 14]. Uncontrolled MMP activity results in tissue damage and functional alterations. In the current era of cancer genomics and proteomics, numerous studies have shown positive correlation between elevated MMP levels within the tumor stroma and tumor cells invasion or metastasis[15]. This finding suggests that MMP/TIMPphysiological equilibrium is shifted in malignant tissues.Changes in theexpression level of adhesion-related molecules, including MMPs and TIMPs, may be utilized as prognostic factors in cancer development and potentially be exploited as therapeutic targets [16].
2. Ligands, receptors and the extracellular matrix (ECM)
2.1 Ligands
The tissue matrix may be classified into interstitial connective, or stromal matrix, that supports individual cells, and a very specialized structure, which forms a continuous sheet called the basement membrane (BM) that supports cell layers, such as the epithelium and endothelium. The ECM refers to a cell secreted, supporting, connective material of assembled specialized fibrous protein families, including fibronectins, laminins, collagens, proteoglycans (PGs) and tenascins.Detailed description of matrix protein family members, their functions and role in human pathologies are provided in recent reviews [17–21].
ECM proteins are overexpressed in most carcinomas. A recent study also showed that increased stroma fibrosis and stiffness in breast cancer is due to high levels of crosslinked collagen type I, was a marker of poor prognosis for metastatic breast tumors [22]. However, loss of type IV collagen from the BM correlates with metastatic potential [23]. Laminin expression correlates with tumor invasiveness; in particular, laminin-332 (or laminin-5) is deposited by squamous cell carcinomas in the stroma and is shown to interact with collagen type VII to promote invasion [24, 25].Similar to collagens and laminins, PGs are overexpressed in epithelial tumors and play an important role in cell adhesion, migration and growth. The PG syndecan-1 is highly expressed in, and associated with, urothelial carcinoma progression; in vitrosiRNA knockdown was able to induce cellular apoptosis by activating caspases 3 and 8 [26]. In melanoma, chondroitin sulfate PG induces integrin activation and constitutive activation of Erk pathway [27].Fibronectin engagement by prostate tumor cells PC-3 enhances cell survival by upregulating anti-apoptotic protein survivin levels and protecting cells from tumor necrosis factor-α (TNF-α) induced apoptosis [28]. More recently, microRNA MiR-17, part of the tumor suppressor microRNA cluster 17–92 that wasshown to be frequently deleted from ovarian, breast and melanoma cancers, was demonstrated to target and repress fibronectin expression [29]. Increased stroma fibronectin levels are also indicative of an EMT and tumor progression [30].
2.2 Receptors
Cell adhesion is primarily achieved through cell surface (receptors) that belong to five major classes: integrins, immunoglobulin superfamily (Ig-CAMs), cadherins, selectins and the hyaluronan receptor CD44[31]. Integrins are composed of two subunits, the α (alpha) and the β (beta) [32]. Upon ligand binding, integrins undergo conformational changes,followed by transmission of ‘outside-to-inside’ signals through pathwaysinvolving Focal Adhesion Kinase, (FAK), or RTK mediated signaling, that are described in detail elsewhere [33, 34]. The type of integrins expressed dictate selective cellular functions including adhesion, contraction, survival, proliferation and migration, through initiation of specific ‘inside-to-ouside’ cellular response[7].Cadherins are transmembrane monomeric glycoproteins that mediate cell-cell adhesion through binding to the ectodomain of homotypic cadherins on neighbor cells.Their intracellular C-terminus interacts with another group of proteins the catenins (α, β, γ and p120)that regulate the stability of cell-cell adhesions[6, 35]. A dimeric interaction has also been reported to occur between E-cadherin and RTKs that leads to modulation of cell signaling [9].Selectins are a family of single-chain transmembrane proteins expressed on endothelial cells, leukocytes and platelets. They facilitate leukocyte rolling on the blood vessel wall during inflammation and immunity.Ig-CAMsare expressed in leukocytes, epithelial, endothelial and neuron cells andassociate throughhomoor heterotypic interactions with members of the same family, as well as proteins in the ECM[6]. Finally, CD44 is a ubiquitous ECM component that contains 20 exons and multiple isoforms resulting in a multifunctional family of proteins [36].
Integrin/RTK joint signaling is significantly enhanced during tumor progression and ultimately assures tumor cell survival and proliferation. The role of integrins in the progression of specific types of human cancer and metastasis is well described in recent reviews [33, 37]. Frequently,the FAK signal transduction pathway is overexpressed in many tumors, including lung, breast, prostate, colon and ovarian leading to increased tumor growth and upregulation of anti-apoptotic mechanisms [38].The E-cadherin/β-catenin interaction is fundamental for cell adhesion, cytoskeletal signaling and cell proliferation [39]. Since most human malignancies are of epithelial origin, loss of E-cadherin or mutations in the β-catenin binding domain on E-cadherin protein leads to complex disruption, reduced cell-cell adhesion and enhanced tumor invasion[9]. Whereas loss of E-cadherin from the cell junctions promotes EMT, N-cadherin expression has been associated with the mesenchymal phenotype of epithelial tumors that renders them invasive and migratory. N-cadherin (neural) is highly expressed during embryonic development in neurons and glial cells and is also associated with tumor progression, EMT and metastasis [8]. Selectins are upregulated particularly during the process of tumor cell extravasation and associated with increased metastastic potential. E-selectin and vascular cell adhesion molecule-1 (VCAM-1) overexpression on endothelial cells facilitates colon cancer cell adhesion to the endothelium and ultimately their homing to the liver[40].The activated endothelium also attracts inflammatory cells and monocytes to the tumor cell foci initiating the formation of pre-metastatic microenvironment [41].Finally, tumor cells express increased number of CD44 isoforms that are normally absent in normal cells. CD44 involvement in malignancies, both as promoting growth (eg. lymphomas, breast cancer) and suppressing metastasis (eg. prostate cancer), is primarily through CD44/hyaluronan-mediated oncogenic signals[42, 43].
2.3 ECM
Extracellular matrices do not merely provide a structural barrier to the tissue, organ or cell they support but also actively participate in important cellular decisions such as proliferation or cell cycle arrest, motility or immobility, survival or apoptosis [44].In physiological conditions, defective or lack of interaction between matrix and cells triggers a type of cell death called anoikis, a mechanism that various cancers develop resistant too, an characteristically referred to as adhesion-independent growth[45, 46]. The adhesive interactions between the cell, its ECM and the microenvironment are bidirectional, a dynamic cross-talk achieved by receptors and ligands followed by recruitment of cytoskeletal proteins, nuclear chromatin activation and ultimately resulting in altered gene expression, which in turn influences the tumor microenvironment. This model termed dynamic reciprocity applies to organ and tissue interactions in development and extended to disease states[2]. Matrix remodeling in cancer is primarily caused by MMP activity and this process is essential for solid tumors to progression[47–49]. The extensive structural changes also facilitate the initiation of a variety of host responses that havelong been associated with tumorigenicity, such as tumor inflammation and angiogenesis. Essentially, tumor cells create their own microenvironment that ultimately supports growth, inhibits apoptotic signals and, as several clinical studies have shown, contributes to drug resistance.
Coordinated cellular events may be launched via cellular attachment to different structural entities within the matrix. For instance, ECM proteins (eg. fibronectin, vitronectin) using their specialized sequence motifs, such as the RGD tripeptide, bind directly to the appropriate integrins (eg. α5β1, αvβ3,αvβ1, αvβ5, αvβ6, αvβ8) and initiate attachment and cell signaling through cytoskeletal rearrangement[50]. Synthetic peptides containing the RGD motif have been successfully used in numerous in vitro and in vivo models to inhibit tumor cell invasion and metastasis. a bio-bank, a repository of inactive growth factors and other hidden bioactive molecules that become unleashed upon matrix degradation. ECM contains embedded growth factors that when released from the matrix, they initiate growth factor receptor signaling [51]. ECM PGs also function tokeep the growth factors inactive and bound to the matrix. It seems that solid-supported growth factors are essential matrix components and play a key role in the overall ECM contribution to tissue homeostasis. Matrix proteins are also found to contain potential growth factor-like domains, which upon proteolytic degradation become soluble and may bind to cognate growth factor receptors inducing their activation. Collagen and fibronectin are able to activate an integrin-dependent, transforming growth factor (TGF)-β/Smad signaling, independently of TGF-β or TGF-β receptor[52]. This finding enhances even further the contribution of ECM modulation of cellular behavior.
The multiple contributions of ECM in etiological association with human genetic diseases and pathologies have been reported in mouse knockout or mutant models [53, 54]. Whereas a number of ECM proteins, including laminins and collagen l or II deleted or introduced as mutant transgenes are embryonic lethal, mice with spontaneous mutations show abnormalities related to human disorders (eg. muscular dystrophy). ECM also contains cryptic fragments generated from cleaved collagens that act as angiogenesis inhibitors (see section 3.2); mice lacking these fragments show increased tumor growth and tumor-associated angiogenesis. Taken together, mouse models reveal significant details on ECM component functions, enabling us to better understand the steps involved in cancer development, including tumor cell attachment, matrix proteolysis, tumor cell release and migration. In particular, tumor proteolytic activity positively correlates with cancer aggressiveness, and inhibition of protease enzymatic activity inhibits tumor invasion. There are four protease families: seryl-, aspartyl-, cyctyl- and metallo-proteases (MMPs). Studies over the last forty years have provided ample evidence to suggest that MMPs directly regulate tumor cell invasiveness and metastasis.
3. MMPs and their role in cancer
3.1 MMPs family
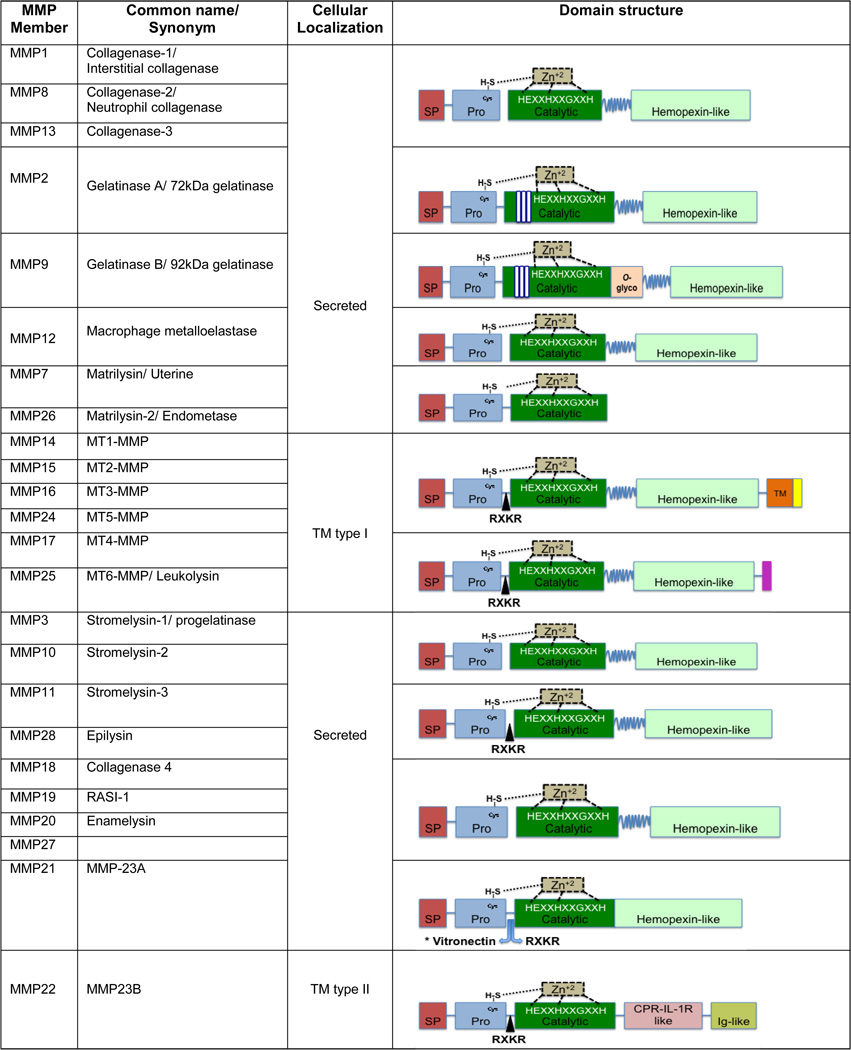
MMPs belong to a family of zinc-dependent endopeptidases intrinsically responsible for the degradation of a vast number of protein targets by cleavage of internal peptide bonds[55, 56]. Currently, there are over 20 human MMP members divided in two groups based on their cellular localization (secreted versus membrane-bound), or in five main groups according to their structure and substrate specificity: collagenases, gelatinases, membrane type, stromelysins and matrilysins. MMPs are synthesized as inactive proenzymes (pro-MMPs), and they shareseveral conserved structural domains, although additional domains are unique for a number of MMP members (Figure 1).Plasma associated MMPs are inhibited by liver secreted α2-macroglobulin, while tissue or extracellular MMPs are regulated by their endogenous inhibitors named Tissue Inhibitor of Metalloproteinases (TlMPs). There are four TIMP members, TIMP-1, -2, -3 and -4 and their contribution in MMP homeostasis and cancer will be discussed in a separate section.
Figure 1. Matrix Metaloproteinases (MMPs) domain structures and functions.
There are 5 main MMP groups based on their structure and substrate: collagenases, gelatinases, matrilysins, membrane type MMPs (MT-MMPs) and stromelysins. The basic domain structure consists of: i) a signal peptide (SP) that directs the protein through the endoplasmic reticulum prior to secretion, ii) a pro-peptide region (PRO) that secures enzymatic latency and inactivity by blocking the catalytic site from becoming accessible to substrates or to inhibitors, iii) a catalytic region that, after the pro-peptide removal, confers the MMPs with their enzymatic activity with the help of zinc (Zn) ions and iv) a hemopexin domain at the C-terminus, that provides the specificity to and interaction with the substrates or the inhibitors (TIMPs), also presents the substrate to the catalytic domain via a high flexible hinge, except for matrilysins that lack the hemopexin-like and the hinge that joints it to the catalytic domain. The prodomain contains a cysteine-to-zinc switch motif where the Zn+2 at the catalytic domain binds to the cysteine at the prodomain and inhibits MMP activation and interaction with the substrate. The zinc in the catalytic domain interacts with the three histidines (H) in a motif sequence (HEXXHXXGXXH). Upon proteolytic processing and removal of the prodomain, the mature MMPs become active and able to catalyze. Although the majority of the MMPs are secreted as inactive zymogens, the MT-MMPs, MMP-11 and MMP-28 (epilysin) contain the furin-like motif RXKR between the proenzyme and catalytic domains allowing them to be activated by intracellular serine proteinases before they get secreted or localize to the cell membrane. The type I transmembrane (TM type I) MMPs are anchored to the membrane with a hydrophobic domain and a short cytoplasmic tail. The two exceptions are the MMP-17 and MMP-25 that instead attach with a glycosyl-phosphatidyl inositol (GPI) anchor domain. Gelatinases A and B or MMP-2 and MMP-9 also contain three collagen-binding fibronectin type II repeats within the catalytic domain. In addition, MMP-9 contains a serine-threonine and prolice rich O-glycosylated domain (O-glyco).

Another zinc protease group of membrane bound and secreted proteins is the ‘a disintegrin and metalloprotease’ (ADAMs) family. ADAMs have both metalloprotease and integrin receptor-binding proterties and are implicated in many pathologies including cancer. MMPs promote carcinogenesis and this has been demonstrated in a number of genetically modified animal models [57]. Several reports support MMPs’ direct role in cell adhesion, migration, EMT, tumor angiogenesis, and proteolytic processing of cytokines, chemokines, growth factors or their receptors, underlying the complex nature of tumorigenesis.
3.2. MMPs role incancercell adhesion
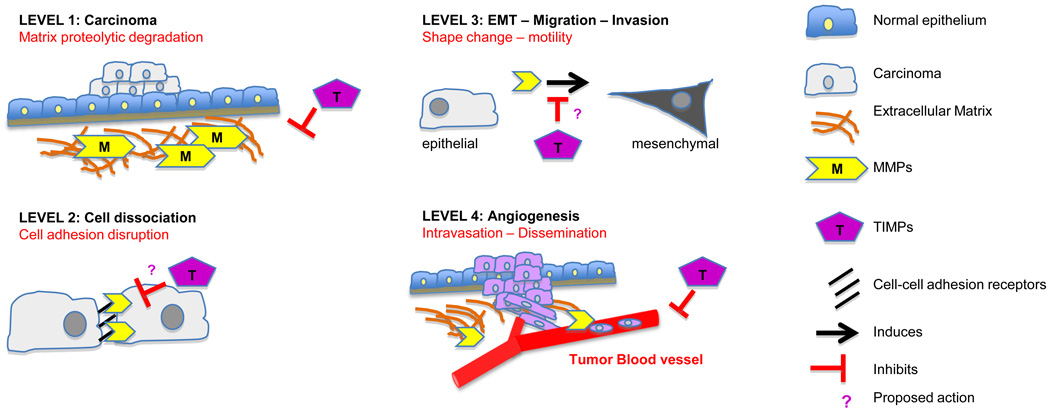
Studies attempted to identify mechanisms by which MMPs affect cell adhesion. Early on, it was shown that MMP-2 processedcell membrane bound componentsand, therefore, directly affects the adhesive cellular properties of human melanoma A2058cellsin vitro[58].Comprehensive analysis on the effects of MMPs-mediated ECM component degradation in various types of cancer systems is described elsewhere [59–64]. Proteolysis of matrix substrates generates degradation fragments that regulate specific pathways. Cleavage of collagen IV and laminin 5 exposes cryptic sites that initiate novel functions including tumor cell growth, migration and angiogenesis[63, 65].More recently it was shown that MT1-MMP-mediated collagen degradation is required for matrix tunnel formation in 3D collagen gels, and this mechanism is essential for tumor and endothelial cell motility in 3D matrices in an MMP-independent mode [66, 67].In invasive tumors, MMPs and integrins are expressed in high levels suggesting that there is possible interaction between the two protein families. Indeed, MMP-2 was shown to interact with αvβ3 on the surface of angiogenic blood vessels and melanoma cells in vivo, contributing to collagen degradation and cell invasion [68]. Later, more studies showed that MMP-9 interacts with αvβ3 promoting migration of breast cancer cells [69]. Active forms of MMP-2 or MT1-MMP also interact with αvβ5 integrin and affect their membrane localization in a number of tumor cell lines including melanoma[70, 71]. MT1-MMPs processesprecursors of αvα5 and α3 but not α2, integrins leading to αvβ3 mediated signaling and migration of breast cancer cells[72, 73]. Cadherins are also targeted by MMPs. MMP-3 and MMP-7 have been shown to cleave membrane bound E-cadherin in MCF-7 breast cancer cells. The released soluble E-cadherin was shown to promote cell invasion;although the mechanism was not shown, it was proposed that the soluble fragment my inhibit the membrane bound E-cadherin function and disrupt cell-cell contacts [74]. ADAM-10 also cleaved E-cadherin and changed its cellular localization and promoted β-catenin translocation to the nucleus. Similarly, N-cadherin was targeted by ADAM-10 and its cleavage enhances migration of human glioblastoma cells [75, 76].More recently, breast cancer cells overexpressing P-cadherin, to mimic clinical breast cancer with poor survival, was shown to promote invasion even in the presence of functional E-cadherin in vitro. P-cadherin induced MMP-1 and MMP-2 expression that led to P-cadherin proteolytic cleavage and induction of invasion [77]. Finally, the hyaluronan receptor CD44 is also cleaved by MT1-MMP releasing extracellular fragments that increase invasion of pancreatic and breast cancer cells [78]. In conclusion, MMPs target both ECM components and adhesion receptors to alter cellular resposes to the environment and this mechanism is highly exploited by tumor cells to promote migration, invasion and metastatic potential (Figure 2).
Figure 2. MMPs and TIMPs input in tumor cell adhesion.
A schematic representation of MMPs and TIMPs role in cell adhesion modulation during different the levels of carcinoma progression (see text). Proposed (?) TIMPs actions remained to be discovered.
MMPs have also been involved in the disruption of endothelial cell ECM resulting in enhanced tumor angiogenesis [79–81]. However, anti-angiogenic fragments derived from the cleavage of collagen XVIII and plasminogen act as small endogenous angiogenesis inhibitors [82–84]. These studies indicate that pro-angiogenic nature of MMPs is highly dependent on the experimental cancer model used and other factors should also be taken into account such as the tumor microenvironment and the matrix complexity. ECM bound factorssuch as insulin-like growth factors (IGFBPs) and TGF-β are MMPs targets. Cleavage of IGF binding proteins (IGFBP) allows IGFs to become available and bind to their receptors resulting in tumor cell growth [85]. MMP-19 proteolysis of IGFBP-3 contributes to IGF-enhanced keratinocyte adhesion on collagen I, whereas MMP-7 mediated cleavage of IGBP-3 induced IGF signaling and protected against apoptosis [86]. Fibronectin-bound latent form TGF-β is released to its active form in the presence of MMP-2 and MMP-9and this activation is CD44 dependent[87]. This study provided a new mechanism for TGF-β activation that tumor cells adopt to enhance proliferation and invasion. TGF-β is also a master EMT inducer in carcinomas. Snail1, a critical transcription factor for EMT induction, was shown to be involved in ECM degradation via an MT1-MMP-dependent mechanism. Snail1 induces non-invasive breast carcinoma cells to perforate the BMby upregulation of MT1-MMP and MT2-MMP expression leading to increased BM degradation [88]. Released TGF-β also induces key transcriptional regulators of EMT including members of the Snail and Zeb families. Recent studies have shown Snail and Zeb induction of MMPs that in turn can induce EMT in different cancers [30, 89]. Frequently activated pathways in cancer such as RTKs, Wnt and TNF-α can upregulate MMPs expression at the mRNA and protein levels [56, 59, 90]. The above studies suggest that the release of growth factors from the ECM degradation by MMPs creates a positive feedback loop that supports the hallmark invasive phenotype of cancer cells. It is therefore up to the endogenous inhibitory mechanisms to control the MMPs pro-tumorigenic effects.
4. TIMPs, the endogenousinhibitors of MMPs, and their role in cancer
4.1 The TIMP family
There are four TIMP family members, TIMP-1, -2, -3 and TIMP-4 and their basic properties are summarized in[91]. Each of their N- and C-terminal domains contains 6 conserved cysteine residues that form three disulfide loops. The N-terminal regionbinds to the MMPs’ catalytic domain and inhibits MMP activity,whereas, the C-terminal region interacts with the the pro-forms of MMP-2 and MMP-9 C-terminal hemopexin domain to stabilize the pro-enzyme inhibitor complex. TIMP-2 is the only TIMP member that specifically interacts on the cell surface with both MT1-MMP and pro-MMP-2 in order to facilitate the activation of pro-MMP-2 [92, 93]. Thus TIMP-2 is unique in that it functions both as an MMP inhibitor and activatior. TIMPs can inhibit all active MMPs, however, not with the same efficacy. TIMP-1 preferentially inhibits MMP-7, MMP-9, MMP-1 and MMP-3, whereas, TIMP-2 is also a more effective inhibitor of MMP-2. TIMP-3 can inhibits MMP-2 and MMP-9 but also the majority of ADAMs, whereas, TIMP-4 inhibits MT1-MMP and MMP-2 catalytic activity. TIMPs function, therefore,is to regulate proteolytic activity and all the MMP-mediated activities. It is only the last ten years that novel biological activities started being reported, attributed specifically to TIMPs’ genetic and protein configuration, rather than a consequence of their proteaseinhibitory ability. Although, they are secreted proteins functioning extracellularly, a series of reports identified TIMPs’ cellular receptors, suggesting that these molecules may modulate cellular behavior from the outside to the inside of the cell.
In the literature, TIMP status is usually portrayedsolely as an MMP inhibitor and, therefore, mechanistic studies explain TIMP effects, in both physiological and pathological conditions, on the restrained MMPs activities and mediated functions. Studies have revealed that TIMPs are involved in several biological activities including cell differentiation, growth, migration, invasion, angiogenesis and apoptosis, and these cellular effects are mediated independently of TIMP inhibition of MMP activity(reviewed in [94]) and Figure 2.
4.2. TIMPsrole in tumor cell adhesion
Early on, TIMP-1, and later TIMP-2, was found to possess erythroid potentiating activity although the significance of this activity remains unclear [95, 96]. It was not long before TIMPs were described to promote tumor cell proliferation or induce apoptosis in some systems and to inhibit tumor growth and promote cell death in other tumor models; the signaling pathways involved in these activitesare reviewed in [13]. TIMPs involvement in cell adhesion process either by direct interaction with cell adhesion molecules or with ECM componentsis less studied. TIMP-1 was shown to engage withits cellular receptor, CD63, a member of the tetraspanins known to interact with cell adhesion molecules including integrins and modulate cell adhesion [97]. It was demonstrated that TIMP-1 interacts with β1 integrin on the cell surface of mammary in a CD63-dependent manner, resulting in inhibition of apoptosis. TIMPs also have anti-angiogenic activities and these are particularly important during tumor growth and metastasis. The pathways involved could be distinct for each TIMP member, as well, MMP-dependent or independent. We have previously shown that TIMP-2 inhibits the endothelial cell growth in vitro and angiogenesis in vivo upon stimulation with endothelial growth factors, VEGF-A or FGF-2. The identified mechanism is independent of TIMP-2-mediated MMP inhibition. In fact, it was shown that extracellular TIMP-2 and Ala+2TIMP-2 (TIMP-2 mutant devoid of MMP inhibitory activity)interact withα3β1, an integrin receptor expressed on the surface of tumor and endothelial cells and induce an integrin-dependent signaling response[98]. In a following up study we demostrated that TIMP-2 also induces RECK expression (the reversion-inducing-cystein-rich protein with Kazal motif) leading to loss of cell migration. RECK is a plasma membrane associated protein shown to inhibit a number of MMPs including MMP-2, -9, MT1-MMP and ADAM10. The pathway involved includes decrease of Src levels, inactivation of Rac1 (a small guanosine triphosphates, GTPase) and activation of another small GTPase, Rap1. TIMP-2 interaction with its receptor α3β enhances RECK expression in endothelial cells resulting in inhibition of endothelial cell migration. TIMP-3’s anti-angiogenic effects occur through different mechanisms including TIMP-3 directly binding to VEGF receptor 2 and acting as antagonist, therefore, blocking VEGF-A mitogenic effects. During inflammation, and in response to TNF-a and IL-1B, endothelial adhesion molecule VCAM-1 ectodomain is released to a soluble VCAM-1 (sVCAM-1) by ADAM-17 proteolysis. TIMP-3 knockout mice exhibit much higher levels of sVCAM-1 suggesting that TIMP-3 regulates cellular responses to inflammation by inhibiting ADAM-17 activity [99]. TIMP-3 promoter was also found hypermethylated in a study of bladder cancer and was identified as a prognostic marker for bladder cancer progression [100]. In the ECM, TIMP-3 has also been shown to specifically interact with heparan sulfate; the significance of this interaction is still unknown, however, it is proposed that maintains TIMP-3 in an inactive form [101]. In the end, TIMPs interaction with ECM components, plasma membrane proteins, RTKs and cell adhesion receptors initiate transduction pathways that influencecell signaling,induce cytoskeletal changes that results in altered cell migration and growth. Inhibition of angiogenesis leads to decrease of tumor cell invasion and extravasation, minimizing the metastatic potential.
5. Epilogue
Induced expression and increased activation are two reasons why MMPs are significant players in tumor cell invasion and metastasis. In fact, MMPs modulation of cancer cell adhesion can be attributed to four events: Firstly, active MMPs members target ECM ligands for proteolytic degradation, including collagens, laminins, fibronectin, vitronectin, resulting in ECM remodelling and tumor cell detachment. Secondly,active MMPs members target tumor cell adhesion receptors, including important non-ECM molecules such as E-cadherin or integrins and, therefore, directly disrupt cell-cell and cell-matrix adhesions. Thirdly, MMPs transcriptional expression being induced by tumor cell specific mechanisms, eg. during epithelial to mesenchymal transition (EMT), or tumor angiogenesis, leads to increased MMPs activity and substrate degradation. Finally, lack of or reduced MMPs inhibitory mechanisms, such as downregulation of their endogenous inihitors TIMPs, would disrupt the ratio of MMPs and TIMPs within the tumor microenvironment promoting tumor metastasis (Figure 2). The identification of MMP-independent TIMPs functions, the significance ofthe cellular receptors that TIMPs interact with (eg. integrins, RTKs) and the implication of these novel interactions, as demonstrated by the activated downstream signaling pathways, clearly demonstratethat there are still activities to be discovered and explained within the broader contextof ECM and the function of the tumor microenvironment.
Acknowledgements
We would like to show appreciation on the work of colleagues we could not cite due to space limitations. This work was supported by intramural research funds from the NCI, Center for Cancer Research Project Z01SC 009179.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 2.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010;112:3–25. doi: 10.1016/j.acthis.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 5.Guan JL. Integrin signaling through FAK in the regulation of mammary stem cells and breast cancer. IUBMB Life. 2010 doi: 10.1002/iub.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- 7.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 8.Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155–163. doi: 10.1196/annals.1294.016. [DOI] [PubMed] [Google Scholar]
- 9.Jeanes A, Gottardi CJ, Yap AS. Cadherins and cancer: how does cadherin dysfunction promote tumor progression? Oncogene. 2008;27:6920–6929. doi: 10.1038/onc.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jodele S, Blavier L, Yoon JM, DeClerck YA. Modifying the soil to affect the seed: role of stromal-derived matrix metalloproteinases in cancer progression. Cancer Metastasis Rev. 2006;25:35–43. doi: 10.1007/s10555-006-7887-8. [DOI] [PubMed] [Google Scholar]
- 11.Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11:143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 12.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359:2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy R, Yang J, Moses MA. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J Clin Oncol. 2009;27:5287–5297. doi: 10.1200/JCO.2009.23.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon MK, Hahn RA. Collagens. Cell Tissue Res. 2010;339:247–257. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339:237–246. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 20.Durbeej M. Laminins. Cell Tissue Res. 2010;339:259–268. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 21.Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 24.Marinkovich MP. Tumour microenvironment: laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7:370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 25.Waterman EA, Sakai N, Nguyen NT, Horst BA, Veitch DP, Dey CN, et al. A laminincollagen complex drives human epidermal carcinogenesis through phosphoinositol-3-kinase activation. Cancer Res. 2007;67:4264–4270. doi: 10.1158/0008-5472.CAN-06-4141. [DOI] [PubMed] [Google Scholar]
- 26.Shimada K, Nakamura M, De Velasco MA, Tanaka M, Ouji Y, Miyake M, et al. Role of syndecan-1 (CD138) in cell survival of human urothelial carcinoma. Cancer Sci. 2010;101:155–160. doi: 10.1111/j.1349-7006.2009.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Price MA, Li GY, Bar-Eli M, Salgia R, Jagedeeswaran R, et al. Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition. Cancer Res. 2009;69:7538–7547. doi: 10.1158/0008-5472.CAN-08-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, et al. Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/survivin pathway. J Biol Chem. 2003;278:50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 29.Shan SW, Lee DY, Deng Z, Shatseva T, Jeyapalan Z, Du WW, et al. MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Nat Cell Biol. 2009;11:1031–1038. doi: 10.1038/ncb1917. [DOI] [PubMed] [Google Scholar]
- 30.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Makrilia N, Kollias A, Manolopoulos L, Syrigos K. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 2009;27:1023–1037. doi: 10.3109/07357900902769749. [DOI] [PubMed] [Google Scholar]
- 32.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–280. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 34.Giancotti FG, Tarone G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol. 2003;19:173–206. doi: 10.1146/annurev.cellbio.19.031103.133334. [DOI] [PubMed] [Google Scholar]
- 35.Ishiyama N, Lee SH, Liu S, Li GY, Smith MJ, Reichardt LF, et al. Dynamic and static interactions between p120 catenin and E-cadherin regulate the stability of cell-cell adhesion. Cell. 2010;141:117–128. doi: 10.1016/j.cell.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Mol Pathol. 1999;52:189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilghman RW, Parsons JT. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin Cancer Biol. 2008;18:45–52. doi: 10.1016/j.semcancer.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Woodward J. Crossing the endothelium: E-selectin regulates tumor cell migration under flow conditions. Cell Adh Migr. 2008;2:151–152. doi: 10.4161/cam.2.3.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laubli H, Spanaus KS, Borsig L. Selectin-mediated activation of endothelial cells induces expression of CCL5 and promotes metastasis through recruitment of monocytes. Blood. 2009;114:4583–4591. doi: 10.1182/blood-2008-10-186585. [DOI] [PubMed] [Google Scholar]
- 42.Bourguignon LY. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. Semin Cancer Biol. 2008;18:251–259. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naor D, Wallach-Dayan SB, Zahalka MA, Sionov RV. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol. 2008;18:260–267. doi: 10.1016/j.semcancer.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakamoto S, Kyprianou N. Targeting anoikis resistance in prostate cancer metastasis. Mol Aspects Med. 2010 doi: 10.1016/j.mam.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakuma Y, Takeuchi T, Nakamura Y, Yoshihara M, Matsukuma S, Nakayama H, et al. Lung adenocarcinoma cells floating in lymphatic vessels resist anoikis by expressing phosphorylated Src. J Pathol. 2010;220:574–585. doi: 10.1002/path.2676. [DOI] [PubMed] [Google Scholar]
- 47.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 48.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996;148:1345–1350. [PMC free article] [PubMed] [Google Scholar]
- 50.Stupack DG. Integrins as a distinct subtype of dependence receptors. Cell Death Differ. 2005;12:1021–1030. doi: 10.1038/sj.cdd.4401658. [DOI] [PubMed] [Google Scholar]
- 51.Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- 52.Garamszegi N, Garamszegi SP, Samavarchi-Tehrani P, Walford E, Schneiderbauer MM, Wrana JL, et al. Extracellular matrix-induced transforming growth factor-beta receptor signaling dynamics. Oncogene. 2010 doi: 10.1038/onc.2009.514. [DOI] [PubMed] [Google Scholar]
- 53.Gustafsson E, Fassler R. Insights into extracellular matrix functions from mutant mouse models. Exp Cell Res. 2000;261:52–68. doi: 10.1006/excr.2000.5042. [DOI] [PubMed] [Google Scholar]
- 54.Aszodi A, Legate KR, Nakchbandi I, Fassler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- 55.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 56.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 58.Ray JM, Stetler-Stevenson WG. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J. 1995;14:908–917. doi: 10.1002/j.1460-2075.1995.tb07072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 60.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 61.Hendrix MJ, Birkedal-Hansen H, Yamada S, Windsor J, Pollard AH, Lyons G, Stetler-Stevenson W, et al. Matrix metalloproteinases. Curr Protoc Cell Biol. 2008;Chapter 10:Unit 10 8. doi: 10.1002/0471143030.cb1008s40. [DOI] [PubMed] [Google Scholar]
- 62.Hendrix MJ, Wood WR, Seftor EA, Lotan D, Nakajima M, Misiorowski RL, et al. Retinoic acid inhibition of human melanoma cell invasion through a reconstituted basement membrane and its relation to decreases in the expression of proteolytic enzymes and motility factor receptor. Cancer Res. 1990;50:4121–4130. [PubMed] [Google Scholar]
- 63.Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, et al. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61:6322–6327. [PubMed] [Google Scholar]
- 65.Xu J, Rodriguez D, Petitclerc E, Kim JJ, Hangai M, Moon YS, et al. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fisher KE, Sacharidou A, Stratman AN, Mayo AM, Fisher SB, Mahan RD, et al. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J Cell Sci. 2009;122:4558–4569. doi: 10.1242/jcs.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, et al. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009;114:237–247. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 69.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:9482–9487. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seftor RE, Seftor EA, Stetler-Stevenson WG, Hendrix MJ. The 72 kDa type IV collagenase is modulated via differential expression of alpha v beta 3 and alpha 5 beta 1 integrins during human melanoma cell invasion. Cancer Res. 1993;53:3411–3445. [PubMed] [Google Scholar]
- 71.Hornebeck W, Emonard H, Monboisse JC, Bellon G. Matrix-directed regulation of pericellular proteolysis and tumor progression. Semin Cancer Biol. 2002;12:231–341. doi: 10.1016/s1044-579x(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 72.Ratnikov BI, Rozanov DV, Postnova TI, Baciu PG, Zhang H, DiScipio RG, et al. An alternative processing of integrin alpha(v) subunit in tumor cells by membrane type-1 matrix metalloproteinase. J Biol Chem. 2002;277:7377–7385. doi: 10.1074/jbc.M109580200. [DOI] [PubMed] [Google Scholar]
- 73.Baciu PC, Suleiman EA, Deryugina EI, Strongin AY. Membrane type-1 matrix metalloproteinase (MT1-MMP) processing of pro-alphav integrin regulates cross-talk between alphavbeta3 and alpha2beta1 integrins in breast carcinoma cells. Exp Cell Res. 2003;291:167–175. doi: 10.1016/s0014-4827(03)00387-2. [DOI] [PubMed] [Google Scholar]
- 74.Noe V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 75.Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci U S A. 2005;102:9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ribeiro AS, Albergaria A, Sousa B, Correia AL, Bracke M, Seruca R, et al. Extracellular cleavage and shedding of P-cadherin: a mechanism underlying the invasive behaviour of breast cancer cells. Oncogene. 2010;29:392–402. doi: 10.1038/onc.2009.338. [DOI] [PubMed] [Google Scholar]
- 78.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 81.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Reilly MS, Wiederschain D, Stetler-Stevenson WG, Folkman J, Moses MA. Regulation of angiostatin production by matrix metalloproteinase-2 in a model of concomitant resistance. J Biol Chem. 1999;274:29568–29571. doi: 10.1074/jbc.274.41.29568. [DOI] [PubMed] [Google Scholar]
- 83.Dong Z, Kumar R, Yang X, Fidler IJ. Macrophage-derived metalloelastase is responsible for the generation of angiostatin in Lewis lung carcinoma. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 84.Wen W, Moses MA, Wiederschain D, Arbiser JL, Folkman J. The generation of endostatin is mediated by elastase. Cancer Res. 1999;59:6052–6056. [PubMed] [Google Scholar]
- 85.Manes S, Llorente M, Lacalle RA, Gomez-Mouton C, Kremer L, Mira E, et al. The matrix metalloproteinase-9 regulates the insulin-like growth factor-triggered autocrine response in DU-145 carcinoma cells. J Biol Chem. 1999;274:6935–6945. doi: 10.1074/jbc.274.11.6935. [DOI] [PubMed] [Google Scholar]
- 86.Miyamoto S, Yano K, Sugimoto S, Ishii G, Hasebe T, Endoh Y, et al. Matrix metalloproteinase-7 facilitates insulin-like growth factor bioavailability through its proteinase activity on insulin-like growth factor binding protein 3. Cancer Res. 2004;64:665–671. doi: 10.1158/0008-5472.can-03-1916. [DOI] [PubMed] [Google Scholar]
- 87.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 88.Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc Natl Acad Sci U S A. 2009;106:20318–20323. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Joseph MJ, Dangi-Garimella S, Shields MA, Diamond ME, Sun L, Koblinski JE, et al. Slug is a downstream mediator of transforming growth factor-beta1-induced matrix metalloproteinase-9 expression and invasion of oral cancer cells. J Cell Biochem. 2009;108:726–736. doi: 10.1002/jcb.22309. [DOI] [PubMed] [Google Scholar]
- 90.Clark IM, Swingler TE, Sampieri CL, Edwards DR. The regulation of matrix metalloproteinases and their inhibitors. Int J Biochem Cell Biol. 2008;40:1362–1378. doi: 10.1016/j.biocel.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 91.Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27:57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hernandez-Barrantes S, Toth M, Bernardo MM, Yurkova M, Gervasi DC, Raz Y, et al. Binding of active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP) to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP processing and pro-MMP-2 activation. J Biol Chem. 2000;275:12080–12089. doi: 10.1074/jbc.275.16.12080. [DOI] [PubMed] [Google Scholar]
- 93.Strongin AY, Collier I, Bannikov G, Marmer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 94.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gasson JC, Golde DW, Kaufman SE, Westbrook CA, Hewick RM, Kaufman RJ, et al. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. Nature. 1985;315:768–771. doi: 10.1038/315768a0. [DOI] [PubMed] [Google Scholar]
- 96.Stetler-Stevenson WG, Bersch N, Golde DW. Tissue inhibitor of metalloproteinase-2 (TIMP-2) has erythroid-potentiating activity. FEBS Lett. 1992;296:231–234. doi: 10.1016/0014-5793(92)80386-u. [DOI] [PubMed] [Google Scholar]
- 97.Jung KK, Liu XW, Chirco R, Fridman R, Kim HR. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J. 2006;25:3934–3942. doi: 10.1038/sj.emboj.7601281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 99.Singh RJ, Mason JC, Lidington EA, Edwards DR, Nuttall RK, Khokha R, et al. Cytokine stimulated vascular cell adhesion molecule-1 (VCAM-1) ectodomain release is regulated by TIMP-3. Cardiovasc Res. 2005;67:39–49. doi: 10.1016/j.cardiores.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 100.Hoque MO, Begum S, Brait M, Jeronimo C, Zahurak M, Ostrow KL, et al. Tissue inhibitor of metalloproteinases-3 promoter methylation is an independent prognostic factor for bladder cancer. J Urol. 2008;179:743–747. doi: 10.1016/j.juro.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu WH, Yu S, Meng Q, Brew K, Woessner JF., Jr TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]