Abstract
Purpose
To evaluate the role of Anti-mullerian hormone (AMH) in predicting cumulative pregnancy outcome during in-vitro fertilization (IVF) treatment.
Methods
Serum AMH levels on day 6 of ovarian stimulation were taken from 180 women undergoing IVF with or without intracytoplasmic sperm injection (ICSI). The main outcome measures were ongoing pregnancy in the fresh cycle, cumulative ongoing pregnancy and ovarian response.
Results
There was a trend of higher median AMH levels in subjects achieving ongoing pregnancy in the fresh IVF cycle. The median AMH levels were significantly higher in subjects attaining ongoing pregnancy cumulatively and in subjects showing ovarian hyper-response in the stimulated cycle. Areas under the ROC curves were 0.606 and 0.792 for the prediction of cumulative ongoing pregnancy and ovarian hyper-response respectively.
Conclusions
Serum AMH concentration on day 6 of stimulation was significantly higher in subjects who achieved cumulative ongoing pregnancy in IVF compared to those who did not. Serum AMH is a reasonably good predictor of ovarian hyper-response.
Keywords: Anti-mullerian hormone, Assisted conception, Cumulative ongoing pregnancy, Ovarian response
Introduction
A number of parameters known as ovarian reserve markers have been proposed to predict ovarian response to gonadotropin, as well as pregnancy outcome during assisted reproduction treatment (ART) [1]. Early follicular phase serum FSH has been widely used and is a better predictor of ovarian response than the women’s age alone [2, 3]. Subsequently, ultrasound assessment of the antral follicle count (AFC) has been found to be a better clinical predictor of ovarian response than FSH level [4, 5]. Despite a correlation between these markers and ovarian response to stimulation, studies have consistently revealed very limited accuracy of these tests in predicting pregnancy outcome in IVF-Embryo transfer (ET) cycles [1].
More recently serum AMH has been found to be significantly correlated with AFC and the number of oocytes retrieved, and associated with higher fertilization rate and lower incidence of miscarriage after IVF cycles [6–8]. A recent meta-analysis [9] revealed that both AMH and AFC were comparable, they were reasonably good in predicting poor ovarian response but similarly poor in prediction of non-pregnancy. All these studies, however, were reporting on the pregnancy outcome in fresh stimulation cycles.
With the advent of embryo cryopreservation technology and the fact that a considerable proportion of IVF cycles result in surplus good quality embryos, frozen embryo transfer (FET) has become an integral part of assisted reproduction programmes. Indeed, the cumulative pregnancy rate per stimulated cycle, i.e. the combined pregnancy rate after transfer of fresh and frozen-thawed embryos, has been increasingly recognized as the most relevant standard of success in assessing an assisted reproduction programme [10–12]. Therefore, we carried out this retrospective analysis to evaluate the role of serum AMH concentration in predicting cumulative pregnancy outcome in IVF-ET treatment.
Materials and methods
Subject recruitment
This was a retrospective cohort analysis of 180 women undergoing IVF-ET treatment with or without ICSI in Edinburgh Fertility and Reproductive Endocrine Centre, Royal Infirmary of Edinburgh, during the period from October 2007 to March 2008 and who had serum AMH taken on day 6 of ovarian stimulation along with serum estradiol. These were patients undergoing long GnRH agonist protocol for ovarian stimulation after pituitary down-regulation. Patients using the GnRH antagonist protocol and those undergoing oocyte donation cycles were excluded from this retrospective analysis. The AMH assay was performed as part of our routine clinical ART programme when it was first introduced on a pilot basis, and this report was based on a retrospective analysis of data when we audited the performance of our assay.
Stimulated cycles
All women were pre-treated for pituitary down-regulation with buserelin (Suprecur®, Hoechst, Frankfurt, Germany) subcutaneous injection 0.5 ml daily. They were instructed to start using buserelin from the mid-luteal phase of the cycle proceeding the stimulation cycle if the menstrual cycles are regular. Patients with irregular cycles start downregulation on day one. Following at least 14 days of downregulation they received either recombinant FSH or human menopausal gonadotropin (HMG) for ovarian stimulation at a starting dose ranging from 100 to 300 IU per day according to clinical judgment. Recombinant human chorionic gonadotropin (hCG, Ovitrelle®,Merck Serono) 250 mcg was given subcutaneously when there were at least 3 follicles with mean diameter ≥18 mm seen on ultrasound scan.
Transvaginal ultrasound-guided oocyte retrieval under conscious sedation was carried out 36 h after the hCG trigger. Fertilization was carried out in-vitro either by conventional insemination or ICSI depending on semen parameters and previous fertilization history. Patients were allowed to have a maximum of two embryos replaced into the uterine cavity two to three days after oocyte retrieval. ET was performed under transabdominal ultrasound guidance. Prior to ET, embryos were graded for quality according to the criteria described by Zeibe et al. [13] and Van Royen et al. [14]. This included an assessment of the speed of cell division (number of blastomeres at any given stage of development), regularity of cell division and degree of fragmentation. On the basis of this assessment, the best quality embryo(s) were selected for transfer and any further good quality embryos remaining in the cohort were selected for cryopreservation. Luteal phase support was given in all cycles with progesterone suppository 200 mg twice daily from the day of embryo transfer and continued for 12 days. Serum beta hCG was measured 2 weeks following oocyte recovery and a concentration of ≥50 mIU/ml was considered positive indicator of pregnancy. Three weeks later the appearance of gestational sac with or without heart pulsations was considered as clinical pregnancy. All fresh embryos with good quality were cryopreserved if patients had developed symptoms suggestive of ovarian hyperstimulation.
Cryopreservation and frozen-thawed embryo transfer cycles
Excess good quality embryos were frozen on the day of ET. A slow freezing protocol using a programmable freezer was adopted.
Frozen-thawed embryo transfers were carried out in natural cycles or artificial hormone replacement cycles. For those FETs carried out in natural cycles, the patient was monitored for serum E2 and luteinizing hormone (LH) concentrations in the pre-ovulatory period, and FET was performed 2 days from the peak of LH surge. For patients undergoing artificial hormonal replacement cycles for FET, they received pituitary downregulation by Buserelin subcutaneous injection (0.5 ml once a day) for 2 weeks. The downregulation is confirmed by the scan, i.e. endometrium <4 mm or E2 < 150 pmol/L. Then the patient will continue on downregulation and estradiol valerate 6 mg daily and attend a scan after 2 weeks. If the endometrial thickness measures >8 mm, progesterone pessary was commenced 3 days prior to FET. FET was carried out using a soft transcervical catheter under transabdominal ultrasound guidance and a maximum of 2 frozen embryos was transferred. A serum hCG test was done 2 weeks after FET. If it was positive, ultrasound examination was performed 3 weeks later to confirm intrauterine pregnancy and to determine the number of gestational sacs present.
Outcome analysis
Primary outcomes include: (1) on-going pregnancy rate (viable pregnancies beyond 8–10 weeks of gestation), and (2) cumulative ongoing pregnancy rate (combined ongoing pregnancies from the fresh and all frozen-thawed cycles derived therein). Secondary outcomes include the duration and dosage of gonadotropin used, as well as the number of oocytes collected and fertilized.
Hormone assays
AMH concentration was determined with ELISA using a commercial kit (ACTIVE MIS/AMH ELISA, DSL-10-14400). The assay had a sensitivity of 0.04 pmol/l, and the intra-assay and inter-assay coefficients of variation were within 4.6% and 8.0% respectively.
Statistics
The predictive value of Day 6 serum AMH level on the primary and secondary outcomes was analyzed using receiver operator characteristic (ROC) curve. The correlation between Day 6 serum AMH level and the secondary outcomes were analyzed by Spearman’s test. Multiple regression analysis was applied to evaluate the predictive value of serum AMH level and other clinical and demographic parameters on the primary outcomes. The statistical analyses were performed using the SPSS 13.0 software. Since the distribution of AMH concentration was skewed, non-parametric analysis was used throughout and the values are expressed as median (range) in the following discussions.
Assuming a cumulative pregnancy rate in patients with AMH level below and above a cut-off value to be 20% and 40% respectively, a sample size of at least 164 in total would be adequate to determine a statistical significance at power of 80% and type I error of 5%.
Results
A total of 180 cycles of IVF-ET during the assessment period which fulfilled the inclusion criteria were analyzed. Their demographic characteristics are shown in Table 1. Regarding the cause of subfertility, 42.2% were due to male factor, 31.1% were unexplained, 11.7% and 6.1% were due to tubal factor and endometriosis respectively, 2.2% were due to anovulation who had failed ovulation induction treatment, and 6.7% due to mixed and other factors.
Table 1.
Demographic and clinical characteristics of the subjects (n = 180)
| Median | Range | |
|---|---|---|
| Age of woman (years) | 36 | 27–44 |
| Serum AMH concentration (pmol/l) | 10.07 | 0.14–94.96 |
| Serum FSH concentration (IU/l) | 6.3 | 1.6–19.4 |
| Serum estradiol concentration on Day 6 of stimulation (pmol/l) | 535 | 73–4,308 |
| Total dose of gonadotropin used (IU) | 2,488 | 1,000–6,300 |
| Duration of stimulation (days) | 12 | 8–21 |
| Number of oocytes retrieved | 8 | 0–38 |
Among the 180 subjects, 10 of them had not conceived yet but still had frozen embryos in storage. The remaining 170 subjects were entered into cumulative pregnancy outcome analysis.
AMH and ongoing pregnancy in the fresh cycle
There were 85 subjects (47.2%) with a positive pregnancy test in the fresh cycle, and 62 (34.4%) had ongoing pregnancy. The comparison of the clinical and demographic characteristics of subjects with and without attaining ongoing pregnancy in the fresh treatment cycle is shown in Table 2. The two groups did not differ in age. Those subjects who achieved ongoing pregnancy in the first cycle showed a trend towards higher serum AMH level (median 11.92 pmol/l, range 1.50–94.96 pmol/l) compared to those who did not (median 8.53 pmol/l, range 0.29–64.26 pmol/l), although statistical significance was not achieved (p = 0.079).
Table 2.
Comparison of demographic and clinical characteristics of subjects with relation to ongoing pregnancy outcome in the fresh treatment cycle (n = 180)
| Median (range) | Ongoing pregnancy | P value | |
|---|---|---|---|
| Yes (n = 62) | No (n = 118) | ||
| Age (years) | 36 (27–41) | 37 (27–44) | 0.102 |
| Serum AMH (pmol/l) | 11.92 (1.50–94.96) | 8.53 (0.29–64.26) | 0.079 |
| Serum FSH (IU/l) | 6.0 (2.0–17.6) | 6.6 (1.6–13.3) | 0.466 |
| Day 6 serum estradiol (pmol/l) | 610 (73–3,171) | 463 (73–4,308) | 0.121 |
| Total dose of gonadotropin administered (IU) | 2,475 (1,200–4,200) | 2,700 (1,000–6,300) | 0.107 |
| Duration of stimulation (days) | 12 (9–19) | 12 (8–21) | 0.148 |
| No. of oocytes retrieved | 9 (2–21) | 7 (1–24) | 0.020a |
| No. of oocytes fertilized | 6 (1–17) | 5 (1–16) | 0.008a |
aStatistically significant (p < 0.05, Mann-Whitney U test)
AMH and cumulative ongoing pregnancy rate per stimulated cycle
Of the 170 subjects included in the cumulative pregnancy analysis, 85 (50.0%) had positive pregnancy test, and 78 (45.9%) achieved ongoing pregnancy cumulatively after fresh plus frozen embryo replacements. Table 3 depicts the clinical and demographic characteristics of subjects who attained an ongoing pregnancy cumulatively versus those who did not. Subjects who had cumulative ongoing pregnancy were significantly younger in age (p = 0.010), having significantly higher serum AMH concentration (p = 0.017) and higher serum estradiol concentration (p = 0.010) on day 6 of ovarian stimulation than those who did not.
Table 3.
Comparison of demographic and clinical characteristics of subjects with relation to cumulative ongoing pregnancy in fresh together with frozen-thawed cycles (n = 170)
| Median (range) | Cumulative ongoing pregnancy | P value | |
|---|---|---|---|
| Yes (n = 78) | No (n = 92) | ||
| Age (years) | 36 (27–41) | 37 (27–44) | 0.010a |
| Serum AMH (pmol/l) | 12.78 (1.50–94.96) | 8.10 (0.14–64.26) | 0.017a |
| Serum FSH (IU/l) | 6.1 (1.7–17.6) | 6.7 (1.6–19.4) | 0.404 |
| Day 6 serum estradiol (pmol/l) | 630 (73–3,171) | 406 (73–4,308) | 0.010a |
| Total dose of gonadotropin administered (IU) | 2,400 (1,000–4,200) | 2,700 (1,000–6,300) | 0.004a |
| Duration of stimulation (days) | 12 (9–19) | 12 (8–21) | 0.073 |
| No. of oocytes retrieved | 9 (2–36) | 6 (0–34) | <0.001a |
| No. of oocytes fertilized | 7 (1–23) | 3 (0–16) | <0.001a |
Subjects not achieving pregnancy and not yet replacing all frozen embryos were excluded in this analysis
aStatistically significant (p < 0.05, Mann-Whitney U test)
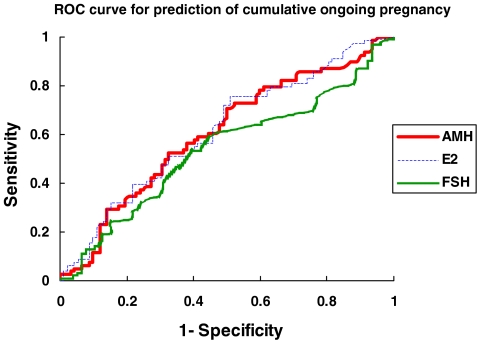
Figure 1 displayed ROC curve analysis of serum AMH level in predicting cumulative ongoing pregnancy. The area under the curve (AUC) for AMH was 0.606 (95% confidence interval (CI) 0.521–0.691), which was comparable to serum estradiol concentration on day 6 of ovarian stimulation (AUC = 0.615; 95% CI 0.530–0.699) and better than baseline serum FSH concentration (AUC = 0.537; 95%CI 0.450–0.624) before treatment. Considering a serum AMH concentration of 5 pmol/l as cut-off, the sensitivity and specificity of predicting cumulative ongoing pregnancy were 86% and 28% respectively. With this cut-off, the cumulative ongoing pregnancy rates in those subjects with serum AMH above and below 5.0 pmol/l were 50.4% and 29.7% respectively (p = 0.026, χ2-test).
Fig. 1.
Receiver-operator characteristic (ROC) curve analysis of serum AMH concentration, serum FSH concentration and day 6 serum estradiol in predicting cumulative ongoing pregnancy from IVF-ET treatment. Serum AMH (area under the curve = 0.606; 95% CI 0.521–0.691). Serum FSH (area under the curve = 0.537; 95% CI 0.450–0.624). Day 6 serum estradiol (area under the curve = 0.615; 95% CI 0.530–0.699)
When age of the women, early follicular serum FSH concentration, day 6 serum AMH concentration and day 6 serum estradiol concentration were entered in a forward stepwise fashion in the binary logistic regression analysis for cumulative ongoing pregnancy, age was the only factor which significantly predicted the likelihood of cumulative ongoing pregnancy (B = 0.010, p = 0.018).
Other clinical and demographic factors and pregnancy outcome
As shown in Table 2, subjects achieving ongoing pregnancy in the first treatment cycle had significantly higher number of oocytes retrieved and oocytes fertilized than those who did not, but the two did not differ in serum FSH, day 6 serum estradiol, total dose of gonadotropin administered and duration of stimulation. As shown in Table 3, subjects who attained cumulative ongoing pregnancy had higher day 6 serum estradiol concentrations (p = 0.010), lower total dose of gonadotropin administered (p = 0.004), and more oocytes retrieved (p < 0.001) and fertilized (p < 0.001), compared to those who did not. There were no differences in serum FSH between these two groups (p = 0.404).
Correlation of AMH concentration with clinical and demographic characteristics
By Spearman’s test, day 6 serum AMH concentration was significantly correlated with age of the women, serum FSH level, day 6 serum estradiol level, total dose of gonadotropin, duration of stimulation, number of oocytes retrieved and number of oocytes fertilized (Table 4).
Table 4.
Correlation between serum AMH concentration and other demographic factors and clinical parameters in the fresh treatment cycle
| Clinical/demographic parameter | Correlation coefficient | P-value |
|---|---|---|
| Age | −0.285 | <0.001a |
| Serum FSH concentration | −0.248 | 0.001a |
| Serum estradiol level on day 6 | 0.299 | <0.001a |
| Total dose of gonadotropin | −0.486 | <0.001a |
| Duration of stimulation | −0.274 | <0.001a |
| Number of oocytes collected | 0.575 | <0.001a |
| Number of oocytes fertilized | 0.469 | <0.001a |
aStatistically significant (p < 0.05, Spearman’s test)
AMH concentration and ovarian over-response
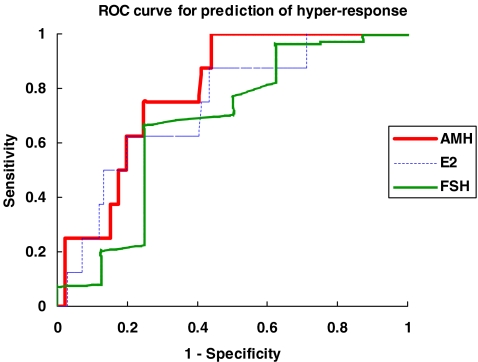
During the assessment period, in 8 of the fresh cycles, ET was withheld and all embryos were cryopreserved because of ovarian hyper-response as reflected by a high number of oocytes retrieved. These 8 cases with hyper-response had significantly higher serum AMH level (median 23.49 pmol/l, range 11.28–52.84 pmol/l) compared with the 166 subjects who had fresh embryo transfer (median 9.53 pmol/l, range 0.29–94.96 pmol/l) (p = 0.005). When plotted on a ROC curve for prediction of over-response, the AUC for AMH was 0.792 (95% CI 0.682–0.902) (Fig. 2), which was higher than that of FSH (AUC = 0.672; 95% CI 0.442–0.903) and day 6 serum estradiol (AUC = 0.737; 95% CI 0.579–0.895). A cut-off value of AMH concentration at 11.2 pmol/l gave a sensitivity of 100% and specificity of 56% in predicting over-response. There was only one case of cycle cancellation due to no response which was therefore too small a number for analysis. Her AMH concentration was 0.14 pmol/l.
Fig. 2.
Receiver-operator characteristic (ROC) curve analysis of day 6 serum AMH concentration, day 3 FSH concentration and day 6 serum estradiol concentration in predicting hyper-response to ovarian stimulation in the fresh treatment cycle of IVF-ET. Day 6 AMH (area under the curve = 0.792; 95% CI 0.682–0.902). Serum FSH (area under the curve = 0.672; 95% CI 0.442–0.903). Day 6 serum estradiol (area under the curve = 0.737; 95% CI 0.579–0.895)
Discussions
AMH is gaining acceptance as a useful marker of female reproductive function, especially with ovulatory disorders and IVF outcomes [15]. AMH is a dimeric glycoprotein member of the transforming growth factor-beta superfamily that is structurally related to inhibin and activin. In females, AMH levels are almost undetectable at birth. After an initially slight increase in the weeks after birth, AMH levels increase, peaking during late puberty [16] and then show a progressive decline throughout reproductive life as the follicular reserve becomes depleted [16, 17], and finally becoming undetectable after menopause [17]. In reproductive aged women, AMH is expressed by granulosa cells of early developing follicles and inhibits the transition from the primordial to the primary follicular stage. Serum levels of AMH correlate strongly with the number of antral follicles, suggesting that AMH levels reflect the size of primordial follicle pool.
AMH inhibits excessive follicular recruitment by FSH and therefore has a critical role in folliculogenesis [17, 18] It was previously reported that AMH serum levels do not significantly change throughout the menstrual cycle [19–21]. However, recent reports revealed statistically significant cyclical fluctuations in AMH levels with a rapid decrease in AMH levels in the early luteal phase [22, 23]. Likewise, AMH level also declines gradually during controlled ovarian stimulation [24]. However, the clinical significance of such intra-cycle variation is uncertain. Assessment of the ovarian reserve is particularly important in the IVF clinic, where AMH may be useful as a predictor of poor response [25]. One of the main advantages of AMH measurement in assisted reproductive technology (ART) compared with the other markers of ovarian reserve may derive from its low inter- and intra-cycle variability.
Although AMH is initially observed in granulosa cells of primary follicles, maximal expression occurs in pre-antral and small antral follicles. AMH expression declines as antral follicles increase in size, with nominal expression restricted to the granulosa cells of the cumulus [26]. This loss of AMH expression during the FSH-dependent final stages of follicular growth, and the lack of expression by atretic follicles suggests that basal levels of AMH may more accurately reflect the total developing follicular cohort and consequently potential ovarian response to FSH [27]. But other studies [6, 28] suggest that AMH concentrations obtained early in the follicular phase during ovarian stimulation under pituitary suppression for assisted reproduction are better predictors of ovarian under-response than basal AMH measurements, however it was not a good predictor of pregnancy outcome in ART.
To our knowledge, there has been no published report on the correlation between AMH and the cumulative pregnancy outcome. We analyzed the role of the serum AMH level during ovarian stimulation in predicting cumulative pregnancy outcome in IVF-ET treatment. It confirmed favorably a positive correlation between day 6 serum AMH level and the cumulative on-going pregnancy rate, although in the fresh cycle it did not differ between those who attained ongoing pregnancy and those who did not. We have considered cumulative success rate as a better outcome measure of our retrospective analysis. Indeed, with the availability of embryo cryopreservation as part of the routine IVF programme, the cumulative pregnancy outcome has greater clinical implication and meaning to the patient than the outcome of just the fresh cycle.
We compared the relative performance of AMH with pretreatment early follicular phase FSH, day 6 estradiol and maternal age as a predictor of cumulative ongoing pregnancy rate. The area under the ROC curve of 0.606 for serum AMH did not indicate that it is a very good predictor. However, it still exceeded that for FSH, which is the ovarian reserve marker commonly used in IVF-programmes at the moment. Yet when the factors were entered into a logistic regression analysis, only age of the women at stimulation remained as a significant independent factor determining cumulative ongoing pregnancy.
Theoretically serum AMH concentration could reflect treatment outcome after assisted conception through two ways. Firstly, AMH is a well accepted marker of ovarian reserve [15, 29], and hence it could well serve as a predictor of ovarian response, which in turn dictates the number of oocytes retrieved and the number of embryos available for transfer. The latter would determine the chance of cumulative pregnancy, which was also illustrated in our findings. Secondly, as a marker of ovarian reserve, a low serum AMH concentration could also reflect ovarian aging, which theoretically has a negative effect on the oocyte quality and hence treatment outcome. Several authors have found a significant positive correlation between AMH levels and oocyte quality [30–32] and embryo morphology [32]. However, this relationship has not been confirmed by others [33, 34]. Moreover, in a recent study, no consistent correlation between AMH and embryo morphology, and most importantly embryo aneuploidy rate, was demonstrable [34]. Hence the possible prediction of qualitative aspects of ART programs by AMH measurement remains largely controversial.
With regard to ovarian response, there have been a number of reports on the role of AMH in predicting ovarian response in the fresh cycle of IVF-ET treatment. It has been demonstrated that higher AMH concentrations were associated with a greater number of mature oocytes, higher fertilization rates, a greater number of embryos, lower incidence of miscarriage during fresh transfers and ultimately a higher clinical pregnancy rate [7, 30]. In line with this, our data did show a significant positive correlation between AMH concentration and day 6 serum estradiol, number of oocytes collected and number of fertilized oocytes, and a significant inverse correlation with serum FSH, total dose of gonadotropin used and total duration of stimulation. The currently available literature indicates that AMH may be a superior marker for predicting ovarian response over either age of the patient, day 3 FSH, estradiol or inhibin B, whereas the vast majority of studies have found AMH and AFC to have similar predictive value for poor response [9, 17]. In the absence of a perfect predictive marker at the moment, serum AMH concentration could well serve as a reference in conjunction with the other factors in pre-treatment counseling. In our cohort, there was only one non-responder and hence statistical analysis was not possible, though it was noteworthy that she had an AMH concentration approaching zero (0.14 pmol/l).
On the other hand, there have also been reports on the role of serum AMH in prediction of excessive ovarian response in IVF treatment [15, 27, 35]. Our finding was in line with these. ROC curve analysis suggested that AMH is superior to FSH and day 6 estradiol in prediction of hyper-response. There was no case of hyper-response where serum AMH concentration was less than 11.2 pmol/l. Whether or not this can be applied to triage the dose of gonadotropin stimulation to prevent hyper-response would require further evaluation. Currently, mild stimulation is generally advocated to reduce the risk of ovarian hyperstimulation syndrome. There is one recent report suggesting dose tailoring based on AMH as a useful approach [36]. It demonstrated that the live birth rate dramatically increases with increasing basal AMH value. They suggested that use of circulating AMH to individualize treatment strategies for controlled ovarian stimulation may result in reduced clinical risk, optimized treatment burden and maintained pregnancy rates Although there is a clear association between AMH and oocyte yield, however, they demonstrated that AMH performs relatively poorly as a screening test for either potential cycle cancellation or poor response to controlled ovarian stimulation according to their ROC analyses. Therefore it is not feasible to suggest that a woman should not undergo controlled ovarian stimulation based on a low plasma AMH value [27]. There has been a case report in a 34-year-old woman with isolated negligible (<0.5 ng/mL or <3.6 pmol/L) AMH level and poor response who had controlled ovarian hyperstimulation resulting in a live birth at term [37].
In our data presented, we arbitrarily set a cut-off value of serum AMH concentration at 5.0 pmol/l which has been suggested as a cut-off for poor ovarian response [36]. From the ROC curve, this gave a sensitivity and specificity of 85.9% and 28.3% respectively. Women with AMH above 5.0 pmol/l had a significantly higher cumulative ongoing pregnancy rate than those below 5.0 pmol/l (50.4% vs. 29.7%). This indicated that AMH levels below this cut-off value of 5.0 pmol/l are reasonably associated with non-successful pregnancy but this cut-off is not specific as a predictor of cumulative ongoing pregnancy.
In conclusion, we found that serum AMH concentration was significantly higher in subjects with successful cumulative ongoing pregnancy in assisted conception treatment compared with those failing treatment but this difference was insignificant after adjusting for age. Serum AMH level is a reasonably good predictor of ovarian hyper-response.
Acknowledgements
We thank the staff at Edinburgh Fertility and Reproductive Endocrine centre, Royal Infirmary of Edinburgh, UK for their support and management of patients.
Footnotes
Capsule
Serum Anti-mullerian hormone concentration was significantly higher in subjects with successful cumulative ongoing pregnancy in assisted conception treatment compared to those who were not pregnant.
References
- 1.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 2.Cahill DJ, Prosser CJ, Wardle PG, Ford WC, Hull MG. Relative influence of serum follicle hormone, age and other factors on ovarian response to gonadotropin stimulation. Br J Obstet Gynaecol. 1994;101(11):999–1002. doi: 10.1111/j.1471-0528.1994.tb13047.x. [DOI] [PubMed] [Google Scholar]
- 3.Sharif K, Elgendy M, Lashen H, Afnan M. Age and basal follicle stimulating hormone as predictors of in vitro fertilization outcome. Br J Obstet Gynaecol. 1998;105(1):107–12. doi: 10.1111/j.1471-0528.1998.tb09360.x. [DOI] [PubMed] [Google Scholar]
- 4.Ng EH, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an in-vitro fertilization programme. Hum Reprod. 2000;15(9):1937–42. doi: 10.1093/humrep/15.9.1937. [DOI] [PubMed] [Google Scholar]
- 5.Nahum R, Shifren JL, Chang YC, Leykin L, Isaacson K, Toth TL. Antral follicle assessment as a tool for predicting outcome in IVF—is it a better predictor than age and FSH? J Assist Reprod Genet. 2001;18(3):151–5. doi: 10.1023/A:1009424407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ficicioglu C, Kutlu T, Baglam E, Bakacak Z. Early follicular antimullerian hormone as an indicator of ovarian reserve. Fertil Steril. 2006;85(3):592–6. doi: 10.1016/j.fertnstert.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Mullerian hormone as a predictor of IVF outcome. Reprod Biomed Online. 2007;14(5):602–10. doi: 10.1016/S1472-6483(10)61053-X. [DOI] [PubMed] [Google Scholar]
- 8.Kwee J, Schats R, McDonnell J, Themmen A, Jong F, Lambalk C. Evaluation of anti-Mullerian hormone as a test for the prediction of ovarian reserve. Fertil Steril. 2008;90(3):737–43. doi: 10.1016/j.fertnstert.2007.07.1293. [DOI] [PubMed] [Google Scholar]
- 9.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullierian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril. 2009;91(3):705–14. doi: 10.1016/j.fertnstert.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Tiitinen A, Hyden-Granskog C, Gissler M. What is the most relevant standard of success in assisted reproduction? The value of cryopreservation on cumulative pregnancy rates per single oocyte retrieval should not be forgotten. Hum Reprod. 2004;19(11):2439–41. doi: 10.1093/humrep/deh446. [DOI] [PubMed] [Google Scholar]
- 11.Ubaldi F, Rienzi L, Baroni E, Ferrero S, Iacobelli M, Minasi MG, Sapienza F, Martinez F, Anniballo R, Cobellis L, Tesarik J, Greco E. Cumulative pregnancy rates after transfer of fresh and thawed embryos. Eur J Obstet Gynecol Reprod Biol. 2004;115S:S106–9. doi: 10.1016/j.ejogrb.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Borini A, Cattoli M, Bulletti C, Coticchio G. Clinical efficiency of oocyte and embryo cryopreservation. Ann NY Acad Sci. 2008;1127:49–58. doi: 10.1196/annals.1434.012. [DOI] [PubMed] [Google Scholar]
- 13.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12(7):1545–9. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 14.Royen E, Mangelschots K, Vercruyssen M, Neubourg D, Valkenburg M, Ryckaert G, Gerris J. Multinucleation in cleavage stage embryos. Hum Reprod. 2003;18(5):1062–9. doi: 10.1093/humrep/deg201. [DOI] [PubMed] [Google Scholar]
- 15.Nakhuda GS. The role of mullerian inhibiting substance in female reproduction. Curr Opin Obstet Gynecol. 2008;20(3):257–64. doi: 10.1097/GCO.0b013e3282fe99f2. [DOI] [PubMed] [Google Scholar]
- 16.Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, Hasegawa Y, Noto RA, Schoenfeld D, MacLaughlin DT. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–6. doi: 10.1210/jc.81.2.571. [DOI] [PubMed] [Google Scholar]
- 17.Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS. ESHRE Special Interest Group for Reproductive Endocrinology—AMH Round Table: Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24(9):2264–75. doi: 10.1093/humrep/dep210. [DOI] [PubMed] [Google Scholar]
- 18.Singer T, Barad DH, Weghofer A, Gleicher N. Correlation of antimüllerian hormone and baseline follicle-stimulating hormone levels. Fertil Steril. 2009;91(6):2616–9. doi: 10.1016/j.fertnstert.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Hehenkamp WJ, Looman CW, Themmen AP, Jong FH, Velde ER, Broekmans FJ. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057–63. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 20.Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21(12):3103–7. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 21.Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy Ch, Englert Y. Stable serum levels of anti-Müllerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22(7):1837–40. doi: 10.1093/humrep/dem101. [DOI] [PubMed] [Google Scholar]
- 22.Wunder DM, Bersinger NA, Yared M, Kretschmer R, Birkhäuser MH. Statistically significant changes of antimüllerian hormone and inhibin levels during the physiologic menstrual cycle in reproductive age women. Fertil Steril. 2008;89(4):927–33. doi: 10.1016/j.fertnstert.2007.04.054. [DOI] [PubMed] [Google Scholar]
- 23.Streuli I, Fraisse T, Chapron C, Bijaoui G, Bischof P, Ziegler D. Clinical uses of anti-Müllerian hormone assays: pitfalls and promises. Fertil Steril. 2009;91(1):226–30. doi: 10.1016/j.fertnstert.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 24.Fanchin R, Schonäuer LM, Righini C, Frydman N, Frydman R, Taieb J. Serum anti-Müllerian hormone dynamics during controlled ovarian hyperstimulation. Hum Reprod. 2003;18(2):328–32. doi: 10.1093/humrep/deg043. [DOI] [PubMed] [Google Scholar]
- 25.Visser JA, Jong FH, Laven JS, Themmen AP. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131(1):1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 26.Weenen C, Laven JS, Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 27.Nelson SM, Yates RW, Fleming R. Serum anti-mullerian hormone and FSH: prediction of live birth and extremes of response in stimulated cycles—implications for individualization of therapy. Hum Reprod. 2007;22(9):2414–21. doi: 10.1093/humrep/dem204. [DOI] [PubMed] [Google Scholar]
- 28.Peñarrubia J, Fábregues F, Manau D, Creus M, Casals G, Casamitjana R, Carmona F, Vanrell JA, Balasch J. Basal and stimulation day 5 anti-Mullerian hormone serum concentrations as predictors of ovarian response and pregnancy in assisted reproductive technology cycles stimulated with gonadotropin-releasing hormone agonist—gonadotropin treatmen. Hum Reprod. 2005;20(4):915–22. doi: 10.1093/humrep/deh718. [DOI] [PubMed] [Google Scholar]
- 29.Marca A, Volpe A. Anti-mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol. 2006;64(6):603–10. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 30.Hazout A, Bouchard P, Seifer DB, Aussage P, Junca AM, Cohen-Bacrie P. Serum antimüllerian hormone/müllerian-inhibiting substance appears to be a more discriminatory marker of assisted reproductive technology outcome than follicle-stimulating hormone, inhibin B, or estradiol. Fertil Steril. 2004;82(5):1323–9. doi: 10.1016/j.fertnstert.2004.03.061. [DOI] [PubMed] [Google Scholar]
- 31.Silberstein T, MacLaughlin DT, Shai I, Trimarchi JR, Lambert-Messerlian G, Seifer DB, Keefe DL, Blazer AS. MIS levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21(1):159–63. doi: 10.1093/humrep/dei270. [DOI] [PubMed] [Google Scholar]
- 32.Ebner T, Sommergruber M, Moser M, Shebl O, Schreier-Lechner E, Tews G. Basal level of AMH is associated with oocyte quality in stimulated cycles. Hum Reprod. 2006;21(8):2022–6. doi: 10.1093/humrep/del127. [DOI] [PubMed] [Google Scholar]
- 33.Smeenk JM, Sweep FC, Zielhuis GA, Kremer JA, Thomas CM, Braat DD. Antimüllerian hormone predicts ovarian responsiveness, but not embryo quality or pregnancy, after in vitro fertilization or intracyoplasmic sperm injection. Fertil Steril. 2007;87(1):223–6. doi: 10.1016/j.fertnstert.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Lie Fong S, Baart EB, Martini E, Schipper I, Visser JA, Themmen AP, Jong FH, Heuvel-Eibrink MM. Anti-Mullerian hormone: a marker for oocyte quantity, oocyte quality and embryo quality? Reprod Biomed Online. 2008;16(5):664–70. doi: 10.1016/S1472-6483(10)60480-4. [DOI] [PubMed] [Google Scholar]
- 35.Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, Laing I. Circulating basal anti-mullerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586–93. doi: 10.1016/j.fertnstert.2008.08.127. [DOI] [PubMed] [Google Scholar]
- 36.Nelson SM, Yates RW, Lyall H, Jamieson M, Traynor I, Gaudoin M, Mitchell P, Ambrose P, Fleming R. Anti-Mullerian hormone based approach to controlled ovarian stimulation for assisted conception. Hum Reprod. 2009;24(4):867–75. doi: 10.1093/humrep/den480. [DOI] [PubMed] [Google Scholar]
- 37.Tocci A, Ferrero S, Iacobelli M, Greco E. Negligible serum anti-mullerian hormone: pregnancy and birth after a 1-month course of an oral contraceptive, ovarian hyperstimulation, and intracytoplasmic sperm injection. Fertil Steril. 2009;92(1):395.e9–.e12. doi: 10.1016/j.fertnstert.2009.03.044. [DOI] [PubMed] [Google Scholar]