Abstract
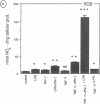
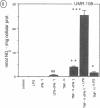
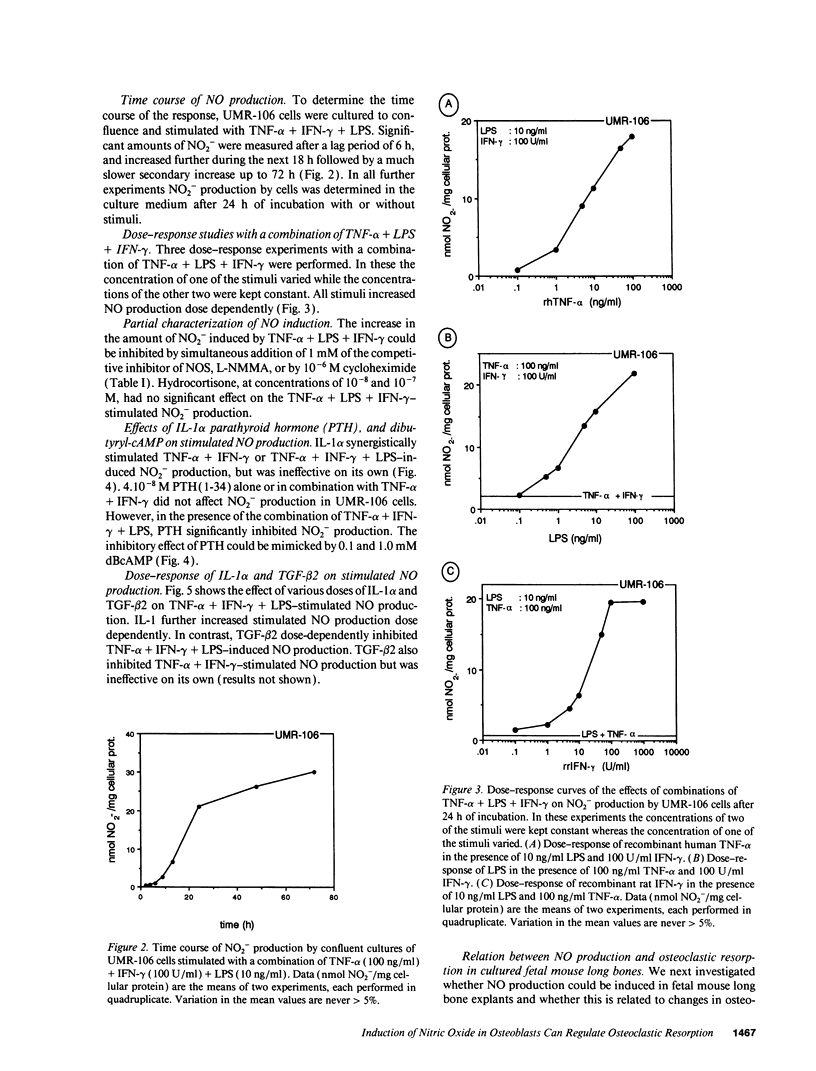
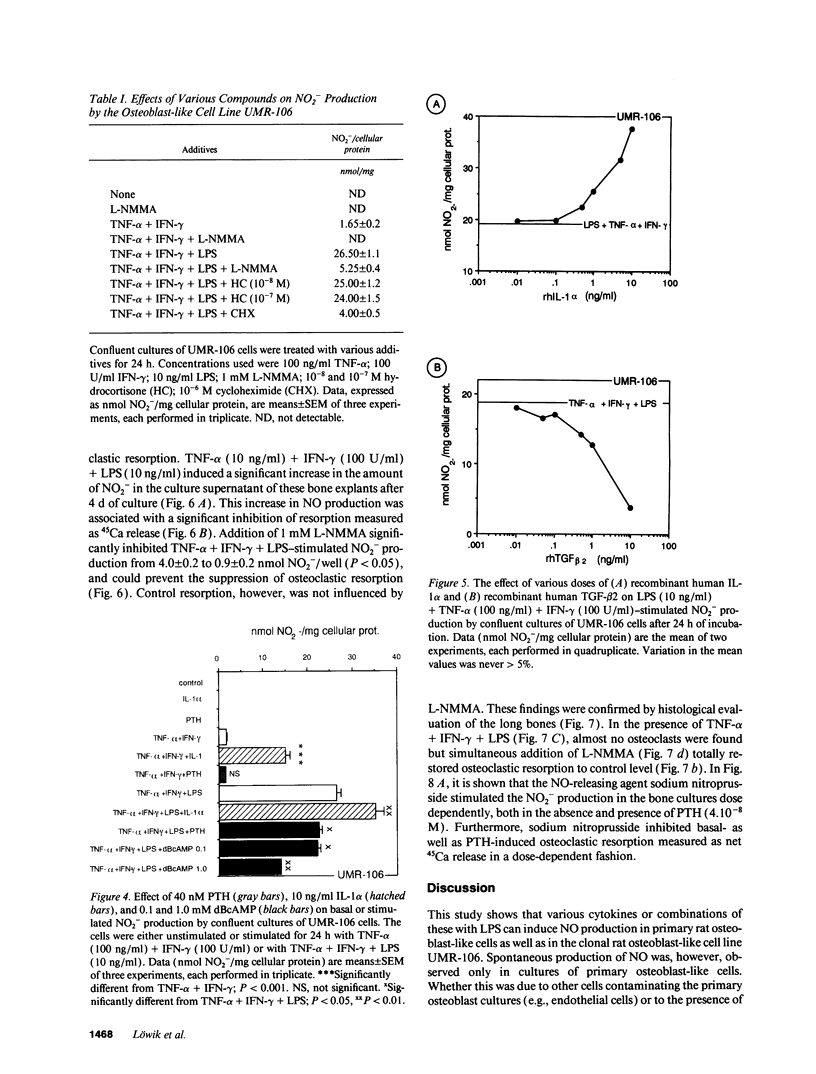
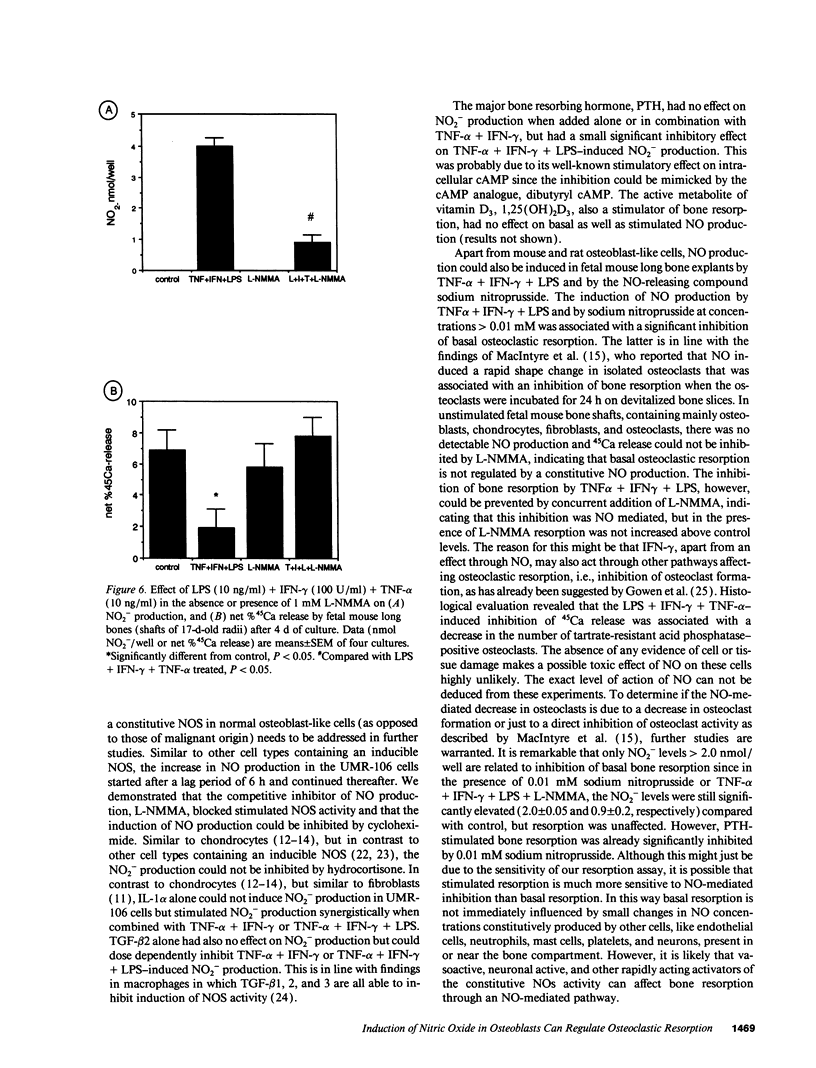
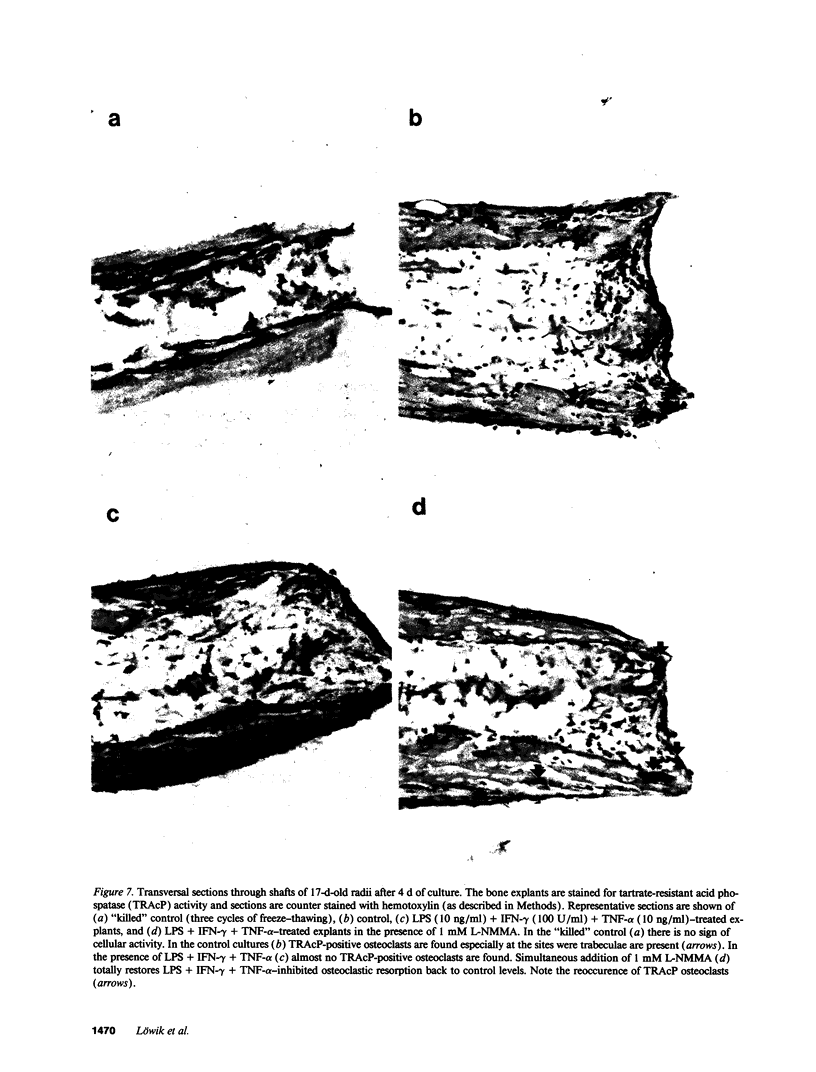
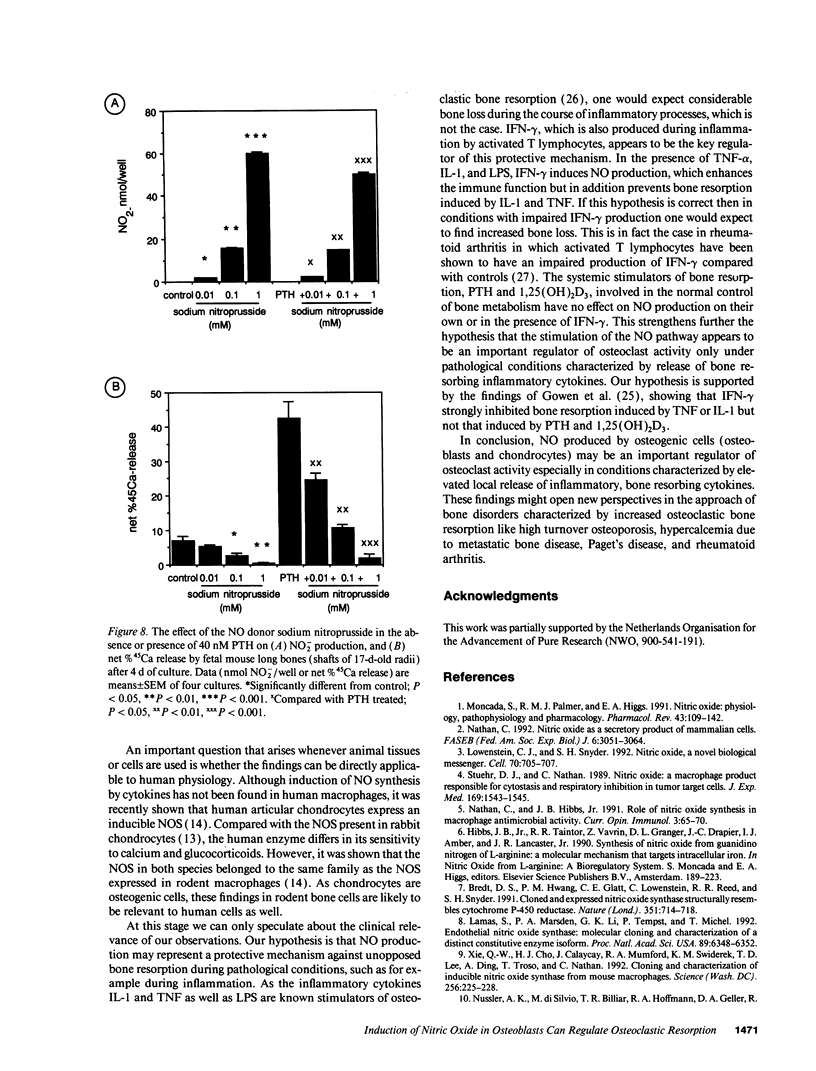
Nitric oxide (NO) has been suggested to be involved in the regulation of osteoclast activity. Since osteoblasts, through the release of various factors, are the main regulators of osteoclastic resorption, first we have investigated whether osteoblast-like cells and fetal mouse long bone explants are able to produce NO. Second, we have assessed the effect of NO on osteoclastic resorption in whole bone cultures. In this study we show that primary rat osteoblast-like cells as well as the clonal rat osteoblast-like cell line UMR-106, stimulated with IFN-gamma together with TNF-alpha and LPS, produce NO, measured as nitrite production. IL-1 alpha enhanced while TGF-beta 2 inhibited TNF-alpha + IFN-gamma + LPS-stimulated NO production in UMR-106 cells dose dependently. Both the cytokines, however, had no effect when given alone. The competitive inhibitor of NO production, NG-monomethyl-arginine (L-NMMA), and cycloheximide abolished the increase in nitrite production induced by TNF-alpha + IFN-gamma + LPS, while hydrocortisone had no effect, as previously reported for chondrocytes. Calciotropic hormones had either no effect [1,25(OH)2D3] or had a small inhibitory effect (parathyroid hormone) on stimulated NO production. Furthermore, we found that in cultured fetal mouse long bone explants the combination of TNF-alpha + IFN-gamma + LPS as well as the NO donor sodium nitroprusside could inhibit osteoclastic resorption, measured as 45Ca release. The inhibition of resorption was prevented by concurrent administration of L-NMMA. Histological evaluation revealed that the TNF-alpha + IFN-gamma + LPS-induced inhibition of 45Ca release was associated with a decrease in the number of tartrate-resistant acid phosphatase-positive osteoclasts. We propose that the NO production by osteogenic cells (osteoblasts and chondrocytes) may represent an important regulatory mechanism of osteoclastic activity especially under pathological conditions characterized by release of bone-resorbing inflammatory cytokines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Radomski M., Carnuccio R., Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- Ding A., Nathan C. F., Graycar J., Derynck R., Stuehr D. J., Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFN-gamma. J Immunol. 1990 Aug 1;145(3):940–944. [PubMed] [Google Scholar]
- Gowen M., Nedwin G. E., Mundy G. R. Preferential inhibition of cytokine-stimulated bone resorption by recombinant interferon gamma. J Bone Miner Res. 1986 Oct;1(5):469–474. doi: 10.1002/jbmr.5650010511. [DOI] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- MacIntyre I., Zaidi M., Alam A. S., Datta H. K., Moonga B. S., Lidbury P. S., Hecker M., Vane J. R. Osteoclastic inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2936–2940. doi: 10.1073/pnas.88.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M. Inhibition of the induction of nitric oxide synthase by glucocorticoids: yet another explanation for their anti-inflammatory effects? Trends Pharmacol Sci. 1991 Apr;12(4):130–131. doi: 10.1016/0165-6147(91)90528-z. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nussler A. K., Di Silvio M., Billiar T. R., Hoffman R. A., Geller D. A., Selby R., Madariaga J., Simmons R. L. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992 Jul 1;176(1):261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Andrews T., Foxwell N. A., Moncada S. Glucocorticoids do not affect the induction of a novel calcium-dependent nitric oxide synthase in rabbit chondrocytes. Biochem Biophys Res Commun. 1992 Oct 15;188(1):209–215. doi: 10.1016/0006-291x(92)92371-4. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Hickery M. S., Charles I. G., Moncada S., Bayliss M. T. Induction of nitric oxide synthase in human chondrocytes. Biochem Biophys Res Commun. 1993 May 28;193(1):398–405. doi: 10.1006/bbrc.1993.1637. [DOI] [PubMed] [Google Scholar]
- Partridge N. C., Alcorn D., Michelangeli V. P., Ryan G., Martin T. J. Morphological and biochemical characterization of four clonal osteogenic sarcoma cell lines of rat origin. Cancer Res. 1983 Sep;43(9):4308–4314. [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33(4):349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- Scheven B. A., Kawilarang-De Haas E. W., Wassenaar A. M., Nijweide P. J. Differentiation kinetics of osteoclasts in the periosteum of embryonic bones in vivo and in vitro. Anat Rec. 1986 Apr;214(4):418–423. doi: 10.1002/ar.1092140413. [DOI] [PubMed] [Google Scholar]
- Stadler J., Stefanovic-Racic M., Billiar T. R., Curran R. D., McIntyre L. A., Georgescu H. I., Simmons R. L., Evans C. H. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991 Dec 1;147(11):3915–3920. [PubMed] [Google Scholar]
- Stolzenburg T., Binz H., Fontana A., Felder M., Wagenhaeuser F. J. Impaired mitogen-induced interferon-gamma production in rheumatoid arthritis and related diseases. Scand J Immunol. 1988 Jan;27(1):73–81. doi: 10.1111/j.1365-3083.1988.tb02324.x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Felmayer G., Werner E. R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Tetrahydrobiopterin-dependent formation of nitrite and nitrate in murine fibroblasts. J Exp Med. 1990 Dec 1;172(6):1599–1607. doi: 10.1084/jem.172.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]