Abstract
Purpose
To determine the effects of α-tocopherol supplementation to oocyte maturation media and embryo culture media on the yield of ovine embryos.
Methods
α-tocopherol, at concentrations of 0, 50, 100, 200, 400 and 500 µM was supplemented to ovine oocyte or embryo culture media and cultured at 5% or 20% O2 levels. Percentages of cleavage, morula and blastocyst, total cell count and comet assay were taken as indicators of developmental competence of embryos.
Results
200 µM α-tocopherol in embryo culture medium at 20% O2 level significantly increased the rates of cleavage (P < 0.05), morulae (P < 0.05) and blastocyst (P < 0.01) formation and blastocyst total cell number (P < 0.01) when compared with control. The rates of blastocyst formation were also significantly higher in 100 µM (P < 0.01) and 400 µM (P < 0.05) supplemented groups than control.
Conclusion
α-tocopherol supplementation may enhance the in vitro developmental competence of ovine embryos by protecting them from oxidative damage.
Keywords: Vitamin E, Ovine, In vitro fertilization, Oxidative stress, Embryo culture
Introduction
Sheep is an important livestock that acts as a source of wool, meat and milk to millions around the globe. Sheep, being seasonal breeders, do not yield sufficient lamb crops to meet the demand. Hence assisted reproduction technologies (ART) have been developed over the past few decades to produce high-yielding lambs in large numbers. As with other technologies, in vitro embryo production technologies have their share of problems and failures [1] and therefore need to be optimized to produce healthy and viable lamb crops. In vitro fertilization (IVF) technique is a commonly used ART.
A major problem encountered in IVF is that of oxidative stress [2]. In their natural environment, oocytes and embryos are protected from oxidative damage by free radical scavengers present in oviductal and follicular fluids and also by antioxidant enzyme systems such as glutathione peroxidase, superoxide dismutase etc [3, 4]. However, during in vitro fertilization, oocytes and embryos are exposed to an environment lacking such sophisticated protection and tend to experience greater oxidative stress. Oxidative damage to gametes and embryos occurs due to free radicals generated by endogenous processes such as normal cellular metabolism and exogenous factors such as chemicals added to culture media, hyperoxia, exposure to light etc. The innate antioxidant defenses in embryos are not sufficient to counter the oxidative stress encountered during in vitro culture. Several studies have documented the damages caused by pro-oxidants and reactive oxygen species (ROS) on in vitro cultured murine [5], bovine [6] and porcine [7] gametes and embryos. Similar observations have also been made in human IVF study [8]. Therefore, during in vitro gamete or embryo culture, this excessive oxidative stress must be controlled by addition of antioxidants to culture media.
Numerous antioxidant chemicals have been added as supplements to culture media in mammalian in vitro embryo culture (IVEC). Some of these include proteins, vitamins, antioxidant enzymes, metal chelators, thiol compounds etc [2]. Vitamin E represents a group of lipid-soluble compounds that are well-known for their antioxidant properties [9]. α-tocopherol and its derivatives act as antioxidants both in vivo [10, 11] and in vitro [12]. Antioxidant vitamins such as α-tocopherol help reduce oxidant damage by acting as a sink to the spare electrons [13].
Earlier studies in porcine indicate that the blastocyst quality of in vitro fertilized and somatic cell nuclear transferred embryos was improved when embryo culture media was supplemented with α-tocopherol [14]. Studies on bovine suggest that culture of embryos with vitamin E resulted in development of more numbers of embryos to early and expanded blastocysts than that of the control group [12]. Studies in human sperm indicate that α-tocopherol supplementation improved the baseline DNA integrity and decreased the level of damage to the human sperm DNA following X-ray irradiation [15]. Since the effect of α-tocopherol has not been experimented in sheep, the present study attempted to determine the role of α-tocopherol in in vitro sheep oocyte maturation and embryo culture through its supplementation in oocyte maturation medium or embryo culture medium.
Materials and methods
Unless otherwise stated, all chemicals used in this experiment were purchased from Sigma-Aldrich Chemicals Pvt. Ltd., Bangalore, India.
Collection of oocytes
Sheep ovaries were obtained from a local abattoir and transported to the laboratory suspended in 0.9% saline supplemented with 50 µg/ml gentamycin in insulated containers within an hour of slaughter. Upon arrival, the ovaries were washed repeatedly in normal saline, trimmed free of extraneous tissue and rinsed in normal saline. The cumulus-oocyte complexes (COCs) were isolated from follicles by slicing method [16] and subsequently washed thrice in Tyrode’s lactate–N–[2-hydroxyethyl] piperazine–N’–[2-ethanesulphonic acid] (TL-HEPES) medium. The COCs were assessed morphologically and only those that had a compact non-atretic cumulus oophorus–corona radiata and a homogenous ooplasm were selected for in vitro maturation.
Maturation of oocytes in vitro
The COCs were washed thrice–first in TL-HEPES medium and subsequently in maturation medium composed of TCM 199 (Invitrogen Corporation, USA) supplemented with 10% (v/v) fetal calf serum (FCS) (Gibco Laboratories, Grand Island, USA), 5.5 mg/ml sodium pyruvate, 25 µg/ml gentamycin sulphate, 5.0 µg/ml LH (ovine LH; Sigma, L5269), 0.5 µg/ml FSH (porcine FSH; Sigma, F8001) and 1 µg/ml Estradiol (E2). Twenty COCs were placed in 100 µl droplets of maturation medium and matured for 24 h at 39°C at 5% CO2 in air (which is approximately 20% O2) or at 5% CO2, 5% O2 and 90% N2, based on the design of the experiment.
Fertilization of oocytes in vitro
Sheep testis obtained from the local abattoir was transported to the laboratory suspended in 0.9% saline supplemented with 50 µg/ml gentamycin in insulated container within an hour of slaughter. The procedure for extracting semen from testes was partly similar to that followed in earlier ovine in vitro study [17]. The main difference was that the swim-up procedure was used as sperm separation technique by these authors. Briefly, sheep testis was washed in saline, trimmed free of covering tissues and the tail of epididymis, presumed to contain mature sperms, was cut using a sterile blade. The sperm-rich fluid that oozed out was directly laid on Bovine Serum Albumin-free Brackett and Oliphant (BSA free BO) medium [18] (containing 10 mM caffeine sodium benzoate and 10 µg/ml heparin) in a petridish. Sperm selection was carried out in a Percoll (Pharmacia, Uppsala) density gradient (45% / 90%) placed in CO2 incubator at 39°C for two hours. Approximately 2–3 ml of BSA free BO medium containing the semen sample was layered over the pre-incubated gradient solution in sterile centrifuge tubes, and centrifuged at 600 × g for 10 min at room temperature. The supernatant was discarded and the sperm sediment was rewashed twice by centrifugation at 600 × g for 10 min in BSA-free BO medium described above. The final pellet was resuspended in 1 ml of BSA-free BO medium diluted with 1 ml BO medium containing 20 mg/ml BSA supplemented with 10 µg/ml heparin. Spermatozoa were capacitated in this medium for 60 min in a stoppered tube at 39°C in air. A final sperm concentration of approximately 1–2 × 106 per ml BO medium was used for fertilization. Mature sheep COCs were then washed in BO medium and distributed at a rate of 20 per 100 µl drop of fertilization medium under mineral oil. 2 µl of capacitated spermatozoa were added to these fertilization drops and incubated for 18 h at 39°C at 5% CO2, 5% O2 and 90% N2.
Embryo culture in vitro
Upon completion of the incubation period, the oocytes were washed to remove the cumulus cells by repeated pipetting through a small-bore pipette. They were then cultured in modified synthetic oviductal fluid medium [19] containing 2% (v/v) BME essential amino acids and 1% (v/v) MEM non-essential amino acids, 3 mg/ml BSA, 0.6 mM sodium pyruvate, 10 µg/ml gentamycin at a rate of 20 embryos per 100 µl droplet for 8 days at 39°C under 5% CO2 in air (which is approximately 20% O2) or under 5% CO2, 5% O2 and 90% N2 based on the design of the experiment. The medium was changed once every 48 h to replenish the nutrients.
Blastocyst cell number analysis
Blastocyst cell number analysis was done to assess the morphological quality of embryos. Expanded day 8 blastocysts from each treatment group were fixed and stained in accordance with earlier reports [20]. These expanded blastocysts were then individually transferred onto glass microscopic slides and dried at room temperature. They were then fixed with 70% ethanol for 24 h. The fixed blastocysts were then stained with 10 µg/ml bisbenzamide (Hoechst 33342) and 2.3% sodium citrate. The slides were observed under an epifluorescence microscope, fitted with excitation filter (330–380 nm) and barrier filter (420 nm). The total numbers of nuclei in each blastocyst were counted.
Comet assay
DNA damage in individual embryos cultured for 3 days under 20% O2, in the presence / absence of vitamin E supplementation was assessed by comet assay [21]. 10 embryos were washed twice in a mixture of phosphate buffered saline (PBS) and polyvinylpyrrolidone (4 mg/ml). Then, embryos from each experimental group were transferred to a 200 µl drop of 1% low-melting temperature agarose (Genei, Bangalore) in PBS at 39°C; the agarose drop was placed on a 35 mm plastic petridish. Using a stereo-dissection microscope to visualize the embryos, the embryos were gently mixed with the 1% low-melting temperature agarose and then captured in a total volume of about 10 µl using a mouth-operated glass pipette. The embryos were then quickly transferred onto a glass microscopic slide pre-coated with 1% high melting temperature agarose (Genei, Bangalore). Then, the slides were placed for 5 min on ice to solidify the agarose. The embryos were then lysed by incubating the slides for 3 h at ambient temperature in lysing buffer composed of 10 mM Tris, pH 10, containing 100 mM sodium EDTA, 2.5 mM NaCl, 10 µg/ml proteinase K, 1% sodium sarcosinate and 1% Triton X-100. Then, the slides were removed from the lysing solution and placed on a horizontal gel electrophoresis unit. The unit was filled with fresh electrophoresis buffer (1 mM sodium EDTA, 300 mM NaOH) to a level 0.25 cm above the slides, followed by equilibration of the slides in electrophoresis buffer for 20 min. Electrophoresis was then carried out at 25 V for 20 min. The slides were then neutralized by immersing in 0.4 M Tris-HCl (pH 7.5) for 5 min at ambient temperature. Staining of DNA was carried out by adding a 20 µl drop of acridine orange (5 µg/ml) to the slide for 2 min followed by 1 min of washing in sterile distilled water. Stained DNA was observed under fluorescence microscope. Quantification of DNA damage was done by measuring the length of the streak of DNA comet tail. The length was calculated by comparing with a photograph of a micrometer of the same magnification as that of the embryos.
Experimental design
α-tocopherol was first dissolved in 95% ethyl alcohol as a 2000-strength stock solution and stored at 4°C in dark; about 18 h prior to culture, appropriate dilutions of this stock solution were made in culture medium to attain the required working solution concentration [12]. The ethanol concentration during oocyte maturation or embryo culture was less than 0.05%. Based on the experimental design, in control groups, 0.05% ethanol was added to the oocyte maturation or embryo culture media.
In Experiment I, varying concentrations of α-tocopherol (0, 50,100, 200, 400 and 500 µM) were added to oocyte maturation medium followed by maturation of oocytes in 5% O2 environment. The embryo culture medium was not supplemented with α-tocopherol. Embryo culture was also carried out under 5% O2 environment. In Experiment II, the aforesaid concentrations of α-tocopherol were added to oocyte maturation medium followed by oocyte maturation in 20% O2 environment. However, the embryos that formed were subsequently cultured in 5% O2 environment. As with Experiment I, in Experiment II also the embryo culture medium was not supplemented with α-tocopherol. Experiment III was carried out in 5% O2 environment wherein varying concentrations of α-tocopherol (0, 50,100, 200. 400 and 500 µM) were added to embryo culture medium. However, α-tocopherol was not supplemented to oocyte maturation medium. In Experiment IV, the aforesaid concentrations of α-tocopherol were added to embryo culture medium followed by culture in 20% O2 environment. However, the oocyte maturation medium was not supplemented with α-tocopherol and oocytes were matured under 5% O2 environment.
Thus the experimental design can be summarized as follows:
Oocyte maturation with α-tocopherol at 5% O2 environment and embryo culture without α-tocopherol at 5% O2 environment.
Oocyte maturation with α-tocopherol at 20% O2 environment and embryo culture without α-tocopherol at 5% O2 environment.
Oocyte maturation without α-tocopherol at 5% O2 environment and embryo culture with α-tocopherol at 5% O2 environment.
Oocyte maturation without α-tocopherol at 5% O2 environment and embryo culture with α-tocopherol at 20% O2 environment.
Statistical analysis
Statistical Package for Social Sciences (SPSS 11.O, Chicago, USA) software was used for statistical analysis. In each experimental group, oocytes were randomly distributed. The percentages of oocytes that were fertilized and embryos that had developed to morula and blastocyst were subjected to arcsine transformation before analysis. The total cell count data (of blastocyst) were directly subjected to analysis. All data were subjected to one-way ANOVA followed by Tukey’s test to determine differences among experimental groups. Differences P < 0.05 were considered statistically significant.
Results
Experiment I
The results of Experiment I are shown in Table 1. Addition of α-tocopherol to in vitro oocyte maturation medium and subsequent culture under 5% O2 environment did not result in significant increases in the percentage of cleavages, morula, blastocyst or total cell count when compared to control.
Table 1.
Effect of α-tocopherol supplementation to oocyte maturation medium on development of preimplantation sheep embryos cultured in 5% oxygen environment
| α-tocopherol conc. (µM) | No. of oocytes inseminated | Cleavage (%) | Morula (%) | Blastocyst (%) | No. of blastocysts evaluated | Total cell number |
|---|---|---|---|---|---|---|
| 0 | 360 | 65.51 ± 2.07a | 33.23 ± 2.17a | 10.66 ± 1.02a | 18 | 89.50 ± 3.39a |
| 50 | 384 | 65.86 ± 1.63a | 33.55 ± 1.70a | 9.96 ± 1.17a | 19 | 87.67 ± 2.94a |
| 100 | 372 | 64.48 ± 1.82a | 31.84 ± 2.34a | 8.94 ± 1.08a | 18 | 90.83 ± 3.88a |
| 200 | 369 | 67.19 ± 1.85a | 33.74 ± 2.34a | 11.14 ± 1.29a | 17 | 94.33 ± 4.38 a |
| 400 | 392 | 66.91 ± 1.18a | 34.17 ± 1.68a | 11.58 ± 1.07a | 20 | 89.00 ± 4.37a |
| 500 | 375 | 65.26 ± 0.94a | 33.60 ± 1.23a | 10.82 ± 0.56a | 19 | 88.33 ± 2.84a |
Values are listed as mean±SEM; means in the same column with different superscripts were significantly different
Experiment II
Data from Experiment II are shown in Table 2. No significant change was observed upon supplementation of α-tocopherol to in vitro oocyte maturation medium and subsequent culture under 20% O2 environment with respect to rates of cleavage, embryos that developed to morulae and blastocyst or blastocyst total cell number when compared with control.
Table 2.
Effect of α-tocopherol supplementation to oocyte maturation medium on development of preimplantation sheep embryos cultured in 20% oxygen environment
| α-tocopherol conc. (µM) | No. of oocytes inseminated | Cleavage (%) | Morula (%) | Blastocyst (%) | No. of blastocysts evaluated | Total cell number |
|---|---|---|---|---|---|---|
| 0 | 382 | 59.06 ± 0.95a | 26.16 ± 1.73a | 8.90 ± 1.44a | 19 | 88.17 ± 3.11a |
| 50 | 364 | 57.82 ± 1.18a | 24.32 ± 2.02a | 8.09 ± 0.79a | 17 | 85.83 ± 2.93a |
| 100 | 377 | 58.30 ± 1.16a | 24.87 ± 2.19a | 8.63 ± 0.99a | 15 | 88.33 ± 4.03a |
| 200 | 395 | 60.31 ± 1.62a | 27.08 ± 2.10a | 9.86 ± 1.74a | 18 | 92.17 ± 3.53a |
| 400 | 365 | 61.21 ± 0.78a | 27.92 ± 1.78a | 10.62 ± 1.83a | 18 | 94.50 ± 3.92a |
| 500 | 383 | 59.57 ± 1.34a | 27.79 ± 2.29a | 10.40 ± 1.66a | 20 | 91.17 ± 4.31a |
Values are listed as mean±SEM; means in the same column with different superscripts were significantly different
Experiment III
The effects of α-tocopherol in embryo culture medium at 5% oxygen levels are shown in Table 3. No significant difference was observed with respect to rate of cleavage, morula, blastocyst formation or blastocyst total cell number between control and supplemented groups. However, significant increases in rates of morulae (P < 0.05) and blastocyst (P < 0.01) formation were observed in the 200 µM supplemented group when compared with the 500 µM supplemented group. No such differences were observed with respect to blastocyst total cell number.
Table 3.
Effect of α-tocopherol supplementation to embryo culture medium on development of preimplantation sheep embryos cultured in 5% oxygen environment
| α-tocopherol conc. (µM) | No. of oocytes inseminated | Cleavage (%) | Morula (%) | Blastocyst (%) | No. of blastocysts evaluated | Total cell number |
|---|---|---|---|---|---|---|
| 0 | 373 | 65.49 ± 1.27a | 33.11 ± 1.93ab | 10.54 ± 0.86ab | 17 | 88.33 ± 2.82a |
| 50 | 386 | 66.36 ± 1.26a | 34.82 ± 1.97ab | 11.80 ± 0.66ab | 18 | 91.50 ± 4.26a |
| 100 | 368 | 68.55 ± 1.33a | 36.47 ± 2.43ab | 12.69 ± 1.84ab | 16 | 92.67 ± 4.28a |
| 200 | 377 | 68.91 ± 0.81a | 39.20 ± 1.90a | 14.54 ± 0.64a | 19 | 94.17 ± 4.90a |
| 400 | 391 | 64.70 ± 2.14a | 34.44 ± 2.45ab | 10.39 ± 0.98ab | 17 | 90.33 ± 3.67a |
| 500 | 369 | 64.34 ± 0.54a | 29.19 ± 1.76b | 9.12 ± 0.47b | 19 | 88.50 ± 4.09a |
Values are listed as mean±SEM; means in the same column with different superscripts were significantly different
Experiment IV
The results of Experiment IV are shown in Table 4. The cleavage rate was significantly higher in 200 µM supplemented group when compared with control (P < 0.05). The rate of embryos that developed to compact morulae was significantly higher (P < 0.05) in 200 µM supplemented group when compared with control and 500 µM supplemented group. The rates of blastocyst formation were significantly higher in 100 µM (P < 0.01), 200 µM (P < 0.001) and 400 µM (P < 0.05) supplemented groups when compared with control. The rate of blastocyst formation was also significantly higher in 200 µM supplemented group when compared with 50 µM supplemented group (P < 0.01) and 500 µM supplemented group (P < 0.05). The blastocyst total cell count was significantly higher in 200 µM supplemented group when compared with control (P < 0.01) and 500 µM supplemented group (P < 0.05).
Table 4.
Effect of α-tocopherol supplementation to embryo culture medium on development of preimplantation sheep embryos cultured in 20% oxygen environment
| α-tocopherol conc. (µM) | No. of oocytes inseminated | Cleavage (%) | Morula (%) | Blastocyst (%) | No. of blastocysts evaluated | Total cell number |
|---|---|---|---|---|---|---|
| 0 | 369 | 57.17 ± 1.26b | 24.08 ± 2.14b | 8.24 ± 0.69c | 18 | 88.00 ± 4.42b |
| 50 | 394 | 57.94 ± 1.16ab | 25.66 ± 1.36ab | 11.49 ± 1.13bc | 17 | 95.33 ± 4.77ab |
| 100 | 386 | 59.34 ± 0.96ab | 28.33 ± 1.03ab | 15.41 ± 0.87ab | 20 | 101.33 ± 2.36ab |
| 200 | 378 | 62.31 ± 1.09a | 32.12 ± 1.93a | 17.60 ± 1.73a | 19 | 111.33 ± 3.14a |
| 400 | 365 | 61.56 ± 1.20ab | 28.07 ± 1.20ab | 14.05 ± 1.28ab | 16 | 101.50 ± 4.01ab |
| 500 | 372 | 58.08 ± 1.25ab | 24.70 ± 2.27b | 12.49 ± 0.56bc | 16 | 94.50 ± 3.18b |
Values are listed as mean±SEM; means in the same column with different superscripts were significantly different
Thus it can be stated that α-tocopherol supplementation is important when embryos are cultured under 20% O2 environment.
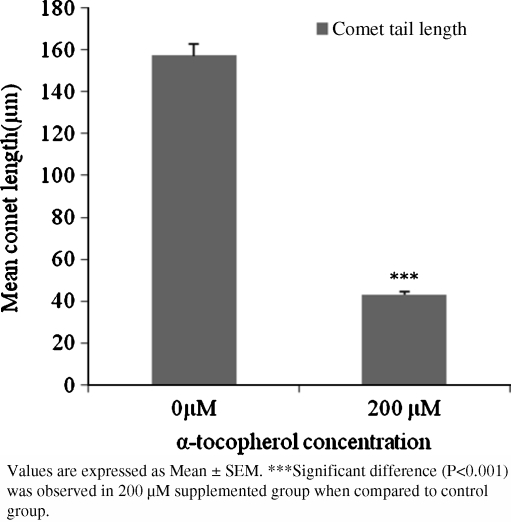
Figure 1 demonstrates the extent of DNA damage in individual embryos on day 3 of culture at 20% oxygen levels, in absence or presence respectively of α-tocopherol supplementation. Under the fluorescence microscope, DNA from individual embryos that migrated in the gel was visualized as a comet tail like streak. Supplementation of 200 µM α-tocopherol resulted in a significant reduction (P < 0.001) in comet tail length when compared with that of control.
Fig. 1.
Effect of α-tocopherol supplementation to Embryo Culture Medium at 20% oxygen levels on DNA damage in individual sheep embryos determined via comet assay
Discussion
The present study was conducted to determine whether the antioxidant α-tocopherol would protect in vitro matured ovine oocytes and in vitro-produced ovine embryos from oxidative damage encountered during in vitro culture. Several authors in the past have preferred culturing gametes and embryos at 5% oxygen atmosphere (5% CO2, 5% O2 and 90% N2) than at 20% oxygen atmosphere (5% CO2 in air) due to the fact that there is lesser oxidative stress in the 5% oxygen atmosphere compared to the 20% oxygen atmosphere [12, 22]. Higher developmental rates were observed in embryos cultured in vitro under 5% O2 environment than those cultured under 20% O2 environment [23]. Greater rates of cleavage and blastocyst-output seventh day post fertilization were reported in embryos obtained from oocytes fertilized at 20% oxygen atmosphere [24]. Also, the antioxidant hypotaurine was reported to exert beneficial effects on in vitro bovine embryo development in both 5% and 20% oxygen atmospheres [25]. Hence we intended to determine whether the antioxidant vitamin E also shows such effects upon supplementation to oocyte maturation media and embryo culture media irrespective of the gaseous environment for gamete and embryo culture.
The results of the present study indicate that α-tocopherol supplementation to oocyte maturation medium did not cause any significant change with respect to the rate of oocyte maturation and / or embryo formation and development, irrespective of the environmental oxygen concentration. Similar observation was also made in bovine in vitro studies, wherein it was reported that the active form of vitamin E in maturation medium did not have any effect on developmental competence of oocytes and embryos [26]. In vitro studies in porcine suggested that the quality of immature oocytes and the composition of culture medium were the critical factors for in vitro maturation of oocytes and that the presence of cumulus cells during in vitro maturation was of prime importance in protecting oocytes from apoptosis induced by oxidative stress [27]. On the contrary, later reports on bovine IVF suggested that ROS production in denuded oocytes was unaltered by maturation, indicating that the culture conditions employed were not responsible for oxidative stress in the oocytes [28].
In the present study, α-tocopherol supplementation to embryo culture medium and subsequent culture at 5% oxygen levels did not result in significant increases in rates of cleavage, morula or blastocyst development or blastocyst total cell count when compared to control. However, the observed variations in rates of morulae and blastocyst between 200 µM and 500 µM supplemented groups possibly indicate that at low oxygen levels in culture environment, higher concentrations of α-tocopherol may not effectively promote embryo development.
One of the critical factors that vary between in vitro and in vivo gamete and embryo culture environments is oxygen tension. Atmospheric levels of oxygen are known to retard in vitro development of mammalian embryos by formation of free oxygen radicals [29]. The effects of ROS on gamete and embryo development are not yet very clearly established. Although some reports suggest that prolonged, experimentally induced ROS production severely affects embryo development [2], ROS are also known to act as effective second messengers in cellular signaling pathways in mammals [30]. Alterations in ROS concentration also helps activate several genes including those for protein kinases, tyrosine kinases and growth factors [31, 32]. The reduction–oxidation (REDOX) state regulation (that is the balance between ROS production and elimination) rather than ROS themselves are thought to mediate these effects. Hence, REDOX state regulation is the key to achieve optimal levels of growth [31]. Therefore, the observed variation in developmental competence of gametes and embryos in a dose-dependent concentration of antioxidant supplement may be attributed to shift in the REDOX status.
The above outcomes indicate that α-tocopherol improves embryo development rates under conditions of greater oxidative stress. Comet assay was performed only for embryos that were cultured in 20% oxygen environment because these embryos are exposed to higher levels of oxygen for longer period of time (due to greater oxygen concentration in culture environment) and hence significant difference is more likely to occur upon antioxidant supplementation to this group of embryos than to any other group. The results of comet assay reveals that embryos cultured at 20% oxygen levels without α-tocopherol supplementation underwent more DNA damage than the 200 µM supplemented group, suggesting that the enhanced developmental competence of ovine embryos may be due to the antioxidant effect of α-tocopherol. The increased rates of blastocyst formation in 100 µM and 400 µM supplemented groups cultured at 20% oxygen levels could also be attributed to the antioxidant effect of α-tocopherol, albeit to a lesser extent than that observed in the 200 µM supplemented group. Also, the observed variations in rates of morulae, blastocyst and blastocyst total cell number between 200 µM and 500 µM supplemented groups indicates that higher concentrations of α-tocopherol may reverse the beneficial effects that it provides at optimal concentrations.
Thus, although, the effectiveness of α-tocopherol as an agent for development of embryos during in vitro culture has been well established, its role in in vivo reproductive physiology still needs to be deciphered clearly. Also, the molecular mechanisms by which tocopherols and tocotrienols suppress oxidative stress and promote fertility need to be investigated in detail in future studies.
Conclusions
The results of this study help to better understand the culture conditions at which α-tocopherol supplementation would enhance ovine embryo in vitro development. Although the antioxidant properties of α-tocopherol are well known, its effects are more pronounced under conditions of greater oxidative stress to the embryos. Our results demonstrate that supplementation of 200 µM α-tocopherol to embryo culture media followed by culture at 20% oxygen levels could enhance the in vitro developmental competence of ovine embryos to the blastocyst stage. However, α-tocopherol supplementation does not improve rate of oocyte maturation irrespective of the oxygen concentration in the culture environment.
Acknowledgments
The technical expertise provided by Dr. Palanisamy and technical assistance provided by Mr. Kartheeswaran, Mr. Shankar and Ms. Nithya, Department of Animal Biotechnology, Tamilnadu Veterinary and Animal Sciences University, Chennai are gratefully acknowledged.
Footnotes
Capsule
Oxidative stress during in vitro ovine oocyte and embryo culture results in lesser yields of viable embryos. α- tocopherol supplementation helps improve the yield.
References
- 1.Camargo LSA, Viana JHM, Sa WF, Ferreira AM, Ramos AA, Filho VRV. Factors influencing in vitro embryo production. Anim Reprod. 2006;3(1):19–28. [Google Scholar]
- 2.Guerin P, Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7(2):175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- 3.Lapointe S, Sullivan R, Sirard MA. Binding of a bovine oviductal fluid catalase to mammalian spermatozoa. Biol Reprod. 1998;58(3):747–753. doi: 10.1095/biolreprod58.3.747. [DOI] [PubMed] [Google Scholar]
- 4.Oyawoye O, Gadir AA, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003;18(11):2270–2274. doi: 10.1093/humrep/deg450. [DOI] [PubMed] [Google Scholar]
- 5.Nasr-Esfahani MM, Johnson MH. The origin of reactive oxygen species in mouse embryos cultured in vitro. Development. 1991;113(2):551–560. doi: 10.1242/dev.113.2.551. [DOI] [PubMed] [Google Scholar]
- 6.Silva PFN, Gadella BM, Colenbrander B, Roelen BAJ. Exposure of bovine sperm to pro-oxidants impairs the developmental competence of the embryo after the first cleavage. Theriogenology. 2007;67(3):609–619. doi: 10.1016/j.theriogenology.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa Y, Suzuki K, Yoneda A, Watanabe T. Effects of oxygen concentration and antioxidants on the in vitro developmental ability, production of reactive oxygen species (ROS), and DNA fragmentation in porcine embryos. Theriogenology. 2004;62(7):1186–1197. doi: 10.1016/j.theriogenology.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Bedaiwy MA, Falcone T, Mohamed MS, Aleem AAN, Sharma RK, Worley SE, Thornton J, Agarwal A. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril. 2004;82(3):593–600. doi: 10.1016/j.fertnstert.2004.02.121. [DOI] [PubMed] [Google Scholar]
- 9.Sies H, Stahl W. Vitamins E and C, β-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995;62:1315S–1321S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- 10.Tappel AL. Vitamin E as the biological lipid antioxidant. Vitam Horm. 1962;20:493–510. doi: 10.1016/S0083-6729(08)60732-3. [DOI] [Google Scholar]
- 11.Miller JK, Brzezinska-Slebodzinska E, Madsen FC. Oxidative stress, antioxidants and animal function. J Dairy Sci. 1993;76(9):2812–2823. doi: 10.3168/jds.S0022-0302(93)77620-1. [DOI] [PubMed] [Google Scholar]
- 12.Olson SE, Seidel GE., Jr Culture of in vitro-produced bovine embryos with vitamin E improves development in vitro and after transfer to recipients. Biol Reprod. 2000;62(2):248–252. doi: 10.1095/biolreprod62.2.248. [DOI] [PubMed] [Google Scholar]
- 13.Carlson JC, Wu XM, Sawada M. Oxygen radicals and the control of ovarian corpus luteum function. Free Radic Biol Med. 1993;14(1):79–84. doi: 10.1016/0891-5849(93)90511-R. [DOI] [PubMed] [Google Scholar]
- 14.Jeong YW, Park SW, Hossein MS, Kim S, Kim JH, Lee SH, Kang SK, Lee BC, Hwang WS. Antiapoptotic and embryotrophic effects of alpha-tocopherol and L-ascorbic acid on porcine embryos derived from in vitro fertilization and somatic cell nuclear transfer. Theriogenology. 2006;66(9):2104–2112. doi: 10.1016/j.theriogenology.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. The effects of antioxidant supplementation during percoll preparation on human sperm DNA integrity. Hum Reprod. 1998;13(5):1240–1247. doi: 10.1093/humrep/13.5.1240. [DOI] [PubMed] [Google Scholar]
- 16.Pawshe CH, Totey SM, Jain SK. A comparison of three methods of recovery of goat oocytes for in vitro maturation and fertilization. Theriogenology. 1994;42(1):117–125. doi: 10.1016/0093-691X(94)90668-9. [DOI] [PubMed] [Google Scholar]
- 17.Wani NA, Wani GM, Khan MZ, Salahudin S. Effect of oocyte harvesting techniques on in vitro maturation and in vitro fertilization in sheep. Small Rumin Res. 2000;36(1):63–67. doi: 10.1016/S0921-4488(99)00097-8. [DOI] [Google Scholar]
- 18.Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod. 1975;12(2):260–274. doi: 10.1095/biolreprod12.2.260. [DOI] [PubMed] [Google Scholar]
- 19.Tervit HR, Whittingham DG, Rowson LE. Successful culture in vitro of sheep and cattle ova. J Reprod Fertil. 1972;30(3):493–497. doi: 10.1530/jrf.0.0300493. [DOI] [PubMed] [Google Scholar]
- 20.Mermillod P, Vansteenbrugge A, Wils C, Mourmeaux JL, Massip A, Dessy F. Characterization of the embryotrophic activity of exogenous protein-free oviduct-conditioned medium used in culture of cattle embryos. Biol Reprod. 1993;49(3):582–587. doi: 10.1095/biolreprod49.3.582. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Keicho K, Takahashi H, Ogawa H, Schultz RM, Okano A. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Theriogenology. 2000;54(1):137–145. doi: 10.1016/S0093-691X(00)00332-0. [DOI] [PubMed] [Google Scholar]
- 22.Bernardi ML, Flechon JE, Delouis C. Influence of culture system and oxygen tension on the development of ovine zygotes matured and fertilized in vitro. J Reprod Fertil. 1996;106(2):161–167. doi: 10.1530/jrf.0.1060161. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JGE, Simpson AC, Pugh PA, Donnelly PE, Tervit HR. Effect of oxygen concentration on in vitro development of preimplantation sheep and cattle embryos. J Reprod Fertil. 1990;89:573–578. doi: 10.1530/jrf.0.0890573. [DOI] [PubMed] [Google Scholar]
- 24.Leoni GG, Rosati I, Succu S, Bogliolo L, Bebbere D, Berlinguer F, Ledda S, Naitana S. A low oxygen atmosphere during IVF accelerates the kinetic of formation of in vitro produced ovine blastocysts. Reprod Domest Anim. 2007;42(3):299–304. doi: 10.1111/j.1439-0531.2006.00783.x. [DOI] [PubMed] [Google Scholar]
- 25.Fujitani Y, Kasai K, Ohtani S, Nishimura K, Yamada M, Utsumi K. Effect of oxygen concentration and free radicals on in vitro development of in vitro-produced bovine embryos. J Anim Sci. 1997;75:483–489. doi: 10.2527/1997.752483x. [DOI] [PubMed] [Google Scholar]
- 26.Dalvit G, Llanes SP, Descalzo A, Insani M, Beconi M, Cetica P. Effect of alpha-tocopherol and ascorbic acid on bovine oocyte in vitro maturation. Reprod Dom Anim. 2005;40(2):93–97. doi: 10.1111/j.1439-0531.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 27.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805–810. doi: 10.1095/biolreprod63.3.805. [DOI] [PubMed] [Google Scholar]
- 28.Dalvit GC, Cetica PD, Pintos LN, Beconi MT. Reactive oxygen species in bovine embryo in vitro production. Biocell. 2005;29:209–212. [PubMed] [Google Scholar]
- 29.Johnson MH, Nasr-Esfahani MH. Radical solutions and cultural problems: could free oxygen radicals be responsible for the impaired development of preimplantation mammalian embryos in vitro? Bioessays. 1994;16(1):31–38. doi: 10.1002/bies.950160105. [DOI] [PubMed] [Google Scholar]
- 30.Schreck R, Baeuerle PA. A role for oxygen radicals as second messengers. Trends Cell Biol. 1991;1(2–3):39–42. doi: 10.1016/0962-8924(91)90072-H. [DOI] [PubMed] [Google Scholar]
- 31.Burdon RH. Control of cell proliferation by reactive oxygen species. Biochem Soc Trans. 1996;24(4):1028–1032. doi: 10.1042/bst0241028. [DOI] [PubMed] [Google Scholar]
- 32.Nose K. Role of reactive oxygen species in the regulation of physiological functions. Bio Pharm Bull. 2000;23(8):897–903. doi: 10.1248/bpb.23.897. [DOI] [PubMed] [Google Scholar]