Abstract
Purpose
The aim of this study was to investigate the protective effect of green tea extracts against doxorubicin-induced damage in the mouse testes correlating with telomerase activity.
Methods
Green tea extracts were administered orally. Doxorubicin was coadministered intraperitoneally. These testes were evaluated histologically and the telomerase activity was analyzed. Additional immunostaining was carried out.
Results
Both the sperm density and sperm motility were significantly increased in green tea extracts coadministration groups as compared to the doxorubicin-treated groups. By histological analysis, germ cell damage was greatly attenuated by green tea extracts coadministration. Telomerase activity significantly increased in association with the coadministration of green tea extracts as compared to that of doxorubicin-only groups. In all groups, human telomerase reverse transcriptase signals were mainly observed in the spermatocytes and spermatids.
Conclusions
These findings suggest that green tea extracts exert protective effects against doxorubicin-induced spermatogenic disorders in conjunction with higher telomerase activity levels.
Keywords: Green tea, Catechins, Doxorubicin, Telomerase, Testes
Introduction
Tea is among the most highly consumed beverages worldwide. Derived from the plant Camellia sinensis, tea is consumed in different parts of the world as green, black, or oolong tea. Green tea is favored in Japan and China, and it contains characteristic polyphenolic compounds, including (−)-epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), (−)-epicatechin (EC), (+)-gallocatechin (GC), and (+)-catechin (C). To date, tea catechins have attracted considerable interest due to the potentially health-promoting properties of these substances, including strong antioxidant activity and cancer chemopreventive effects [1–3]. The exact mechanisms of action of green tea polyphenols remain obscure, but it is known that these substances possess antioxidative capacity derived from their ability to scavenge reactive oxygen species and trap hydroxyl and peroxyl radicals. The in vitro antioxidant capacity of teas and tea polyphenols has been assessed using several different approaches [4–6]. Moreover, antioxidative effects of tea polyphenols have been reported in humans [7–9].
Doxorubicin (DXR), an anthracycline antibiotic, is a widely used anticancer agent. The pharmacological effect of DXR is to inhibit DNA synthesis via intercalation as well as generate toxic reactive oxygen species (ROS) [10, 11]. Therefore, the agent interferes with cell proliferation. In the testes of experimental animals, DXR has been shown to disturb spermatogenesis in a dose-dependent manner [12, 13]. A number of reports have demonstrated decreases in DXR-induced toxicity using various natural or artificial compounds [14–16].
To date, telomerase activity has been shown to be specifically expressed in germ cells, immortal cell lines, and most tumor cells [17–22]. The telomerase hypothesis suggests that telomerase activity is high in germ cells, whereas this activity decreases in somatic tissues during development and differentiation [23]. Several studies have yielded positive telomerase activity results for mouse, rat, and human spermatogonia, primary spermatocytes, secondary spermatocytes, and round spermatids, whereas testicular and epididymal spermatozoa were found to be negative for such activity [17, 23–25]. Thus, we assayed telomerase activity as a functional parameter in germ cells. The detection of telomerase activity using a fluorescence-based telomeric repeat amplification protocol (F-TRAP) assay is a highly quantitative approach that has been shown to be sufficiently sensitive [26, 27]. Such a system employs fluorescence-labeled primers and an automatic DNA sequencer to detect and quantify telomerase activity, which is represented by fluorocurves, with the height and area of each peak calculated automatically. We also performed an immunohistochemical analysis using commercially available anti-human telomerase reverse transcriptase (hTERT) antibodies to examine formalin-fixed and paraffin-embedded tissues.
Defective sperm function has traditionally been difficult to evaluate and treat, and yet is the most common cause of infertility. Therefore, further clarification of the mechanisms of spermatogenesis remains desirable. The aim of the present study was to observe the protective effects of green tea catechins with analyzing the involvement of telomerase activity in a DXR-induced germ cell toxic model.
Materials and methods
Chemicals
In the present study, commercially supplied green tea extracts (GTE), i.e., decaffeinated Sunphenon DCF-1® (Taiyo Kagaku Co. Ltd., Yokkaichi, Japan), were used. These GTE consisted of 90% polyphenols, and the residual caffeine content was 0.07%. The content of total polyphenols and total catechins in the EGCG was 34.7% and 47.8%, respectively. The DXR (Adriacin) was obtained from the Kyowa Hakkou Co. (Tokyo, Japan).
Animals
Male ICR mice, 6 weeks old, were purchased from Charles River Japan (Yokohama, Japan), housed in hanging wire mesh cages (five animals per cage) under controlled lighting conditions (12 h light [commencing at 6:00 a.m.] and 12 h dark) at a temperature of 20–24°C with free access to diet and water throughout the experimental period. The range of body weights at the start of dosing was 33 g to 34 g. The animals were handled daily for 1 week before the experiments were performed.
Treatment protocols
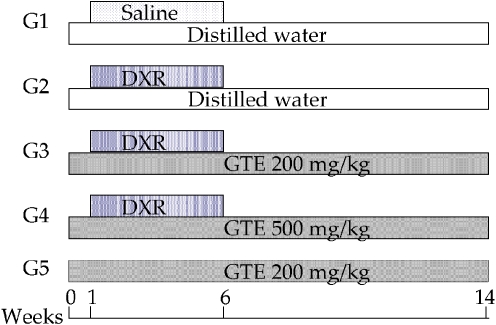
As summarized in Fig. 1, the animals were allocated to five groups (n = 10, respectively). G1 and G2 were fed a standard mouse diet. G3, G4 and G5 were fed a standard diet supplemented with 200 mg/kg, 500 mg/kg, and 200 mg/kg of GTE respectively daily for 14 weeks. Starting at the second week, G2, G3 and G4 were intraperitoneally administered 0.15 mg/kg of DXR twice weekly for 5 weeks (total: 1.5 mg/kg). G1 and G5 were treated with saline (i.p. injection) twice weekly for 5 weeks. At 14 weeks after initiation of the experiment, the animals were sacrificed, and the bilateral testes were removed and weighed. The testes were frozen immediately in liquid nitrogen and stored at −80°C for TRAP assay or were fixed in Bouin’s solution for histopathological examination.
Fig. 1.
Study design. G1: Control, G2: DXR, G3: DXR + GTE (200 mg/kg), G4: DXR + GTE (500 mg/kg), G5: GTE. Ten mice in each group
The experimental protocol was approved by the Animal Research and Care Committee of the School of Medicine, Keio University.
Epididymal sperm count and motility analysis
The spermatozoa examined were collected from the caudal epididymis of each testicular sample, as previously reported [28]. Briefly, the epididymal cauda was cut into small pieces in a 35 mm Petri dish and the pieces were placed in a centrifuge tube containing 3 ml of normal saline in order to allow the sperm to swim for 10 min at 37°C. After specimens were diluted with trypan blue staining solution, they were transferred to a hemocytometer counting chamber with 20 μm depth, where they were left to stand for 5 min. Sperm concentration was determined by counting the number of cells. The number of sperm cells/ml was calculated by multiplying the mean value by 104. Sperm motility was assessed by scoring the number of all progressive sperm and the non progressive and the immotile sperm in the same field. All sperm counts were carried out by the same investigator in order to ensure reproducible results.
Histopathological examination
The fixed testes were embedded in paraffin. Each section was stained with hematoxylin and eosin (H&E). All slides were examined under a light microscope. To evaluate quantitatively, thirty seminiferous tubules per group were randomly examined for the calculation of Sertoli cell index (SCI). Based on the fact that Sertoli cells do not disappear even at very high testicular failure, SCI is the ratio of the number of germ cells to the number of Sertoli cells [29].
Fluorescence-based telomeric repeat amplification protocol assay
A quantitative modification of the telomeric repeat amplification protocol (TRAP) method was applied using a TRAP-ezeTM telomerase detection kit (Oncor Inc.–Kyowa Co., Tokyo, Japan) according to the manufacturer’s instructions [26, 27]. Briefly, frozen testicular samples (20 mg for each assay) were homogenized in 100 µl of ice-cold CHAPS lysis buffer (TRAP-ezeTM Telomerase Detection Kit; Oncor Inc.–Kyowa Co., Tokyo, Japan) and were incubated for 30 min on ice. After incubation, the lysates were centrifuged at 12,000 g for 20 min at 4 C. The supernatants were rapidly frozen and were stored at −80°C. The protein concentration was determined, and an aliquot of extract containing 1 µg of protein was used for each TRAP assay. Aliquots of the extract were incubated with 0.1 ng Cy-5-labeled TS primer (TRAP-eze™). Following a 30-min incubation at 30°C, polymerase chain reaction (PCR) was carried out as follows: 94°C (30 s), then 60°C (30 s) and 72°C (45 s) for 30 cycles. The external control was TSR8 (TRAP-ezeTM) as a positive control. The products were applied to a 10% denaturing gel containing 6 mol/l urea fitted to an automated DNA sequencer (ABI PRISMTM310 Genetic Analyzer, Applied Biosystems Japan). Data from the DNA Sequencer were collected and analyzed automatically. Each peak was quantified in terms of size, peak height, and peak area. Telomerase activity was quantified according to the following formula: TPG (total product generated) units/μg protein = (A × B−1/C × D−1) × 100, where A is the measured total area of telomerase activity (50, 56 bp…), B is the measured area of the internal control (36 bp), C is the measured total area of telomerase activity (50, 56 bp…) in the positive control, and D is the measured area of internal control (36 bp) in the positive control [26].
Immunohistochemistry
A rabbit polyclonal antibody raised against a recombinant protein corresponding to amino acids 900–1,130 mapped at the carboxy terminus of hTERT was used as the primary antibody (Calbiochem-Novabiochem Corp., San Diego, CA, USA). This anti-hTERT antibody reacts with TERTs of mouse, rat, and human origin, as determined by immunohistochemical analyses. Immunohistochemical assays for hTERT were performed as previously described, with slight modifications [30]. Deparaffinized sections were treated by microwaving them in citrate buffer (0.1 M, pH 6.0) for 5 min at 500 W to retrieve the antigenicity. The sections were treated with 0.3% hydrogen peroxide in methanol for 30 min at room temperature to block endogenous peroxidase activity. Then, the tissue sections were incubated for 2 h at room temperature with anti-hTERT antibody at a dilution of 1:750. After the samples were washed, the stain was developed using ENVISION + systems (Dako Japan Co., Kyoto, Japan) according to the manufacturer’s instructions. Since 3, 3′-diaminobenzidine tetrahydrochloride (DAB) was used as the substrate, a positive reaction was detected as a brown stain. The sections were then lightly counterstained with 0.5% methyl green and mounted. The reactions were considered to be positive or negative according to whether or not TERT protein was detected in the spermatogenic cells.
Statistical analysis
Telomerase activity was analyzed in at least three independent experiments per sample. Statistical analysis was performed using ANOVA followed by a Tukey-Kramer’s multiple comparison test. Values are expressed as mean±SD. A probability of P < 0.05 was considered to be statistically significant.
Results
Physical symptoms, body and testes weight
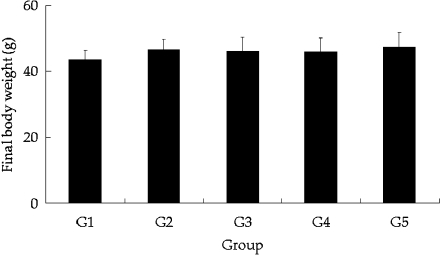
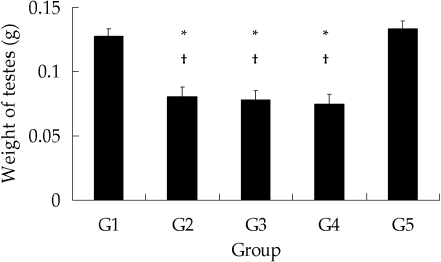
All animals survived the experimental period, and no abnormal behavior considered to be related to DXR treatment was observed in any of the treated groups during the course of the study. While bodyweight did not decrease with DXR treatment (Fig. 2), testes weight was significantly lower in animals exposed to DXR (p < 0.01) than in controls, and no compensation for weight loss by GTE coadministration was observed (Fig. 3).
Fig. 2.
Final body weight of mice. Body weight did not decreased with DXR treatment, with or without GTE exposure (G2, G3 and G4). Each point represents the mean±SD from samples from ten mice
Fig. 3.
Testes weight. The weight of the DXR-treated testes (G2, G3 and G4) was significantly lower than that of controls (p < 0.01), and testes weight loss was not compensated with GTE coadministration (G3, G4). Each point represents the mean±SD from tissues from ten mice. *, P < 0.01 vs. G1; †, P < 0.01 vs. G5
Sperm parameters
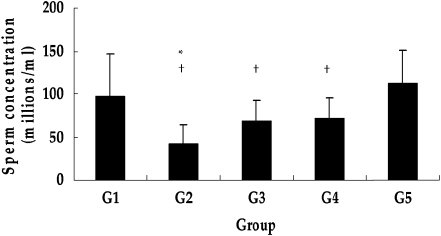
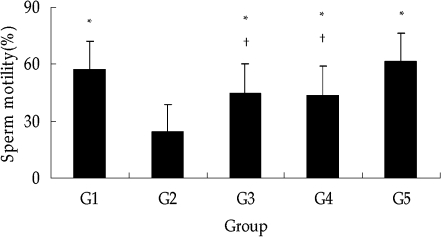
The results of the sperm density and motion analyses are shown in Figs. 4 and 5. Both the sperm density and the percentage of motile sperm were significantly decreased by DXR treatment, while significantly increased by GTE coadministration.
Fig. 4.
Epididymal sperm concentration in mice. The sperm concentration was significantly reduced with DXR treatment (G2), and was significantly increased with GTE coadministration (G3 and G4). Each point represents the mean±SD from tissues from ten mice. *, P < 0.01 vs. G1; †, P < 0.05 vs. G5
Fig. 5.
Epididymal sperm motility in mice. The percentage of motile sperm was significantly higher by coadministration of GTE (G3 and G4) than that of DXR-treated mice (G2). Each point represents the mean±SD from tissues from ten mice. *, P < 0.01 vs. G2; †, P < 0.05 vs. G5
Histopathological findings
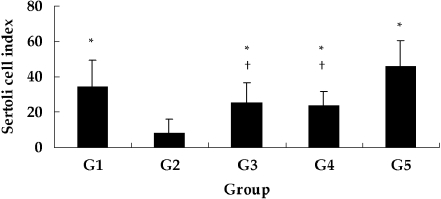
Histological findings in the control and GTE groups were similar (Fig. 6-G1, G5), whereas DXR reduced number of germ cells (Fig. 6-G2). The testicular tubules were also markedly reduced in size, and the seminiferous tubules showed severe vacuolization, with some fibrinoid debris. The primary losses were observed in the counts of spermatogonia, spermatocytes, and round spermatids, while elongated spermatids and spermatozoa were observed in some seminiferous tubules. Widening of the interstitial space and severe vacuolization were also seen in the interstitial tissues, but the number and morphology of Sertoli cells in the shrunken tubules remained normal. However, GTE coadministration was found to inhibit these forms of testicular toxicity (Fig. 6-G3, G4). From a quantitative standpoint, SCI was significantly decreased in the DXR-treated mice (Fig. 7-G2), but this reduction was significantly improved by GTE coadministration (Fig. 7-G3, G4).
Fig. 6.
Histological morphology of testes from all groups of mice examined. Testes from control (G1) and GTE-treated (G5) mice show normal seminiferous epithelium and interstitial tissue features. However, the testes of DXR-treated mice (G2) contained markedly shrunken and empty seminiferous tubules. In samples from mice treated with DXR and GTE (G3:200 mg/kg GTE or G4:500 mg/kg GTE), most tubules were populated with germ cells undergoing maturation to the spermatid stage, although a partial loss of early spermatogenic cells was seen in some seminiferous tubules. G1: Controls, G2: DXR, G3: DXR + GTE (200 mg/kg), G4: DXR + GTE (500 mg/kg), G5: GTE. Hematoxylin-Eosin staining, Magnification: 200×
Fig. 7.
Sertoli cell index (SCI) of testes from all groups of mice. SCI is the ratio of the number of germ cells to the number of Sertoli cells. SCI was significantly decreased in the DXR-treated mice (G2), but this reduction was significantly inhibited by GTE coadministration (G3 and G4). *, P < 0.05 vs. G2; †, P < 0.05 vs. G5
Quantitative analysis of telomerase activity in the mouse testes by telomeric repeat amplification protocol assay
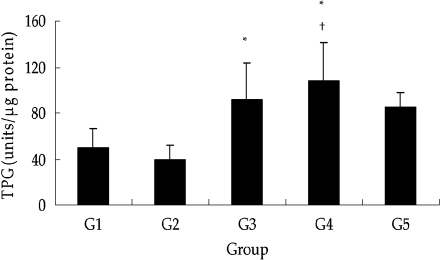
The telomerase activity was significantly increased by coadministration of GTE as compared to control and DXR-treated groups. In addition, the telomerase activity in the GTE groups tended to be higher than that of control groups, while there found no significant change between the control and DXR groups (Fig. 8).
Fig. 8.
Quantification of telomerase activity (TPG) in the testes from each group. Telomerase activity was significantly higher with the coadministration of GTE (G3 and G4) than without GTE treatment (G1 and G2). While, there found no significant change between the control (G1) and DXR-treated groups(G2). *, P < 0.05 vs. G2; †, P < 0.05 vs. G1. G1: Control, G2: DXR, G3: DXR + GTE (200 mg/kg), G4: DXR + GTE (500 mg/kg), G5: GTE. Each point represents the mean±SD from tissues from ten mice
Immunohistochemistry with anti-human telomerase reverse transcriptase antibody
In all groups, hTERT signals were clearly detected in the spermatocytes and spermatids. Furthermore, no such signals were observed in either the stromal cells or spermatozoa. In G3 and G4, increases in hTERT-positive spermatocytes and spermatids were observed as compared to the hTERT-positivity of these structures in G2 (Fig. 9).
Fig. 9.
Immunohistochemical staining of testes treated with DXR and/or GTE. In all groups, hTERT signals were primarily observed in the spermatocytes and the spermatids, while faint signals were seen in the spermatogonia. No significant signals were observed in the stromal cells or spermatozoa. The group that received GTE coadministration (G4) showed greater reactivity than did the DXR-treated groups lacking GTE exposure (G2). G1: Controls, G2: DXR, G4: DXR + GTE (500 mg/kg), G5: GTE. Magnification: 200×
Discussion
Although there have been a number of studies on the antitoxic effects of catechins [31–35], only a few studies of their effects on the testes are available [36, 37]. In the present study, we demonstrated that the testicular toxicity induced by DXR was markedly attenuated by green tea extracts, i.e., GTE.
When 7-week-old mice were treated with a low dose (0.15 mg/kg) of DXR, early spermatogenic cells such as spermatogonia, spermatocytes, and round spermatids disappeared from most seminiferous tubules and ductus epididymides examined. The lesions were greatly attenuated by GTE coadministration, although a partial loss of early spermatogenic cells was observed in some seminiferous tubules.
In general, defect in human spermatogenesis by exposure of toxic substance occur chronically. Thus, it is not appropriate to assess accurately the effect of treatment on acute impairment in spermatogenesis by single injection of anticancer drugs. Therefore, in this experiment we employed the model of chronic and mild dysfunction in spermatogenesis by administering a low-dose doxorubicin (0.15 mg, twice a week) for a long term (5 weeks), caused disabilities only to testes. The duration and dose of doxorubicin were based on the data reported by Sudo K. [38]. The other study had shown that the LD50 of doxorubicin for differentiated spermatogonia in mice given as single injection was at 1 mg/kg [39]. In addition, a single injection of doxorubicin at 10 mg/kg in our preliminary study gave irreversible change on testicular function.
Antioxidants are compounds which dispose, scavenge, and suppress the formation of ROS, or oppose ROS activity. Male germ cell membranes are sensitive to oxygen-induced damage mediated by lipid peroxidation due to their membranes are rich in polyunsaturated fatty acids [40, 41]. Therefore, some antioxidants (e.g., vitamin C, vitamin E, glutathione, and coenzyme Q10) have been demonstrated to help treat male infertility [42–44]. The testicular cytotoxicity of DXR appears to be primarily due to oxygen-related free-radical production, and thus the administration of GTE, which reduce and/or oppose ROS, may inhibit the toxic effects of DXR.
There are few in vivo studies of pure catechin extracts and their detoxification effects, and little is known about the mechanisms of action responsible for metabolic disorders caused by DXR. It has been suggested that components in black tea provide antioxidative effects against carbon tetrachloride (CCl4)-induced toxicity in the liver, kidneys, and testes of rats [36]. It should be noted that the administration of cadmium has been shown to be associated with increased levels of lipid peroxidation products in the rat testes, and green tea catechin does not appear to be helpful against such cadmium toxicity [37].
To date, a number of studies have shown decreases in DXR-induced toxicity with the use of various natural as well as artificial compounds. Amifostine (ethylphosphorothioic acid), a sulfhydryl-containing radioprotective agent, has been found to be a scavenger of the oxygen-free radicals which are produced by DXR. It has also been demonstrated that amifostine exerts significant protective effects against early stages of toxicity of DXR in young, growing rats [16]. On the other hand, amifostine failed to protect germ cells in the testes against this type of cytotoxicity [45]. However, it has been reported that ginseng intestinal metabolite-I, the final intestinal bacterial metabolite of ginseng in humans, has an antioxidant effect, and may be partially protective against doxorubicin-induced testicular toxicity [29].
The F-TRAP assay offers no information at the single-cell level. Each testicular germ cell from rat testis tissue has been isolated with a cell-purity range of 70–98% [25, 46]. Moreover, Tanemura et al. developed an in situ TRAP assay that allows for the detection of telomerase activity in histological sections with single-cell resolution [47].
Telomerase activity has been found to decrease during the process of germ cell differentiation, and to be completely repressed in spermatozoa [25]. On the other hand, Tanemura et al. proposed that telomerase activity disappeared during the pachytene spermatocyte phase [47]. Our TERT study revealed clear TERT-immunopositive signals in both spermatocytes and spermatids, whereas only weak signals were observed in spermatogonia.
Although the precise mechanisms of telomerase activity remain to be elucidated, telomerase appears to play a crucial role in differentiation during spermatogenesis. This hypothesis is supported by analyses of telomerase-deficient mice; sperm from the second generation of telomerase-null mice exhibited lower ratios of fertilization and higher frequencies of early developmental failure as compared to that of controls [48], even though active spermatogenesis has been detected in telomerase-null mice up to the fifth generation [49]. Thus, the observation that telomere extension activity is preserved during germ cell production, even in the absence of the telomerase complex, suggests the existence of an alternative telomere-synthetic pathway; importantly, such an alternative system would be capable of partially complementing the telomerase defect in telomerase-deficient mice [50, 51].
In our study, significantly higher telomerase activity was detected in the DXR treated mice that also received GTE. There is no clear evidence presented that indicates that telomerase or TERT is required for the protective effect of GTE. Rather, the increase in telomerase activity in testes may well be explained by a significantly greater viability of testicular germ cells in GTE-DXR treated animals.
In addition, The dosages of GTE were chosen on the basis of other related literatures [52, 53]. A standard way of preparing Japanese green tea is to soak 10 g of green tea leaves in 430 ml hot water (90°C) for 1 min. The resulting tea beverage contains ∼280 mg tea catechins. Japanese people daily ingest 413 mg tea catechins on average because they ordinarily drink seven to eight cups (740 ml) of green tea per day. On the basis of body weight (average Japanese, 60 kg and mice, 30 g), the intake of 6 mg tea catechins extract by mice per day (GTE 200 mg/kg) corresponds to a human ingesting approximately 30 fold the ordinary intake. However, drinking such volumes can be bypassed because formulations of purified green tea polyphenols are already available as tablets.
In conclusion, GTE could effectively prevent DXR-induced testicular toxicity. Though the effect of GTE on telrmerase activity or TERT in testes remains unclear, the GTE protective effect may be related to the mechanisms involved in its antioxidative effects.
Since DXR-based chemotherapy is still indispensable for treatment of various cancers yet the resultant drug-induced infertility is devastating and often unavoidable, our results raise the hope that co-administration of GTEs with DXR may be a promising solution to this otherwise very serious complication of DXR in testes.
Acknowledgments
We wish to acknowledge Ms. Hiroko Ueno and Ms. Kimiko Tokizawa for their excellent laboratory assistance, Taiyo Kagaku Co. Ltd. for their generous donation of green tea extract.
Footnotes
Capsule
Green tea extracts may be protective against DXR-induced testicular toxicity associated with an increase in telomerase activity.
References
- 1.Mukhtar H, Ahmad N. Green tea in chemoprevention of cancer. Toxicol Sci. 1995;52:111–7. doi: 10.1093/toxsci/52.2.111. [DOI] [PubMed] [Google Scholar]
- 2.Clement Y. Can green tea do that? A literature review of the clinical evidence. Prev Med. 2009;49:83–7. doi: 10.1016/j.ypmed.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Schneider C, Segre T. Green tea: potential health benefits. Am Fam Physician. 2009;79:591–4. [PubMed] [Google Scholar]
- 4.Valcic S, Muders A, Jacobsen NE, Liebler DC, Timmermann BN. Antioxidant chemistry of green tea catechins. Identification of products of the reaction of (−)-epigallocatechin gallate with peroxyl radicals. Chem Res Toxicol. 1999;12:382–6. doi: 10.1021/tx990003t. [DOI] [PubMed] [Google Scholar]
- 5.Rice-Evans C. Plant polyphenols: free radical scavengers or chain-breaking antioxidants? Biochem Soc Symp. 1995;61:103–16. doi: 10.1042/bss0610103. [DOI] [PubMed] [Google Scholar]
- 6.Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys. 1995;322:339–46. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- 7.Sung H, Nah J, Chun S, Park H, Yang SE, Min WK. In vivo antioxidant effect of green tea. Eur J Clin Nutr. 2000;54:527–9. doi: 10.1038/sj.ejcn.1600994. [DOI] [PubMed] [Google Scholar]
- 8.Rietveld A, Wiseman S. Antioxidant effects of tea: evidence from human clinical trials. Nutr. 2003;133:3285S–92. doi: 10.1093/jn/133.10.3285S. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa K, Ninomiya M, Okubo T, Aoi N, Juneja LR, Kim M, et al. Tea catechin supplementation increases antioxidant capacity and prevents phospholipids hydroperoxidation in plasma of humans. J Agri Food Chem. 1999;47:3967–73. doi: 10.1021/jf981195l. [DOI] [PubMed] [Google Scholar]
- 10.Marco A, Gaetani M, Scarpinato B. Adriamycin (NSC-123, 127): a new antibiotic with antitumor activity. Cancer Chemother Rep. 1969;53:33–7. [PubMed] [Google Scholar]
- 11.Quiles JL, Huertas JR, Battino M, Mataix J, Ramirez-Tortosa MC. Antioxidant nutrients and adriamycin toxicity. Toxicology. 2002;30:79–95. doi: 10.1016/S0300-483X(02)00383-9. [DOI] [PubMed] [Google Scholar]
- 12.Imahie H, Adachi T, Nakagawa Y, Nagasaki T, Yamamura T, Hori M. Effects of adriamycin, an anticancer drug showing testicular toxicity, on fertility in male rats. J Toxicol Sci. 1995;20:183–93. doi: 10.2131/jts.20.183. [DOI] [PubMed] [Google Scholar]
- 13.Lui RC, Laregina MC, Herbold DR, Johnson FE. Testicular cytotoxicity of intravenous doxorubicin in rats. J Urol. 1986;136:940–3. doi: 10.1016/s0022-5347(17)45136-6. [DOI] [PubMed] [Google Scholar]
- 14.Dorr RT. Cytoprotective agents for anthracyclines. Semin Oncol. 1996;23:23–34. [PubMed] [Google Scholar]
- 15.Herman EH, Zhang J, Chadwick DP, Ferrans VJ. Comparison of the protective effects of amifostine and dexrazoxane against the toxicity of doxorubicin in spontaneously hypertensive rats. Cancer Chemother Pharmacol. 2000;45:329–34. doi: 10.1007/s002800050048. [DOI] [PubMed] [Google Scholar]
- 16.Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Bauer JA. Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin Pharmacol Toxicol. 2005;96:80–7. doi: 10.1111/j.1742-7843.2005.pto960112.x. [DOI] [PubMed] [Google Scholar]
- 17.Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci USA. 1995;92:4818–22. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 19.Weise JM, Güneş C. Differential regulation of human and mouse telomerase reverse transcriptase (TERT) promoter activity during testis development. Mol Reprod Dev. 2009;76:309–17. doi: 10.1002/mrd.20954. [DOI] [PubMed] [Google Scholar]
- 20.Coussens M, Yamazaki Y, Moisyadi S, Suganuma R, Yanagimachi R, Allsopp R. Regulation and effects of modulation of telomerase reverse transcriptase expression in primordial germ cells during development. Biol Reprod. 2006;75:785–91. doi: 10.1095/biolreprod.106.052167. [DOI] [PubMed] [Google Scholar]
- 21.Fajkus J, Dvorácková M, Sýkorová E. Analysis of telomeres and telomerase. Meth Mol Biol. 2008;463:267–96. doi: 10.1007/978-1-59745-406-3_17. [DOI] [PubMed] [Google Scholar]
- 22.Zalenskaya IA, Zalensky AO. Telomeres in mammalian male germline cells. Int Rev Cytol. 2002;218:37–67. doi: 10.1016/S0074-7696(02)18011-9. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer KM, Gerstein RM, Chiu CP, Conti M, Hsueh AJ. Telomerase activity in female and male rat germ cells undergoing meiosis and in early embryos. Biol Reprod. 1997;56:1120–5. doi: 10.1095/biolreprod56.5.1120. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto Y, Sofikitis N, Ono K, Kaki T, Isoyama T, Suzuki N, et al. Postmeiotic modifications of spermatogenic cells are accompanied by inhibition of telomerase activity. Urol Res. 1999;27:336–45. doi: 10.1007/s002400050160. [DOI] [PubMed] [Google Scholar]
- 25.Achi MV, Ravindranath N, Dym M. Telomere length in male germ cells is inversely correlated with telomerase activity. Biol Reprod. 2000;63:591–8. doi: 10.1095/biolreprod63.2.591. [DOI] [PubMed] [Google Scholar]
- 26.Hisatomi H, Nagao K, Komatsu H. Quantification of telomerase activity in human liver tissues by fluorescence based TRAP analysis. Hepat Res. 1997;7:35–42. doi: 10.1016/S0928-4346(97)00369-1. [DOI] [Google Scholar]
- 27.Ohyashiki JH, Ohyashiki K, Toyama K, Shay JW. A nonradioactive, fluorescence-based telomeric repeat amplification protocol to detect and quantitate telomerase activity. Trends Genet. 1996;12:395–6. doi: 10.1016/S0168-9525(96)90097-9. [DOI] [PubMed] [Google Scholar]
- 28.Ateşşahin A, Türk G, Karahan I, Yilmaz S, Ceribaşi AO, Bulmuş O. Lycopene prevents adriamycin-induced testicular toxicity in rats. Fertil Steril. 2006;85(Suppl. 1):1216–22. doi: 10.1016/j.fertnstert.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Kang J, Lee Y, No K, Jung E, Sung J, Kim Y, et al. Ginseng intestinal metabolite-I (GIM-I) reduces doxorubicin toxicity in the mouse testis. Reprod Toxicol. 2002;16:291–8. doi: 10.1016/S0890-6238(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 30.Kyo S, Masutomi K, Maida Y, Kanaya T, Yatabe N, Nakamura M, et al. Significance of immunological detection of human telomerase reverse transcriptase: re-evaluation of expression and localization of human telomerase reverse transcriptase. Am J Pathol. 2003;163:859–67. doi: 10.1016/S0002-9440(10)63446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kagaya N, Tagawa Y, Nagashima H, Saijo R, Kawase M, Yagi K. Suppression of cytotoxin-induced cell death in isolated hepatocytes by tea catechins. Eur J Pharmacol. 2002;450:231–6. doi: 10.1016/S0014-2999(02)02157-X. [DOI] [PubMed] [Google Scholar]
- 32.Hisano M, Yamaguchi K, Inoue Y, Ikeda Y, Iijima M, Adachi M, et al. Inhibitory effect of catechin against the superantigen staphylococcal enterotoxin B (SEB) Arch Dermatol Res. 2003;295:183–9. doi: 10.1007/s00403-003-0411-x. [DOI] [PubMed] [Google Scholar]
- 33.Raihan SZ, Chowdhury AK, Rabbani GH, Marni F, Ali MS, Nahar L, et al. Effect of aqueous extracts of black and green teas in arsenic-induced toxicity in rabbits. Phytother Res. 2009;23:1603–8. doi: 10.1002/ptr.2827. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Sun CK, Han GZ, Peng JY, Li Y, Liu YX, et al. Protective effect of tea polyphenols against paracetamol-induced hepatotoxicity in mice is significantly correlated with cytochrome P450 suppression. World J Gastroenterol. 2009;15:1829–35. doi: 10.3748/wjg.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin BC, Ryu HH, Chung JH, Lee BR, Kim HL. The protective effects of green tea extract against L-arginine toxicity to cultured human mesangial cells. J Korean Med Sci. 2009;24(Suppl):S204–9. doi: 10.3346/jkms.2009.24.S1.S204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fadhel ZA, Amran S. Effects of black tea extract on carbon tetrachloride-induced lipid peroxidation in liver, kidneys, and testes of rats. Phytother Res. 2002;16:S28–32. doi: 10.1002/ptr.793. [DOI] [PubMed] [Google Scholar]
- 37.Ozdemir S, Dursun S. Role of + (−)catechin against cadmium toxicity in the rat testes. Scand J Urol Nephrol. 2008;23:1–4. doi: 10.1080/00365590802468891. [DOI] [PubMed] [Google Scholar]
- 38.Sudo K. An experimental model of adriamycin-induced spermatogenic disorder in mice (1): histological and functional analysis. J Med Soc Toho. 1991;38:462–75. [Google Scholar]
- 39.Lu CC, Meistrich ML. Cytotoxic effects of chemotherapeutic drugs on mouse testis cells. Cancer Res. 1979;39:3575–82. [PubMed] [Google Scholar]
- 40.Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29:488–98. doi: 10.2164/jandrol.108.005132. [DOI] [PubMed] [Google Scholar]
- 41.Mandal TK, Das NS. Effect of delta-9-tetrahydrocannabinol on altered antioxidative enzyme defense mechanisms and lipid peroxidation in mice testes. Eur J Pharmacol. 2009;607:178–87. doi: 10.1016/j.ejphar.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 42.Tavares DC, Cecchi AO, Antunes LM, Takahashi CS. Protective effects of the amino acid glutamine and of ascorbic acid against chromosomal damage induced by doxorubicin in mammalian cells. Teratog Carcinog Mutagen. 1998;18:153–61. doi: 10.1002/(SICI)1520-6866(1998)18:4<153::AID-TCM1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 43.Balercia G, Mosca F, Mantero F, Boscaro M, Mancini A, Ricciardo-Lamonica G, et al. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril. 2004;81:93–8. doi: 10.1016/j.fertnstert.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal A, Sharma RK, Nallella KP, Thomas AJ, Jr, Alvarez JG, Sikka SC. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86:878–85. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- 45.Hou M, Chrysis D, Nurmio M, Parvinen M, Eksborg S, Söder O, et al. Doxorubicin induces apoptosis in germ line stem cells in the immature rat testis and amifostine cannot protect against this cytotoxicity. Cancer Res. 2005;65:9999–10005. doi: 10.1158/0008-5472.CAN-05-2004. [DOI] [PubMed] [Google Scholar]
- 46.Miller RG, Phillips RA. Separation of cells by velocity sedimentation. J Cell Physiol. 1969;73:191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- 47.Tanemura K, Ogura A, Cheong C, Gotoh H, Matsumoto K, Sato E, et al. Dynamic rearrangement of telomeres during spermatogenesis in mice. Dev Biol. 2005;281:196–207. doi: 10.1016/j.ydbio.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 48.Liu L, Blasco M, Trimarchi J, Keefe D. An essential role for functional telomeres in mouse germ cells during fertilization and early development. Dev Biol. 2002;249:74–84. doi: 10.1006/dbio.2002.0735. [DOI] [PubMed] [Google Scholar]
- 49.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–74. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 50.Niida H, Shinkai Y, Hande MP, Matsumoto T, Takehara S, Tachibana M, et al. Telomere maintenance in telomerase-deficient mouse embryonic stem cells: characterization of an amplified telomeric DNA. Mol Cell Biol. 2000;20:4115–27. doi: 10.1128/MCB.20.11.4115-4127.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddel RR. Alternative lengthening of telomeres, telomerase, and cancer. Cancer Lett. 2003;194:155–62. doi: 10.1016/S0304-3835(02)00702-4. [DOI] [PubMed] [Google Scholar]
- 52.Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134:3431S–40. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi Y, Hayashi M, Yamazoe H, Kunitomo M. Preventive effects of green tea extract on lipid abnormalities in serum, liver and aorta of mice fed a atherogenic diet. Nippon Yakurigaku Zasshi. 1991;97:329–37. doi: 10.1254/fpj.97.6_329. [DOI] [PubMed] [Google Scholar]