Abstract
Purpose
To investigate the protective effect of Lithium against the toxic effect of Cadmium in the rat testes.
Methods
Twenty four adult male Sprague-Dawley rats were treated with four different regimens: Cadmium only, Cadmium and lithium, lithium only and controls. Rats were sacrificed after 6 weeks and testicular levels of pro-inflammatory cytokine (IL-4), anti-inflammatory cytokine (TNF-α), Pro-apoptotic protein (Bax) and anti-apoptotic protein (Bcl-2) were measured by ELISA while serum levels of FSH, LH, Prolactin and Testosterone were measured using the Vidas parametric system. Antioxidant status (MDA, SOD) was also assessed in serum. Histopathological changes of testes were examined using light and electron microscopy. Immunohistochemical staining for Bax, Bcl-2 and Caspase 3 were performed.
Results
Treatment with lithium was associated with significant reduction in the toxic effects of Cadmium as shown by reduced testicular levels of TNF-α, serum levels of Malondialdehyde and testicular level of Bax, and increased levels of IL-4, Zn-Cu SOD, Bcl-2 and Testosterone. Testicular histopathology showed that Cadmium produced an extensive germ cells apoptosis and the addition of lithium in Cadmium-treated rats significantly reduced cadmium-induced testicular damage.
Conclusion(s)
Lithium has a protective effect against cadmium-induced testicular apoptosis in the rat.
Keywords: Apoptosis, Bax, Bcl-2, Lithium, Testes, Cadmium
Introduction
Cadmium is an environmental toxicant and an endocrine disruptor in humans and rodents. It is released into water as a by-product of smelting, into air by combustion of coal and oil, and into soils as impurities [1]. Cadmium is absorbed via the lungs in significant quantities from cigarette smoke and it has toxic effects on the male reproductive system [2–5] which may account for the recent declining fertility in men in developed countries. The role of cadmium in the modulation of male reproductive health stems from the observations that high levels of cadmium in seminal fluid are associated with asthenozoospermia in infertile males [6]. Furthermore, animal studies demonstrated a time- and dose-dependent reduction in sperm motility following cadmium exposures [7]. Cadmium-induced toxicity to the testis is probably the result of interactions of a complex network of causes. This is likely to involve the disruption of the blood-testis barrier via specific signal transduction pathways and signaling molecules, such as p38 mitogen-activated protein kinase. Furthermore, cadmium is associated with its capacity to induce oxidative stress and apoptosis of the germ cells of humans and animals [8–10]. In these studies, cadmium has been shown to increase the expression of proapoptotic proteins p53 and Bax while reducing the expression of Bcl-2, an antiapoptotic protein.
Lithium is frequently used as an effective drug for the treatment of several psychiatric and neurodegenerative diseases in humans based on its neuro-protective effects. Long term treatment with lithium was found to prevent glutamate-induced excitotoxicity which was associated with inhibition of glutamate-induced increase in p53 and Bax expression while at the same time increasing the expression of Bcl-2 leading to an increase in the ratio of Bcl-2/Bax protein levels. Glutamate-induced cytochrome c and caspase-3 release were also blocked by lithium [11]. Long term treatment with lithium has also been shown to protect against thapsigargin-induced cytotoxicity in rat PC 12 cells [12]. This was associated with an enhanced expression of Bcl-2, an anti-apoptotic protein.
It was, therefore, of our interest to determine whether the use of lithium in a therapeutic dose would protect against cadmium-induced toxicity in rats. Such investigation will explore the potential therapeutic or preventive approaches that can be developed in future studies by blocking or minimizing the destructive effects of Cadmium to testicular function in men.
Materials and methods
Animals and experimental design
Adult male Sprague-Dawley rats weighing 250–300 g were used in this study. The experimental protocol followed the Guide for Care and Use of Laboratory Animals. The rats were divided into four groups (6 rats per group) as follows: [1] cadmium only, [2] cadmium and lithium, [3] lithium only and [4] control. Cadmium (5 mg) was administered intraperitoneally in a single dose while lithium was administered orally (0.1 mg/100 gm body weight was added to the drinking water daily) for 6 weeks.
Rats were sacrificed after 6 weeks and blood samples were collected. The serum was separated and stored at −20°C until used for the assays. Serum levels of malondialdehyde (MDA) and superoxide dismutase (SOD) were measured as previously described [13, 14].
Measurement of IL-4 and TNF-α levels in the rat testes
Rat testis was homogenized (1/10) in normal saline and ultracentrifuged at 40,000 rpm for 1 h. The supernatant was stored at −80°C until assay. Tissue levels of IL-4 and TNF-α were assayed using the multiple antibody sandwich ELISA assay system according to the manufacturer’s manual (Quantikine R&D system Inc, Minneapolis, MN). The intra- and inter-assay coefficients of variation were 4.1–7.5% and 4.5–7.5% respectively.
Measurement of Bax and Bcl-2 protein levels in testes
Testicular levels of Bax and BCl-2 were determined by the ELISA method according to the manufacturer’s manual (R&D system Inc). Rat testis was homogenized (1/10) in normal saline and stored at −80°C. Samples were treated with cell lysis buffer modified with PMSF (phenylmethanesulphonylfluoride) and PIC (protease inhibitor cocktail) prior to the assay. Samples were further diluted in assay buffer (provided in the kit). After pipetting 100 µl assay buffer to all wells, 100 µl of sample/standard were added to the wells. The plate was sealed and incubated at room temperature on a plate shaker for 1 h. The wells were washed 5 times with wash buffer and 100 µl of Bax/Bcl-2 antibody was added to each well except blank. Following 1 hr incubation at room temperature, the plate was washed 5 times with wash buffer and 100 µl of conjugate added to all wells except blank. The plate was sealed and incubated for 30 min at room temperature. Well contents were emptied and washed 5 times in wash buffer. 100 µl of substrate was added to each well and color allowed to develop. After 30 min, stop solution was added. The plate was read on an ELISA Plate Reader at OD 450 nm. Linear standard curves were generated in assay buffer to calculate the concentration of Bax and BCl-2 respectively. The intra- and inter-assay coefficients of variation were 4–7% and 10% respectively
Hormone assays
The hormones FSH, LH, Prolactin and Testosterone were measured using the Vidas parametric system. The procedure was as described in the manufacturers’ manual (Diomeriux, MO, USA). All samples for a given experiment were performed within the same assay. Intra- and inter-assay coefficients of variation for the rat assay were 5% and 11%, respectively.
Histological examination
Testicular tissues were fixed in methyl Carnoy’s solution and embedded in paraffin. About 4 µm sections were cut and stained with periodic acid-Schiff (PAS) reagent and counterstained with haematoxylin and eosin. The sections were assessed for histological features using a bright field microscope (Olympus BH2) and evaluated according to Johnsen score [15].
Electron microscopy of rat testis
Electron microscopic examination was performed on selected specimens of testicular tissue fixed in glutaryldehyde solution. Ultrathin sections were cut and stained with uranyl acetate and lead citrate for examination with electron microscopy. Cells were identified as apoptotic if they had condensed or fragmented nuclei, a low cytoplasm to nucleus ratio indicative of cell shrinkage, or surface membrane blebbing. Apoptotic cells were counted in five randomly selected fields and expressed as a percentage of the total number of cells counted.
Immunohistochemistry staining for Bcl-2, Bax and Caspase 3 (C3)
This was done as described by Eguchi et al. [16]. Briefly, after harvesting the testis, cryo-sections (5 µm) of the tissues were air-dried for 2 h and fixed in acetone for 5 min at room temperature. The samples were rehydrated in PBS for 10 min, before staining with Bcl-2, Bax and C3 antibodies respectively. The sections were examined on a Labor Lux microscope (Leitz, Wetzlar, Germany), at a magnification of X100. A positive reaction for Bcl-2, Bax and Caspase 3 (C3) expression was observed. Each section was examined by two observers who were blinded to the source of tissue and they agreed on the intensity of staining according to the following semi-quantitative scale: (+), negative; (++), equivocally positive; (+++), weakly positive; (++++), positive; (+++++), strongly positive. Representative sections were photographed using an Olympus digital camera at X100.
Statistical Analysis Data are shown as means ± SD and were analyzed using one-way ANOVA. Fisher’s Least Significance Difference test was used to look for differences between group means. A P-value < 0.05 was considered statistically significant.
Results
Effects of Lithium on pro- and anti-inflammatory cytokines (TNF-α and IL-4) in cadmium-treated testes
The results showed that TNF-α levels (pro-inflammatory cytokine) were statistically significantly higher in the testes of rats treated with Cadmium as compared with the controls (45.2 ± 21.6 vs. 7.4 ± 4.4, p < 0.001), whereas the IL-4 levels, an anti-inflammatory cytokine, were significantly lower than the controls (undetectable vs. 8.4 ± 3.2, p < 0.05) (Table 1). These effects of Cadmium were prevented in rats treated with lithium in addition. As shown in Table 1, lithium alone had no effect on the concentration of TNF-α while significantly increased IL-4 (anti-inflammatory cytokine) in the testes when compared to the controls. Furthermore, the addition of Lithium significantly reduced the tissue level of TNF-α in rats treated with cadmium by approximately 150% (19.4 ± 12.6 as compared to 45.2 ± 21.6). The level of TNF-α in the testes was however still significantly higher in rats cadmium-treated rats receiving lithium than in control rats (19.4 ± 12.6 vs. 7.4 ± 4.4). Lithium also increased the level of IL-4 in the Cadmium-treated testes by more than 90% (undetectable vs. 7.8 ± 3.6). There was no significant difference between testicular levels of IL-4 in control rats and rats treated with cadmium and lithium (8.4 ± 3.2 vs. 7.8 ± 3.6) indicating almost 100% protection.
Table 1.
Effect of Lithium on cadmium-induced changes of cytokine levels in the rat testes
| Cadmium | Cadmium + Lithium | Lithium | Control | |
|---|---|---|---|---|
| Cytokines (pg/gm) | ||||
| IL-4 | undetectable | 7.8 ± 3.6* | 18.8 ± 4.8** | 8.4 ± 3.2 |
| TNF-α | 45.2 ± 21.6** | 19.4 ± 12.6* | 11.4 ± 6.2 | 7.4 ± 4.4 |
Values are expressed as Mean ± SD
*p < 0.05 relative to cadmium only group
**p < 0.05 relative to control rats
Effect of lithium on cadmium-induced oxidative stress in serum
Oxidative effect of cadmium was assessed by measuring serum levels of MDA. Serum levels of the anti-oxidant Cu-ZnSOD were also measured. As shown in Table 2, the serum levels of MDA was significantly higher in Cadmium treated rats compared to the controls (12.8 ± 4.4 vs. 5.4 ± 2.4; p < 0.001). The levels of Cu-ZnSOD were 5.6 ± 2.4 mmol/l and 2.4 ± 1.2 mmol/l in the control and cadmium-treated rats respectively. The difference is statistically significant (p < 0.001). Treatment with lithium alone had no effect on the serum concentrations of MDA (5.8 ± 2.6 vs. 5.4 ± 2.4) and Cu-ZnSOD (6.2 ± 2.4 vs. 5.6 ± 2.4) as compared to controls. However treatment with lithium resulted in an approximate 70% reduction in serum concentration of MDA (12.8 ± 4.4 mmol/l in cadmium-treated rats compared to 7.6 ± 3.2 mmol/l in rats treated with cadmium and lithium). This is statistically significant (p < 0.001). Treatment with lithium also significantly increased Cu-ZnSOD by approximately 80% (2.4 ± 1.2 mmol/l in cadmium-treated rats compared to 4.4 ± 1.4 in rats treated with cadmium and lithium; p < 0.001).
Table 2.
Effect of Lithium on cadmium-induced changes in circulating levels of malondialdehyde (MDA) and Copper-Zinc superoxide dismutase (Cu-Zn SOD) in the rat
| Cadmium | Cadmium + Lithium | Lithium | Control | |
|---|---|---|---|---|
| Oxidant/antioxidant | ||||
| MDA (mmol/l) | 12.8 ± 4.4** | 7.6 ± 3.2* | 5.8 ± 2.6 | 5.4 ± 2.4 |
| Cu-Zn SOD (mmol/l) | 2.4 ± 1.2** | 4.4 ± 1.4* | 6.2 ± 2.4 | 5.6 ± 2.4 |
Values are expressed as Mean ± SD
*p < 0.05 relative to cadmium only group
**p < 0.05 relative to control rats
Effect of lithium on cadmium-induced changes in circulating hormone levels
The effects of cadmium on circulating levels of LH, FSH, testosterone and prolactin are shown in Table 3. Cadmium significantly reduced the levels of LH by 60% from 10.8 ± 2.4 pg/ml to 4.2 ± 1.4 pg/ml, FSH by 60% from 12.4 ± 4.2 pg/ml to 4.8 ± 2.2 pg/ml, testosterone by 70% from 28.6 ± 8.4 pg/ml to 8.2 ± 3.4 pg/ml. It however significantly increased the circulating level of prolactin by more than 100% from 104.4 ± 43.8 pg/ml to 240.2 ± 48.4 pg/ml. Treatment with lithium alone had no effect on the serum concentrations of LH, FSH, testosterone and prolactin compared with the controls (Table 3). However it significantly increased the circulating levels of LH by 75% from 4.2 ± 1.4 pg/ml to 7.6 ± 2.6 pg/ml, FSH by 90% from 4.8 ± 2.2 pg/ml to 9.2 ± 4.2 pg/ml, testosterone by 150% from 8.2 ± 3.4 pg/ml to 20.8 ± 8.2 pg/ml and significantly reduced the levels of prolactin by 35% from 240.2 ± 48.4 pg/ml to 156.6 ± 52.8 pg/ml in rats treated with lithium and Cadmium compared with those treated with Cadmium only.
Table 3.
Effect of Lithium on cadmium-induced changes in circulating levels of hormones in the rat
| Cadmium | Cadmium + Lithium | Lithium | Control | |
|---|---|---|---|---|
| Hormones | ||||
| LH (IU/l) | 4.2 ± 1.4* | 7.6 ± 2.6** | 9.8 ± 3.2 | 10.8 ± 2.4 |
| FSH(IU/l) | 4.8 ± 2.2* | 9.2 ± 4.2** | 12.2 ± 5.2 | 12.4 ± 4.2 |
| Testosterone (nmol/l) | 8.2 ± 3.4* | 20.8 ± 8.2** | 30.8 ± 9.8 | 28.6 ± 8.4 |
| Prolactin (IU/l) | 240.2 ± 48.4* | 156.6 ± 52.8** | 98.4 ± 46.2 | 104.4 ± 43.8 |
Values are expressed as Mean ± SD
*p < 0.05 relative to control group
**p < 0.05 relative to Cadmium only group
Effect of lithium on cadmium-induced changes in testicular levels of pro-apoptotic and anti-apoptotic proteins
As shown in Table 4, Cadmium significantly (p < 0.001) reduced the testicular levels of Bcl-2 from 286.4 ± 128.4 pg/g to 90.4 ± 32.2 pg/g while significantly increasing the level of Bax by approximately 100% from 106.8 ± 52.6 pg/g to 216.2 ± 92.4 pg/g. Administration of lithium alone had no effect on tissue levels of Bcl-2 or Bax compared to the control rats. However in rats treated with cadmium, lithium increased Bcl-2 level from 90.4 ± 32.2 pg/g to 256.6 ± 105.4 pg/g while decreasing the level of Bax from 216.2 ± 92.4 pg/g to 88.2 ± 44.6 pg/g.
Table 4.
Effect of Lithium on cadmium-induced changes in tissue levels of apoptotic proteins in the rat testes
| Cadmium | Cadmium + Lithium | Lithium | Control | |
|---|---|---|---|---|
| Bcl-2 (pg/g) | 90.4 ± 32.2* | 256.2 ± 105.4** | 364.6 ± 154.2 | 286.4 ± 128.4 |
| Bax (pg/g) | 216.2 ± 92.4* | 88.8 ± 44.6** | 120.6 ± 56.4 | 106.8 ± 52.6 |
Values are expressed as Mean ± SD
*p < 0.05 relative to control group
**p < 0.05 relative to Cadmium only group
Effect of lithium on cadmium-induced changes in histology of the testes
Light microscopy
Assessment of Johnsen scores in various groups revealed significant reduction in Cadmium-treated group compared with the control group. This is partially preserved in Lithium-Cadmium group (Table 5). In addition significant inflammation (90–100%) was noted in testes from rats treated with Cadmium. Lithium alone did not produce any significant inflammation in the testes; it however reduced the inflammation associated with cadmium to 10–20%. No inflammation was observed in testes from control rats (Fig. 1).
Table 5.
Immunohistochemical staining for Bcl-2, Bax and Caspase 3 and Mean Johnsen scores
| Cadmium | Cadmium + Lithium | Lithium | Control | |
|---|---|---|---|---|
| Bcl-2 | + | +++ | +++++ | +++++ |
| Bax | +++++ | ++ | + | + |
| Caspase 3 | +++++ | ++ | + | ++ |
| Mean Johnsen scores | 2.4 ± 0.8 | 6.4 ± 2.0 | 7.8 ± 3.2 | 8.8 ± 3.4 |
Johnsen scores: Scale of 10 down to 1
10- Normal spermatogenesis with open lumen
9- Many spermatozoa with obliteration of lumen
8- Only a few spermatozoa
7- No spermatozoa but many spermatids present
6- No spermatozoa and only a few spermatids
5- No sperms/spermatids but several spermatocytes
4- Only a few spermatocytes present
3- Spermatogonia only germ cells present
2-No germ cells but sertoli cellsonly present
1- No cells in tubular section
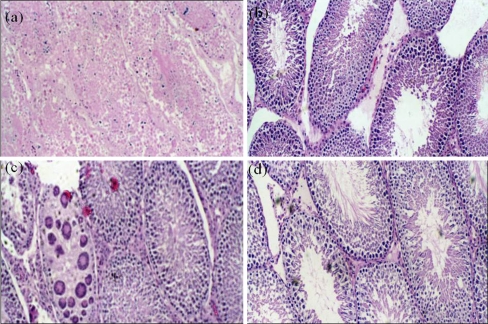
Fig. 1.
Light Microscopy after H and E staining of Rat testes × 100. a Cadmium only: Generalized degeneration of the germ, sertoli and leydig cell. b Cadmium + Lithium: Normal testiclar cells- germ cells, sertoli and leydig cells, with some inflammatory changes (10–25%). c Lithium only: shows almost normal spermatogenesis with normal germ cells, sertoli and leydig cells with scattered inflammatory changes. d Control: Normal Spermatogenesis with normal sertoli and leydig cells
Electron microscopy
Figure 2a–d shows the electron microscopy of different stages of rat spermatogenesis and apoptosis of the germ cells. As shown in Fig. 2a, Cadmium produced marked apoptosis of the germ cells to the extent that only about 20% of the round spermatids and the peripheral vesicles and early mitochondria are distinguishable. There was little degeneration of the testicular tissue in rats treated with lithium alone (Fig. 1c) where about 80% of the round spermatids and the peripheral vesicles and early mitochondria could be distinguished. Least degeneration was observed in the control group (10%) (Fig. 1d). Addition of lithium in Cadmium-treated rats (Fig. 1b) significantly reduced cadmium-induced testicular tissue degeneration and apoptosis of the round spermatids by more than 50%.
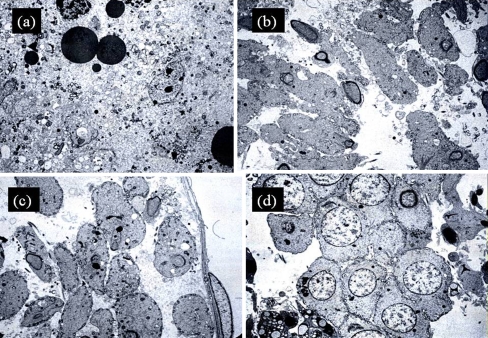
Fig. 2.
Electron microscopy of many stages of rat spermatogenesis- × 1000. a Cadmium only group showed marked apoptosis of the germ cells especially the round spermatids and mitochondria. b Cadmium-Lithium group revealed reduced apoptosis of the germ cells and mitochondria compared to Cadmium group (80% vs. 25%, p < 0.01). c Both Lithium only group (80% vs. 20%, p < 0.01) and d Control group have reduced apoptosis compared to cadmium group (80% vs. 10%, p < 0.01)
Immunohistochemistry staining for Bcl-2, Bax and Caspase 3 (C3)
As shown in Table 5 and Fig. 3, testicular tissues from control and lithium-treated rats stained strongly for Bcl-2 while little or no staining was observed for Bax and Caspase 3. In contrast, testicular tissues from rats treated with cadmium alone stained strongly for Bax and Caspase 3 but only weakly for Bcl-2. This was reversed in the cadmium + lithium group where there was an increase in the expression of Bcl-2 (+++ staining) and reduced expression of Bax and Caspase 3 (++ staining). There was no difference in the expression of Bcl-2, Bax and C3 in the lithium group compared to the control group.
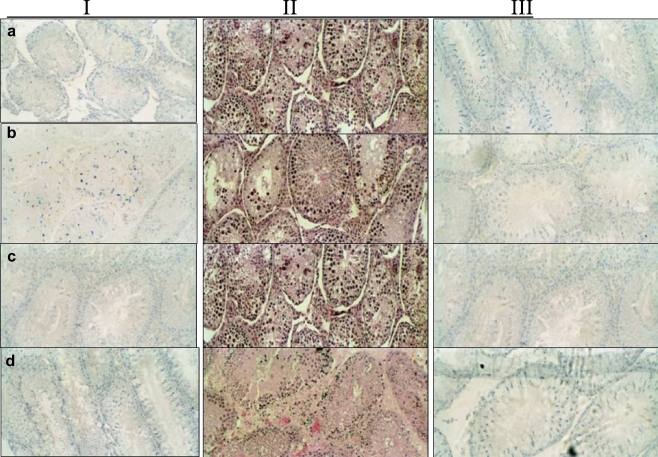
Fig. 3.
Immunohistchemical staining of the rat testis for Bcl-2, Bax and Caspase 3: Staining Columns: I- BcL-2; II- Caspase 3 and III- Bax. a Cadmium only testis stained strongly for Bax and caspase, but not for Bcl-2. b Cadmium + Lithium group showed moderate staining for Bcl-2, Bax and Caspase 3. c Lithium only stained strongly for Bcl-2 and poorly for Bax and Caspase 3. d Control group stained strongly for Bcl-2 and poorly for Bax and Caspase
Discussion
The present study showed the efficacy of low dose Lithium in preventing the toxic effects of Cadmium in the rat testes. It also highlighted the putative mechanisms behind the insult and its prevention. Our data showed that a single administration of cadmium to male rats produced generalized degeneration of the germ, sertoli and Leydig cells confirming previous reports in the literature [2, 5]. Cadmium exposure was associated with alteration in oxidative stress indices and inflammatory markers as evident by high levels of MDA and TNF-α and low levels of the anti-inflammatory cytokine, IL-4. This experimental evidence indicates the involvement of oxidative stress as well as immune system in Cadmium-mediated tissue damage. As has been previously shown, oxidative stress (enhanced ROS and LPO as well as altered anti-oxidant enzymes) is considered as one of the underlying mechanisms of toxicity [17] and antioxidants have been successful in abating some of these deleterious effects [18, 19]. Our data also showed that Cadmium causes imbalance in immune regulation leading to skewing ratio towards Th1-type cytokines (high TNF- α and undetectable IL-4) compared to the controls. In addition, marked apoptosis of the germ cells and the round spermatids and mitochondria in particular was seen in Cadmium-treated group. This was associated with a marked increase in the levels of proapoptotic proteins Bax and caspase and reduced levels of the antiapoptotic protein Bcl-2. Immunohistochemical staining has also revealed intense staining of the testis for Bax and caspase and reduced staining for Bcl-2. The prevalence and cellular localization of these markers in testicular tissues of Cadmium-treated group suggest the possible involvement of Bax/Bcl-2 in Cadmium-induced germ cell apoptosis.
Although the testicular damage induced by Cadmium is well recognized, the precise mechanisms underlying its toxicity to the testes remained unclear. Our data have demonstrated that the Cadmium-induced toxicity to the testes is probably the interactions of a complex network of causes. Recent data have suggested that this insult involves the disruption of the blood-testis barrier via specific signal transduction pathways and signaling molecules, such as p38 mitogen-activated protein kinase [20].
Considering the high sensitivity of the testicular tissue to Cadmium insult, preventive intervention is of major concern. Several investigations have shown that lithium has antiapoptotic properties in low concentrations while it is proapoptotic at higher concentrations [12, 21–25]. Previous studies on the effect of chronic administration of lithium on the male reproductive system have used high doses of lithium even though these doses are therapeutically relevant. Such studies have always reported deleterious effects of lithium on spermatogenesis and serum hormone levels. Ghosh et al. [26] have shown that treatment with high doses of lithium chloride (200 µg/100 g and 400 µg/100 g) daily for 21 days produced a remarkable reduction in testicular gametogenesis and plasma levels of FSH, LH, prolactin and testosterone along with significant diminution in the activities of testicular delta 5-3 beta-hydroxysteroid dehydrogenase (5-3 beta-HSD) and 17 beta-hydroxysteroid dehydrogenase (17 beta-HSD). None of the above parameters was affected by treatment with a lower dose (100 µg/100 g) of lithium. Similarly Thakur et al. [27] have reported that high doses of lithium (800 and 1100 mg/kg in the diet for 90 days) significantly reduced testicular weights and caused degeneration of spermatogenic cells and vacuolization of sertoli cells cytoplasm, whereas no untoward effect was observed with a lower dose (500 mg/kg diet). We have used a low dose of lithium (100 µg/100 g) in our study and have been able to show that this dose of lithium did not produce any structural damage in the rat testis confirming previous report by Ghosh et al. [26]. It also had no effect on serum concentrations of LH, FSH, testosterone, prolactin, MDA and Cu-ZnSOD. It did however significantly increased tissue levels of the anti-inflammatory cytokine, IL-4 while having no effect on tissue levels of TNF-α.
We therefore have used cadmium-induced toxicity as our test model to investigate the antiapoptotic effect of low dose lithium in the rat testis. Results obtained in this study have shown that lithium, in a low dose had no effect on the expression of proapoptotic (Bax and caspase 3) and antiapoptotic (Bcl-2) proteins in the rat testis. However this low dose of lithium significantly reduced cadmium-induced oxidative stress and inflammation characterized by reduced levels of TNF-α and MDA and increased levels of IL-4 and Cu-ZnSOD. Marked apoptosis of the germ cells produced by cadmium was prevented by treatment with low dose lithium. This was characterized histochemically by increased staining of the testis for Bcl-2 but reduced staining for Bax and caspase 3. In addition, the expression of Bcl-2 was increased while that of Bax was reduced indicating an antiapoptotic effect of lithium in this system.
Conclusion
We have, therefore, demonstrated the protective efficacy of low dose Lithium against Cadmium-induced testicular damage. Even though the mechanism of action of lithium at the molecular level cannot be deduced from the results presented above it could possibly be due to a combination of its anti-inflammatory, antioxidant and anti-apoptotic mechanisms. An interaction between the mechanisms is possible. Hockenbery et al. [28] have shown that Bcl-2 completely suppressed lipid generation following an apoptotic stimulus. Several studies have shown that lithium interferes with a number of signal transduction pathways including phosphoinositide hydrolysis, adenyl cyclase, G protein, glycogen synthase kinase-3ß and protein kinase C [29]. In a recent review, Bielecka and Obuchowicz described the influence of Lithium on intracellular apoptotic signaling pathways and the active sites implicated in mediating its action. They described how Lithium blocks the key pro-apoptotic molecules (GSK-3β, Caspase cascades) and enhances survival pathways [30]. It is quite possible that one or all of the above signaling mechanisms could have contributed to the protective effect of lithium seen in this study.
Data from the current study may contribute to the preventive as well as therapeutic measures to Cadmium-induced toxicity on testicular function. Indeed, male infertility account for 30% of all infertility causes and current available treatments are either non-specific or involved costly assisted reproductive technologies (IVF/ICSI). Therefore studies related to the prevention of male infertility in developed countries, where the risk of pollution is maximum, seem to be of great importance.
Acknowledgments
Disclosure information All authors have nothing to declare.
Footnotes
Capsule
Lithium protection against toxic effect of Cadmium in rat testes suggests its potential preventive uses in male infertility among smokers.
References
- 1.Benoff S, Jacob A, Hurley IR. Male infertility and environmental exposure to lead and cadmium. Hum Reprod Update. 2000;6:107–121. doi: 10.1093/humupd/6.2.107. [DOI] [PubMed] [Google Scholar]
- 2.Laskey JW, Rehnberg GL, Laws SC, Hein JF. Reproductive effects of low acute doses of cadmium chloride in adult male rats. Toxicol Appl Pharmacol. 1984;73:250–255. doi: 10.1016/0041-008X(84)90330-2. [DOI] [PubMed] [Google Scholar]
- 3.Laskey JW, Rehnberg GL, Laws SC, Hein JF. Age-related dose response of selected reproductive parameters to acute cadmium chloride exposure in the male Lon-Evans rat. J Toxicol Environ Health. 1986;19:393–401. doi: 10.1080/15287398609530937. [DOI] [PubMed] [Google Scholar]
- 4.Gennart JP, Buchet JP, Roels H, Ghyselen P, Ceulemans E, Lauwerys R. Fertility of male workers exposed to cadmium, lead or manganese. Am J Epidemiol. 1992;135:1208–1219. doi: 10.1093/oxfordjournals.aje.a116227. [DOI] [PubMed] [Google Scholar]
- 5.Foote RH. Cadmium affects testes and semen of rabbits exposed before and after puberty. Reprod Toxicol. 1999;13:269–277. doi: 10.1016/S0890-6238(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 6.Benoff S, Hurley IR, Mandel FS, Paine T, Jacob A, Cooper GW, Hershlag A. Use of mannose ligands in IVF screens to mimic zona pellucida-induced acrosome reactions and predict fertilization success. Mol Hum Reprod. 1997;3(10):839–846. doi: 10.1093/molehr/3.10.839. [DOI] [PubMed] [Google Scholar]
- 7.Xu LC, Wang SY, Yang XF, Wang XR. Effects of cadmium on rat sperm motility evaluated with computer assisted sperm analysis. Biomed Environ Sci. 2001;14(4):312–317. [PubMed] [Google Scholar]
- 8.Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA. Zinc is a potent inhibitor of the apoptotic protease, Caspase-3. A novel target for zinc in the inhibition of apoptosis. Cell Death Differ. 1997;4:29–33. doi: 10.1038/sj.cdd.4400200. [DOI] [PubMed] [Google Scholar]
- 9.Xu G, Zhou G, Jin T, Jin T, Zhou T, Hammarstrom S, Bergh A, Nordberg G. Apoptosis and p53 gene expression in male reproductive tissues of cadmium exposed rats. Biometals. 1999;12:131–139. doi: 10.1023/A:1009273711068. [DOI] [PubMed] [Google Scholar]
- 10.Zhou T, Zhou G, Song W, Eguchi N, Lu W, Lundin E, Jin T, Nordberg G. Cadmium-induced apoptosis and changes in expression of p53, c-jun and MT-I genes in testes and ventral prostate of rats. Toxicology. 1999;142:1–13. doi: 10.1016/S0300-483X(99)00115-8. [DOI] [PubMed] [Google Scholar]
- 11.Chen RW, Chuang DM. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J Biol Chem. 1999;274:6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- 12.Hiroi T, Wei H, Hough C. Leeds, Chaung DM. Protracted lithium treatment protects against the ER stress elicited by thepsigargin in rat PC12 cells: role of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J. 2005;5:102–111. doi: 10.1038/sj.tpj.6500296. [DOI] [PubMed] [Google Scholar]
- 13.Omu AE, Al-Qattan F, Abdul-hadi FM, Fatinikun MT, Fernandes S. Seminal immune response in infertile men with leukocytospermia: effect on antioxidant activity. Eur J Obstet Gynecol Reprod Biol. 1999;86:195–202. doi: 10.1016/S0301-2115(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 14.Kehinde EO, Mojiminiyi OA, Mahmoud AH, Al-Awadi KA, Al-Hunayan A, Omu AE. The significance of measuring the time course of serum malondialdehyde concentration in patients with torsion of the testis. J Urol. 2003;169:2177–2180. doi: 10.1097/01.ju.0000067360.90440.2c. [DOI] [PubMed] [Google Scholar]
- 15.Johnsen SG. Testicular biopsy score-count-a method for registration of spermatogenesis in human testes: normal values and result of 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 16.Eguchi Y, Srinivasan A, Tomaselli KJ, Shimizu S, Tsujimoto Y. ATP-dependent steps in Apoptotic Signal Transduction. Cancer Research. 1999;59:2174–2181. [PubMed] [Google Scholar]
- 17.Ikediobi CO, Badisa VL, Ayuk-Takem LT, Latinwo LM, West J. Response of antioxidant enzymes and redox metabolites to cadmium-induced oxidative stress in CRL-1439 normal rat liver cells. Int J Mol Med. 2004;14:87–92. [PubMed] [Google Scholar]
- 18.El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem Toxicol. 2004;2:1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Yadav N, Khandelwal S. Effect of Picroliv on cadmium induced testicular damage in rat. Food Chem Toxicol. 2008;46:494–501. doi: 10.1016/j.fct.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 20.Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann NY Acad Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- 22.Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- 23.Sahebgharani M, Neijati M, Sepehrizadeh Z. Lithium chloride protects PC12 pheochromocytoma cell line from morphine-induced apoptosis. Arch Iran Med. 2008;11:639–648. [PubMed] [Google Scholar]
- 24.Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Zhou T. Jope RS Lithium facilitates apoptotic signaling induced by activation of the Fas death domain-containing receptor. BMC Neurosci. 2004;5:20. doi: 10.1186/1471-2202-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh D, Biswas NM, Ghosh PK. Effect of lithium chloride on adrenocortical activity in male rats: evidence of dose and duration dependent response. Indian J Physiol Pharmacol. 1990;34:263–266. [PubMed] [Google Scholar]
- 27.Thakur SC, Thakur SS, Chaube SK, Singh SP. Subchronic supplementation of lithium carbonate induces reproductive system toxicity in male rat. Reproductive Toxicol. 2003;17:683–690. doi: 10.1016/S0890-6238(03)00107-2. [DOI] [PubMed] [Google Scholar]
- 28.Hockenbery DM, Oltvai ZN, Yin X-M, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-N. [DOI] [PubMed] [Google Scholar]
- 29.Lenox RH, Hahn CG. Overview of the mechanism of action of lithium in the brain: fifty-year update. J Clin Psychiatry. 2000;61(Suppl 9):5–15. [PubMed] [Google Scholar]
- 30.Bielecka AM, Obuchowicz E. Antiapoptotic action of lithium and valproate. Pharmacol Rep. 2008;60:771–782. [PubMed] [Google Scholar]