Abstract
Purpose
Evaluation of Fas receptor on surface of sperm, as an apoptotic marker, using flow cytometry and confirming the results using an antibody-antigen complex through the classic complement pathway.
Materials and methods
Semen samples were obtained from 10 fertile and 73 infertile individuals with diagnoses of male factor infertility. Expressions of Fas receptor and phosphatidyl serine on sperm were assessed by flow cytometry. Fas expression was further assessed by antibody-antigen complex through the complement pathway. Lysis was detected via PI (propidium iodide) staining.
Results
The mean Fas expression was considerably lower than previously reported values. No significant differences in the percentage of PI were detected before and after activation of the classic complement pathway. Annexin V positive samples showed low Fas expression.
Conclusion
Our results have confirmed the presence of selected apoptotic markers such as Fas or phosphatidyl serine on ejaculate sperm, but suggest that Fas expression is low. Further studies are required to investigate the “abortive apoptosis” mechanism through Fas/Fas L.
Keywords: Apoptosis, Flow cytometry, Infertility, Fas expression, Complement, Lysis
Introduction
In mammalian testis, germ cells expand clonally through many rounds of mitosis before undergoing differentiation steps that result in mature spermatozoa [1]. Apoptosis plays an important role in the testis by controlling germ cell number [2, 3] and 50–70% of germ cells ultimately undergo apoptosis at different stages of spermatogenesis [4]. This process is very specific and solely restricted to the germ cells of the seminiferous tubules [4, 5]. Substantial evidence suggests that apoptosis is under cellular control and involves the Fas/FasL system in testis [6, 7].
Fas (CD95, APO-1) is a 45-kDa type 1 transmembrane receptor protein, which belongs to the tumor necrosis factor/nerve growth factor receptor family [8]. Binding of Fas ligand (FasL) or agonistic anti-Fas antibody to Fas, results in apoptosis [9]. In humans and several other species under normal conditions, Sertoli cells express FasL, which lead to the destruction of Fas-positive germ cells, thereby limiting the numbers of germ cell population to the capacity that Sertoli cells can support [2, 3, 10].
Sakkas et al. first described the presence of Fas on ejaculated sperm and proposed the “abortive apoptosis” theory states that an apoptotic process begins in germ cells but fails to be completed and deleted, can end up as Fas positive sperm in the semen [11]. They also proposed that this theory may explain the presence of abnormal sperm observed in semen samples. In support of this theory, Sakkas et al. showed that 10–50% Fas expression in oligoasthenoteratozoospermia and oligoteratozoospermia samples were significantly higher than the percentage of Fas positive sperm in men with normal semen parameters [12, 13]. In contrast, later studies apparently have reached opposite conclusions which showed that Fas protein was not detected on the ejaculated sperm of normozoospermic and non-normozoospermic men [14, 15]. Considering the presence of substantial evidence that apoptosis is functional in sperm, taken together, these studies suggest that the presence of Fas receptor on sperm membrane remains controversial.
Studies assessing the presence of Fas on sperm have mainly implemented an immunostaining procedure. An alternative method is to use the classic pathway of antibody-antigen complex for detection of cell surface proteins. This procedure has been previously used for the detection of functional proteins on the surface of sperm. We have taken the controversy regarding the presence of Fas receptor on the sperm surface of subfertile individuals into consideration. Therefore, the aim of this study was to evaluate the presence of Fas receptor on the sperm of subfertile individuals and to further assess its presence via antibody-antigen complex through the classic complement pathway.
Material and method
This study was initially approved by the Ethical and Scientific Committee of Royan Institute as well as the Isfahan Fertility and Infertility Center.
Sperm analysis
Semen samples were collected from 10 fertile individuals who attended an embryo donation program and 73 infertile individuals with male factor infertility who were referred to the Isfahan Fertility and Infertility Center. All semen samples were collected by masturbation into sterile containers after 3–4 days of sexual abstinence and were delivered to the laboratory within 45 min after ejaculation. Sperm concentration and motility were assessed according to WHO criteria [16] and sperm morphology was assessed according to strict criteria [17]. The remainder of the semen was washed twice in Ham’s F10+10% human serum albumin (HSA) for evaluation of Fas expression, viability and Annexin V.
Peripheral Blood Mononuclear Cell (PBMCs) analysis
Blood samples were donated by researchers at Royan Institute. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll density gradient centrifugation (Ficoll Biotest, Germany) at 300 g for 15–20 min and washed twice in cold phosphate-buffered saline (PBS). PBMCs were used as a positive control, due to Fas expression on these cells [18].
Determination of Fas on sperm
Samples were washed with Ham’s F10+10% HSA. Then, sperm concentration was adjusted to 1 million per 100 μl. Subsequently, 10 μL fluorescin-isothiocyanate conjugated anti-CD95 mAb clone DX2 (BD Biosciences Pharmingen, San Diego, CA, USA), was added to 100 μl of the adjusted sperm sample for 90 min at 2–8°C. Then, sperm were washed with Ham’s F10+10% HSA and adjusted to a final volume of 500 μl. Percentage Fas expression of each sample was analyzed by flow cytometry. Anti-CD95 mAb clone DX2 is a specific antibody for human Fas when it cross links with Fas and delivers an apoptotic signal which indicates that DX2 recognizes a functional epitope of the CD95 antigen [15, 19]. The same procedure was carried out with lymphocytes.
Fas analysis by flow cytometry
Samples were processed for Fas detection in human spermatozoa, as described by Pentikainen et al. 1999, with minor modifications. The samples were analyzed by a FACSCalibur flow cytometer (Becton Dickinson) equipped with a 15 mW argon-ion laser for excitation (488 nm). For each sample, at a flow rate of <100 cells /second, 10,000 events were recorded within the characteristic flame shaped region in the forward light scatter/side light scatter (FSC/SSC) dot plot, then a gate was used to select single sperm form clumps and debris. Green fluorescence (FITC) was detected in the fluorescence detector 1 (FL-1) with a 530/30 nm band pass filter, while red fluorescence (PI: propidium iodide) was measured in the fluorescence detector 2 (FL-2) with a 585/42 nm band pass filter. Instrument setting was performed using FSC/SSC dot plot in the isotype control for threshold adjusting and debris exclusion. Therefore, quadrant setting was accomplished in the FL-1/FL-2 dot plot that the lower left quadrant was included 99% of the total events. On the other hand, fluorescence compensation was set by acquiring samples separately labeled with green fluorescence and red fluorescence in the sample test. Data obtained from flow cytometry were analyzed using CellQuest Pro and WinMDI 2.9 software [20].
Complement mediated lysis
Complement mediated lysis was performed according to the standard protocol for micro-lympho-cytotoxicity with some alterations. A total of 10,000 cells per ml were treated with 5 μl purified mouse anti-human CD95 mAb clone DX2 (BD Biosciences Pharmingen, San Diego, CA, USA) for 30 min at room temperature, followed by incubation for 1 h at 37°C with 25 μl rabbit complement (Code No:CL3111, Cedarlane, Ontario, Canada) [21]. To demonstrate the effect of complement-activating, and the degree of cell lysis, PI was used [22].
Detection of membrane PS exposure and membrane integrity using the Annexin V assay
Phosphatidyl serine (PS) is located normally to the inner leaflet of the plasma membrane bilayer. Apoptosis causes membrane phospholipid asymmetry and translocation of PS onto the outer leaflet of the membrane. Thus, the detection of PS exposure has been well established as an early apoptotic marker [23]. In the present study, detection of PS externalization in sperm was performed using a Phosphatidyl Serine Detection Kit (IQ, Texas, U.S.A), following the manufacturer’s protocol with modifications. Generally, semen samples containing 1 × 106 sperm were first washed twice (300 g for 10 min at 4°C) with calcium buffer. The washed sperm were resuspended in the AnnexinV labeling solution [containing recombinant AnnexinV protein and Ca+2]. After incubation at room temperature for 15 min, sperm were washed twice with calcium buffer and stained by PI for 10 min. Immediately, samples were analyzed by flow cytometry. Annexin V was detected on the green fluorescence detector 1 (FL-1) with a 530/30 nm band pass filter.
Evaluation of sperm cell viability
A total of 10 μl of PI (Sigma, St. Louis, MO; 1 mg/ml water) was added to a 400 μl sperm suspension for 1 min, and then cells were immediately analyzed by flow cytometry to evaluate the percentage of dead cells (PI-positive) [24].
Statistical analysis
Data was analyzed using the SPSS version 11 software package and a p value lower than 0.05 was considered significant. Significant relationships between the various parameters were evaluated by the Pearson correlation test.
Results
Descriptive analysis
Table 1 shows the descriptive results of semen parameters, % Fas expression, Annexin V positivity and % PI positive in the infertile individuals. Mean sperm concentration (million/ml), percentage motility and abnormal morphology were 21.51 ± 26.39, 27.48 ± 15.61 and 85.78 ± 7.60, respectively. Percentage of PI positive also ranged from 2.87 to 79.04% with a mean of 36.22±17.30. In addition, the mean Annexin V positivity was 50.91 ± 24.81 which ranged from 24.31 to 81.18%.
Table 1.
Descriptive information of semen parameters, percentages of propidium iodide, AnnexinV and Fas positive sperm in infertile group (n = 73)
| Parameters | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Sperm concentration (106/ml) | 0.10 | 120 | 21.51 ± 26.39 |
| Sperm motility (%) | 0.00 | 70 | 27.48 ± 15.61 |
| Abnormal morphology (%) | 60 | 100 | 85.78 ± 7.60 |
| Fas expression (%) | 0.02 | 23.52 | 4.13 ± 5.08 |
| Propidium iodide (%) | 2.87 | 79.04 | 36.22 ± 17.30 |
| Annexin V positive sperm (%) | 24.31 | 81.18 | 50.91 ± 24.81 |
The mean sperm density and motility in the fertile individuals were 48.20 ± 23.78 and 56.40 ± 5.94 and percentage sperm morphology according to strict criteria were 80.20 ± 7.00.
Assessment of Fas expression
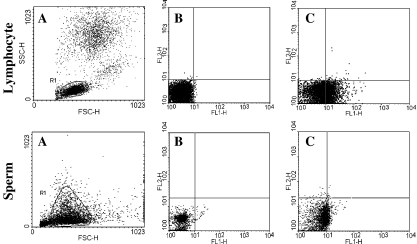
In order to detect Fas expression, lymphocytes were used as a positive control. Figure 1 shows Fas positive cells detectable in the lymphocytes. Fas expression in lymphocytes was 28.16% but Fas expressions of sperm in subfertile individuals were much lower and ranged from 0.02 to 23.52% with a mean of 4.13% (Table 1), and the mean of Fas expression in the fertile individuals was 0.96 ± 0.52.
Fig. 1.
Fas expression on lymphocyte and sperm: a) Gate of lymphocyte and sperm, b) Isotype control, c) Fas staining with FITC-anti CD95 (FL1)
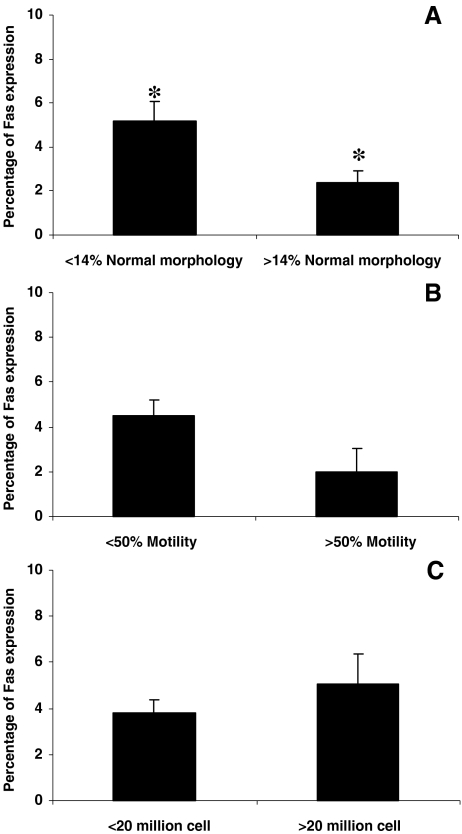
The infertile individuals were also grouped according to WHO criteria for sperm concentrations of less or greater than 20 million/ml, sperm progressive motility of less or greater than 50% and according to strict criteria, in individuals with lower and higher than 14% normal morphology sperm (Fig. 4). The results revealed that the percentage of Fas expression was significantly higher in the group with lower than 14% normal sperm morphology as compared to those with higher than 14% normal morphology (5.17 ± 5.9 vs. 2.40 ± 2.4, p = 0.013). However, the percentage of Fas expression was not significantly different when individuals were grouped according to lower and higher than 50% progressive motility (4.50 ± 5.26 vs. 1.96 ± 2.81, p = 0.25) or sperm concentration of lower or higher than 20 million/ml (3.78 ± 4.25 vs. 5.05 ± 6.35, p = 0.34).
Fig. 4.
Fas expression in73 infertile individuals which were grouped according to sperm morphology (a), motility (b) and sperm concentration (c).* show significant difference between the two group at P < 0.05
Assessment of cell viability post treatment with Fas antibody and complement
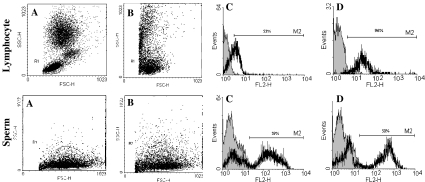
Complement mediated lysis was carried out both on blood and sperm suspensions, and the percentage of cell lysis was assessed by PI stain. Figure 2 shows that lymphocytes treated with purified mouse anti-human CD95 mAb followed by incubation with rabbit complement results in disappearance of Fas positive cells from the cell population and the percentage of PI positive cells to increase. Similar processes were carried out on sperm samples and no difference was observed in the percentage of PI positive sperm before and after treatment with purified mouse anti-human CD95 mAb followed by incubation with rabbit complement.
Fig. 2.
Lymphocytes and sperm treated with purified mouse anti human CD95 mAb followed by incubation with rabbit complement: a) Gate of lymphocyte and sperm before treatment, b) Gate of lymphocyte and sperm after treatment with CD59 mAb followed by incubation with rabbit complement, c) Histogram of PI in untreated lymphocytes and sperm and d) Histogram of PI lymphocytes and sperm after treatment with CD59 mAb followed by incubation with rabbit complement
Assessment of phosphatidyl serine with Annexin V expression
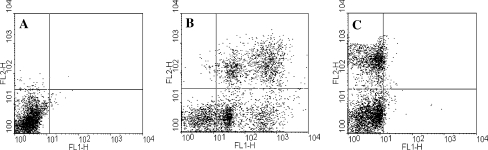
Figure 3 shows Fas and phosphatidyl serine expression with viability. FITC-anti CD95 and PI were used to evaluate Fas and viability, respectively. The result revealed that 3% of Fas positive sperm were nonviable, while 29.05% of Annexin V positive sperm, an apoptotic marker for phosphatidyl serine expression, were PI positive, which represented a state of late apoptosis.
Fig. 3.
Fas (FITC-anti CD95) and phosphatidyl serine (Annexin V) assessment on sperm: a) Control without staining, b) Annexin V and PI staining and c) FITC-anti CD95 & PI staining. FL1 represents CD95-FITC & Annexin V. FL2 represents propidium iodide
Discussion
The results of this study did not reveal complete absence of Fas but showed low Fas expression on the sperm of infertile individuals. The mean value of Fas expression in our study was approximately 4% with a range of 0.02 to 23.5%, far lower than the reported value of 10–50% by Sakkas et al. Considering the present results and discrepancies regarding the Fas expression in sperm, we decided to further verify the results and our procedure. Therefore, the presence of Fas was also assessed on PBMC. The results revealed that PBMC cells are Fas positive, as documented in the literature. To further confirm low Fas expression on sperm, the antibody-complement mediated pathway which has been proven to be a sensitive procedure for determining the presence of cell surface markers was implemented for both PBMC and sperm samples. As shown in Fig. 2, the addition of complement along with Fas antibody increased the percentage of PI cells in lymphocytes. However, this procedure did not significantly increase the percentage of PI in the sperm samples treated with Fas antibody and complement, which further confirmed low Fas expression on the sperm of infertile individuals. Since low Fas expression does not preclude the absence of apoptotic sperm in semen samples, the presence of Annexin V showing phosphatidylserine externalization in apoptotic cells and Fas expression were simultaneously assessed. These results showed that, even in samples with high levels of Annexin V, Fas expression was low (Fig. 3). Thus, this further confirmed the results of a previous study which indicated that apoptotic sperm (Annexin V positive cells) might not express Fas [25].
Sakkas et al. reported semen samples with low sperm concentrations and poor morphology were more likely to show higher levels of Fas expressing cells. In this study individuals were also categorized for motility, morphology and sperm density. Although, in this study we showed that the overall Fas expression was low, however only in individuals with lower and higher that 14% normal morphology, according to strict criteria, was a significant difference observed.
Therefore, this study further confirms the presence of selected apoptotic markers such as Fas or AnnexinV on ejaculate sperm, but suggests that Fas expression is low. Additional studies are required to investigate the true role of “abortive apoptosis” in infertility.
Acknowledgements
The authors express their gratitude to Royan Institute for its financial support, as well as the staff of the Isfahan Fertility and Infertility Center and Isfahan School of Medical Sciences for their kind collaboration.
Footnotes
Capsule Considering controversies regarding the presence of Fas in sperm, our results show low Fas expression on sperm as assessed by flow cytomerty and antibody-antigen complex sperm lysis.
References
- 1.Bartke A. Apoptosis of male germ cells, a generalized ora cell type-specific phenomenon? Endocrinol. 1995;136:3–4. doi: 10.1210/en.136.1.3. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinol. 1997;138:2081–2088. doi: 10.1210/en.138.5.2081. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16:2262–2270. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJ. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubules stages. Endocrinol. 1995;136:5–12. doi: 10.1210/en.136.1.5. [DOI] [PubMed] [Google Scholar]
- 5.Shikone T, Billig H, Hsueh AJW. Experimentally induced cryptorchidism increases apoptosis in rat testes. Biol Reprod. 1994;51:865–872. doi: 10.1095/biolreprod51.5.865. [DOI] [PubMed] [Google Scholar]
- 6.Zhou XC, Wei P, Hu ZY, Gao F, Zhou RJ, Liu YX. Role of Fas/FasL genes in azoospermia or oligozoospermia induced by testosterone undecanoate in rhesus monkey. Acta Pharmacol Sin. 2001;22:1028–1033. [PubMed] [Google Scholar]
- 7.Eguchi J, Koji T, Nomata K, Yoshii A, Shin M, Kanetake H. Fas-Fas ligand system as a possible mediator of spermatogenic cell apoptosis inhuman maturation-arrested testes. Hum Cell. 2002;15:61–68. doi: 10.1111/j.1749-0774.2002.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 8.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 9.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-L. [DOI] [PubMed] [Google Scholar]
- 10.Lue YH, Hikim AP, Swerdloff RS, Im P, Taing KS, Ti Bu, et al. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinol. 1999;140:1709–17. doi: 10.1210/en.140.4.1709. [DOI] [PubMed] [Google Scholar]
- 11.Sakkas D, Mariethoz E, St John JC. Abnormal sperm parameters in humans are indicative of an abortive apoptotic mechanism linked to the Fas-mediated pathway. Exp Cell Res. 1999;251:350–355. doi: 10.1006/excr.1999.4586. [DOI] [PubMed] [Google Scholar]
- 12.Sakkas D, Mariethoz E, Manicardi G, Bizzaro D, Bianchi PG, Bianchi U. Origin of DNA damage in ejaculated human spermatozoa. Rev Reprod. 1999;4:31–37. doi: 10.1530/ror.0.0040031. [DOI] [PubMed] [Google Scholar]
- 13.McVicar CM, McClure N, Williamson K, Dalzell LH, Lewis SE. Incidence of Fas positivity and deoxyribonucleic acid double-stranded breaks in human ejaculated sperm. Fertil Steril. 2004;81:767–774. doi: 10.1016/j.fertnstert.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Castro A, Parodi D, Morales I, Madariaga M, Rios R, Smith R. Absence of Fas protein detection by flow cytometery in human spermatozoa. Fertil Steril. 2004;81:1019–1025. doi: 10.1016/j.fertnstert.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Perticarari S, Ricci G, Boscolo R, Santis M, Pagnini G, Martinelli M, et al. Fas receptor is not present on ejaculated human sperm. Hum Reprod. 2008;23:1271–1279. doi: 10.1093/humrep/den113. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization (1999) WHO Laboratory manual for the examination of human semen and sperm-cervical mucus interaction, 4th En. Cambridge Univ. Press Cambridge
- 17.Menkveld R, Rhemrev JP, Franken DR, Vermeiden JP, Kruger TF. Acrosomal morphology as a novel criterion for male fertility diagnosis: relation with acrosin activity, morphology (strict criteria), and fertilization in vitro. Fertil Steril. 1996;65:637–644. doi: 10.1016/s0015-0282(16)58167-9. [DOI] [PubMed] [Google Scholar]
- 18.Matiba B, Mariani SM, Krammer PH. The CD95 system and the death of a lymphocyte. Semin Immunol. 1997;9:59–68. doi: 10.1006/smim.1996.0054. [DOI] [PubMed] [Google Scholar]
- 19.Zandieh T, Safari Fard A, Aghaipur M. Expression of Fas antigen (CD95) on human leukemic cells and assessment of apoptosis on Fas + and Fas-samples by flowcytometry method. Iran J Allergy Asthma Immunol. 2003;2:198–207. [PubMed] [Google Scholar]
- 20.Pentikainen V, Erkkila K, Dunkel L. Fas regulates germ cell apoptosis in the human testis in vitro. Am J Physiol. 1999;276:10–6. [DOI] [PubMed]
- 21.Terasaki PI (1980) Histocompatibility Testing: Report of an International Workshop and Conference, Los Angeles. Los Angeles, CA. UCLA Tissue Typing Laboratory, UCLA
- 22.Hochgrebe TT, Humphreys D, Wilson MR, Easterbrook-Smith SB. A reexamination of the role of clusterin as a complement regulator. Exp Cell Res. 1999;249:13–21. doi: 10.1006/excr.1999.4459. [DOI] [PubMed] [Google Scholar]
- 23.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-I. [DOI] [PubMed] [Google Scholar]
- 24.Graham JK, Kunze E, Hammerstedt RH. Analysis of sperm cell viability, acrosomal integrity, and mitochondrial function. Biol Reprod. 1990;43:55–64. doi: 10.1095/biolreprod43.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Grunewald S, Paasch U, Glander HJ. Enrichment of nonapoptotic human spermatozoa after cryopreservation by immunomagnetic cell sorting. Cell Tissue Bank. 2001;2:127–133. doi: 10.1023/A:1020188913551. [DOI] [PubMed] [Google Scholar]