Abstract
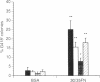
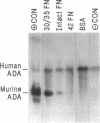
Direct contact between hematopoietic cells and viral packaging cell lines or other sources of stroma has been shown to increase the efficiency of retroviral-mediated gene transfer into these target cells compared with infection with viral supernatant. We have investigated the role of defined bone marrow extracellular matrix molecules (ECM) in this phenomenon. Here we report that infection of cells adhering to the carboxy-terminal 30/35-kD fragment of the fibronectin molecule (30/35 FN), which contains the alternatively spliced CS-1 cell adhesion domain, significantly increases gene transfer into hematopoietic cells. Two retroviral vectors differing in recombinant viral titer were used. Gene transfer into committed progenitor cells and long-term culture-initiating cells, an in vitro assay for human stem cells, was significantly increased when the cells were infected while adherent to 30/35 FN-coated plates compared with cells infected on BSA-coated control plates or plates coated with other bone marrow ECM molecules. Although gene transfer into committed progenitor cells and to a lesser degree into long-term culture-initiating cells was increased on intact fibronectin as well, increased gene transfer efficiency into hematopoietic cells on 30/35 FN was dependent on CS-1 sequence since infection on a similar FN fragment lacking CS-1 (42 FN) was suboptimal. 30/35 FN has previously been shown by our laboratory and other investigators to mediate adhesion of primitive murine and human hematopoietic stem cells to the hematopoietic microenvironment. Additional studies showed that neither soluble 30/35 FN nor nonspecific binding of hematopoietic cells to poly-L-lysine-coated plates had any appreciable effect on the infection efficiency of these cells. Our findings indicate that hematopoietic stem cell adhesion to specific ECM molecules alters retroviral infection efficiency. These findings should aid in the design of gene transfer protocols using hematopoietic progenitor and stem cells for somatic gene therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Human gene therapy. Science. 1992 May 8;256(5058):808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- Bergelson J. M., Shepley M. P., Chan B. M., Hemler M. E., Finberg R. W. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science. 1992 Mar 27;255(5052):1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- Bernardi P., Patel V. P., Lodish H. F. Lymphoid precursor cells adhere to two different sites on fibronectin. J Cell Biol. 1987 Jul;105(1):489–498. doi: 10.1083/jcb.105.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., McDonagh K. T., Brandt S. J., Ney P. A., Agricola B., Byrne E., Nienhuis A. W. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron J. W. Effect of steroids and dibutyryl cyclic AMP on the sensitivity of haemopoietic stem cells to 3 H-thymidine in vitro. Nature. 1971 Nov 5;234(5323):39–40. doi: 10.1038/234039a0. [DOI] [PubMed] [Google Scholar]
- Carter R. F., Abrams-Ogg A. C., Dick J. E., Kruth S. A., Valli V. E., Kamel-Reid S., Dubé I. D. Autologous transplantation of canine long-term marrow culture cells genetically marked by retroviral vectors. Blood. 1992 Jan 15;79(2):356–364. [PubMed] [Google Scholar]
- Correll P. H., Colilla S., Dave H. P., Karlsson S. High levels of human glucocerebrosidase activity in macrophages of long-term reconstituted mice after retroviral infection of hematopoietic stem cells. Blood. 1992 Jul 15;80(2):331–336. [PubMed] [Google Scholar]
- Cotten M., Wagner E., Zatloukal K., Phillips S., Curiel D. T., Birnstiel M. L. High-efficiency receptor-mediated delivery of small and large (48 kilobase gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6094–6098. doi: 10.1073/pnas.89.13.6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiano R. J., Smith L. C., Woo S. L. Hepatic gene therapy: adenovirus enhancement of receptor-mediated gene delivery and expression in primary hepatocytes. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2122–2126. doi: 10.1073/pnas.90.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Einerhand M. P., Bakx T. A., Kukler A., Valerio D. Factors affecting the transduction of pluripotent hematopoietic stem cells: long-term expression of a human adenosine deaminase gene in mice. Blood. 1993 Jan 1;81(1):254–263. [PubMed] [Google Scholar]
- Gordon M. Y., Greaves M. F. Physiological mechanisms of stem cell regulation in bone marrow transplantation and haemopoiesis. Bone Marrow Transplant. 1989 Jul;4(4):335–338. [PubMed] [Google Scholar]
- Gordon M. Y., Riley G. P., Watt S. M., Greaves M. F. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. 1987 Mar 26-Apr 1Nature. 326(6111):403–405. doi: 10.1038/326403a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Hall D. E., Reichardt L. F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C. H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990 Jun;110(6):2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jordan C. T., Lemischka I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990 Feb;4(2):220–232. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsmann M., Griffin J. D., DiCarlo J., Cannistra S. A. Myeloid and erythroid progenitor cells from normal bone marrow adhere to collagen type I. Blood. 1992 Feb 1;79(3):657–665. [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B., Apperley J. F., Orkin S. H., Williams D. A. Long-term expression of human adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8892–8896. doi: 10.1073/pnas.86.22.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luskey B. D., Rosenblatt M., Zsebo K., Williams D. A. Stem cell factor, interleukin-3, and interleukin-6 promote retroviral-mediated gene transfer into murine hematopoietic stem cells. Blood. 1992 Jul 15;80(2):396–402. [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988 Dec;167(2):400–406. [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Moore K. A., Deisseroth A. B., Reading C. L., Williams D. E., Belmont J. W. Stromal support enhances cell-free retroviral vector transduction of human bone marrow long-term culture-initiating cells. Blood. 1992 Mar 15;79(6):1393–1399. [PubMed] [Google Scholar]
- Moore K. A., Fletcher F. A., Villalon D. K., Utter A. E., Belmont J. W. Human adenosine deaminase expression in mice. Blood. 1990 May 15;75(10):2085–2092. [PubMed] [Google Scholar]
- Moritz T., Keller D. C., Williams D. A. Human cord blood cells as targets for gene transfer: potential use in genetic therapies of severe combined immunodeficiency disease. J Exp Med. 1993 Aug 1;178(2):529–536. doi: 10.1084/jem.178.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A. P., Wheldon L. A., Komoriya A., Wayner E. A., Yamada K. M., Humphries M. J. Affinity chromatographic isolation of the melanoma adhesion receptor for the IIICS region of fibronectin and its identification as the integrin alpha 4 beta 1. J Biol Chem. 1990 Mar 5;265(7):4020–4024. [PubMed] [Google Scholar]
- Ohashi T., Boggs S., Robbins P., Bahnson A., Patrene K., Wei F. S., Wei J. F., Li J., Lucht L., Fei Y. Efficient transfer and sustained high expression of the human glucocerebrosidase gene in mice and their functional macrophages following transplantation of bone marrow transduced by a retroviral vector. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11332–11336. doi: 10.1073/pnas.89.23.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. Loss of adhesion of murine erythroleukemia cells to fibronectin during erythroid differentiation. Science. 1984 Jun 1;224(4652):996–998. doi: 10.1126/science.6585955. [DOI] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. The fibronectin receptor on mammalian erythroid precursor cells: characterization and developmental regulation. J Cell Biol. 1986 Feb;102(2):449–456. doi: 10.1083/jcb.102.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda S., Ward M., Himelstein A., Richardson C., de la Flor-Weiss E., Smith L., Gottesman M., Pastan I., Bank A. Transfer and expression of the human multiple drug resistance gene into live mice. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9676–9680. doi: 10.1073/pnas.89.20.9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Schuening F. G., Kawahara K., Miller A. D., To R., Goehle S., Stewart D., Mullally K., Fisher L., Graham T. C., Appelbaum F. R. Retrovirus-mediated gene transduction into long-term repopulating marrow cells of dogs. Blood. 1991 Nov 15;78(10):2568–2576. [PubMed] [Google Scholar]
- Shaw L. M., Messier J. M., Mercurio A. M. The activation dependent adhesion of macrophages to laminin involves cytoskeletal anchoring and phosphorylation of the alpha 6 beta 1 integrin. J Cell Biol. 1990 Jun;110(6):2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siczkowski M., Clarke D., Gordon M. Y. Binding of primitive hematopoietic progenitor cells to marrow stromal cells involves heparan sulfate. Blood. 1992 Aug 15;80(4):912–919. [PubMed] [Google Scholar]
- Sorrentino B. P., Brandt S. J., Bodine D., Gottesman M., Pastan I., Cline A., Nienhuis A. W. Selection of drug-resistant bone marrow cells in vivo after retroviral transfer of human MDR1. Science. 1992 Jul 3;257(5066):99–103. doi: 10.1126/science.1352414. [DOI] [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Eaves A. C., Dragowska W., Lansdorp P. M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989 Oct;74(5):1563–1570. [PubMed] [Google Scholar]
- Toksoz D., Zsebo K. M., Smith K. A., Hu S., Brankow D., Suggs S. V., Martin F. H., Williams D. A. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G., Isberg R. R. Bacterial internalization mediated by beta 1 chain integrins is determined by ligand affinity and receptor density. EMBO J. 1993 May;12(5):1887–1895. doi: 10.1002/j.1460-2075.1993.tb05837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie C. M., McCarthy J. B., McGlave P. B. Differentiation of primitive human multipotent hematopoietic progenitors into single lineage clonogenic progenitors is accompanied by alterations in their interaction with fibronectin. J Exp Med. 1991 Sep 1;174(3):693–703. doi: 10.1084/jem.174.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Zatloukal K., Cotten M., Kirlappos H., Mechtler K., Curiel D. T., Birnstiel M. L. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Rios M., Stephens C., Patel V. P. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991 Aug 1;352(6334):438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- Yurochko A. D., Liu D. Y., Eierman D., Haskill S. Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9034–9038. doi: 10.1073/pnas.89.19.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K. S., Wicha M. S. Extracellular matrix production by the adherent cells of long-term murine bone marrow cultures. Blood. 1983 Mar;61(3):540–547. [PubMed] [Google Scholar]
- van Beusechem V. W., Kukler A., Heidt P. J., Valerio D. Long-term expression of human adenosine deaminase in rhesus monkeys transplanted with retrovirus-infected bone-marrow cells. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7640–7644. doi: 10.1073/pnas.89.16.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]