Abstract
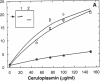
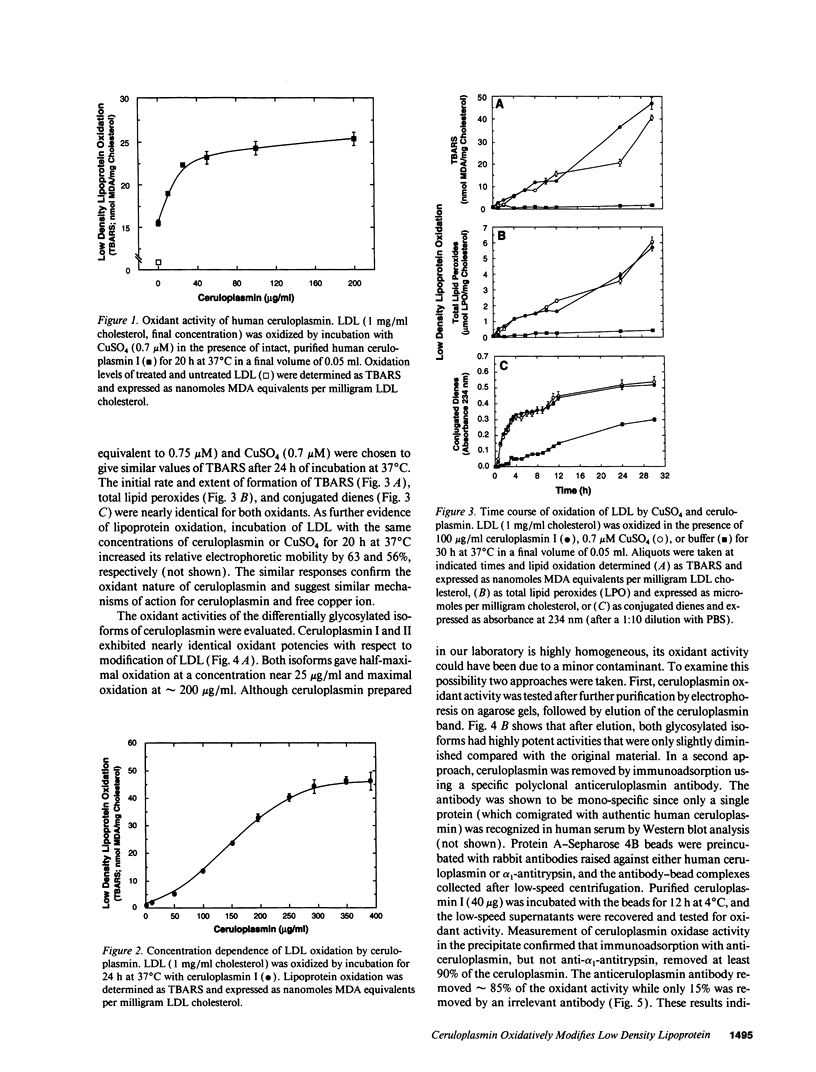
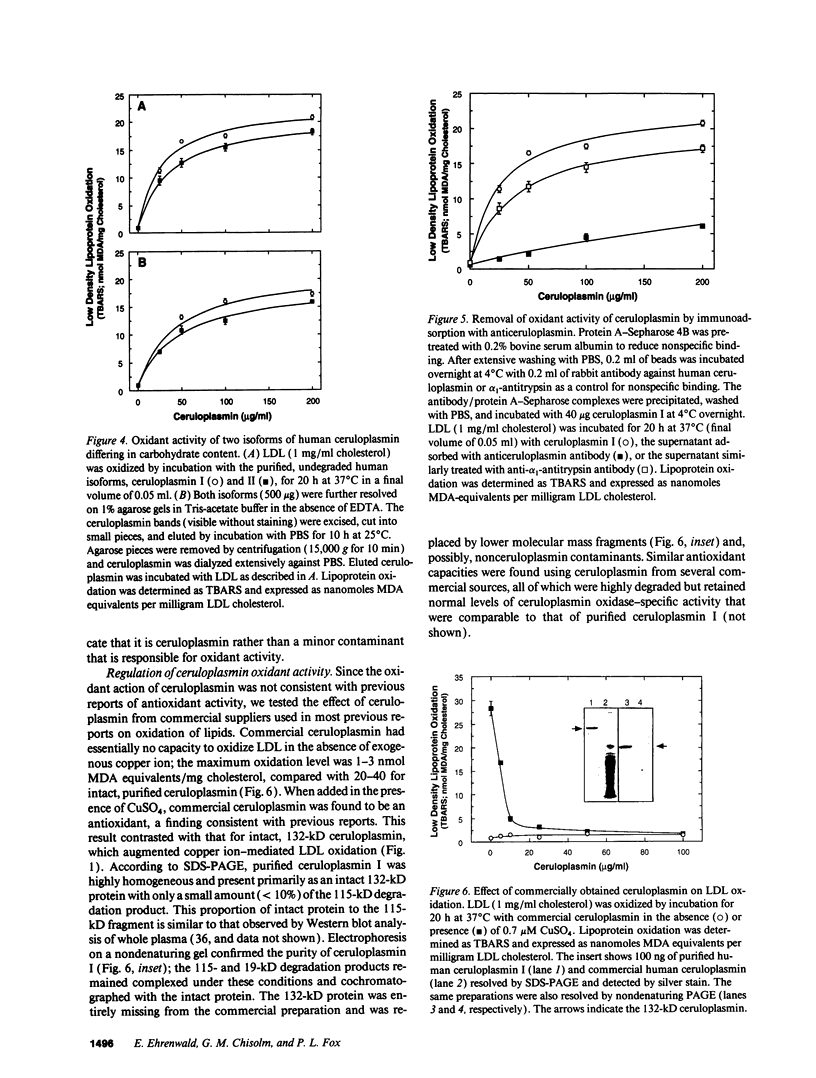
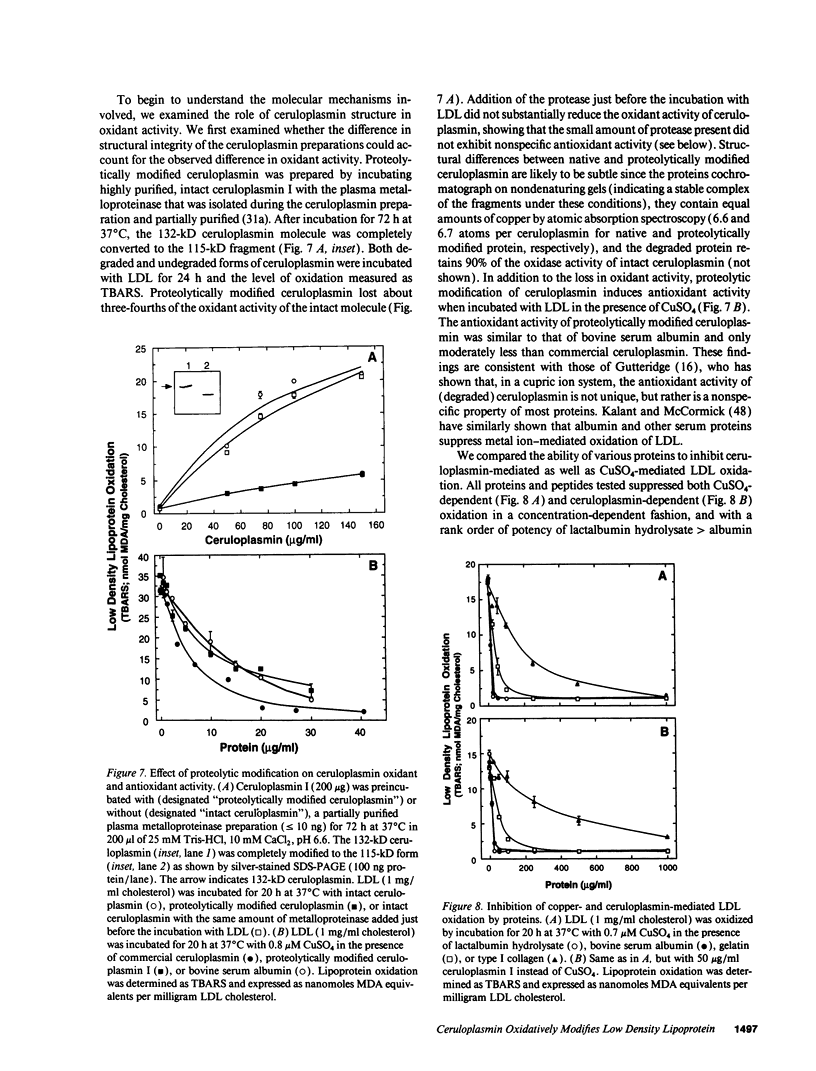
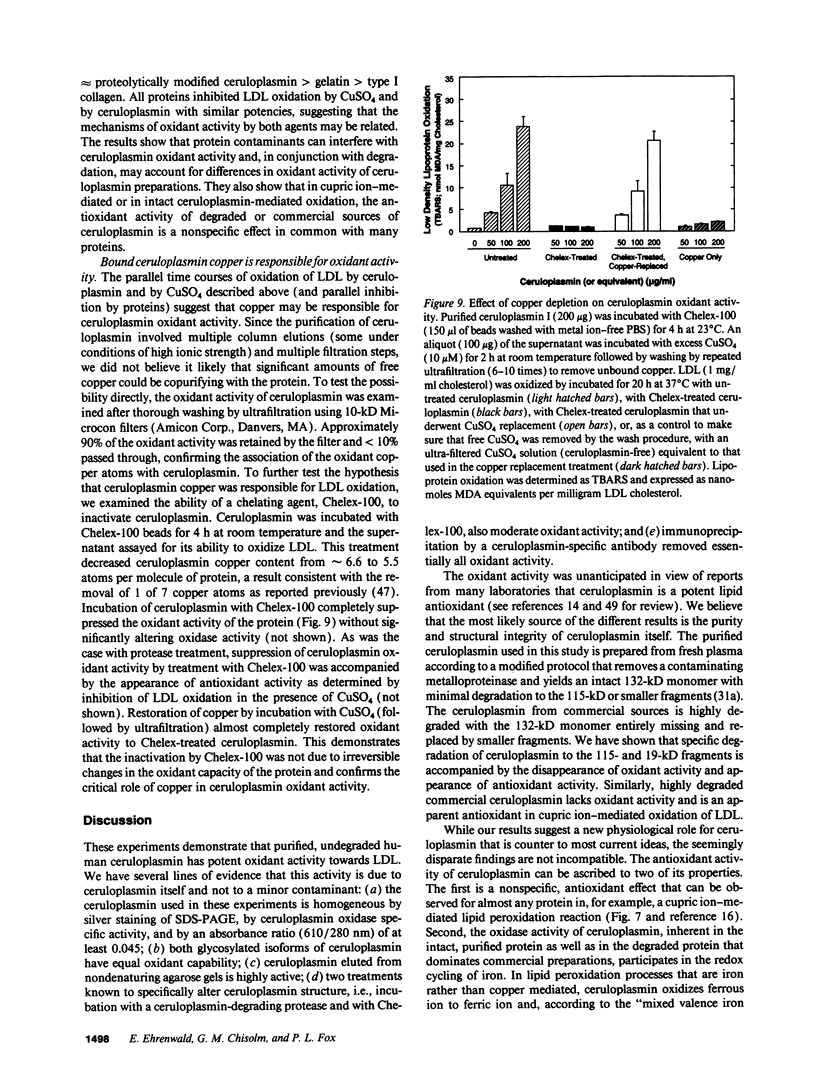
Ceruloplasmin is a plasma protein that carries most of the copper found in the blood. Although its elevation after inflammation and trauma has led to its classification as an acute phase protein, its physiological role is uncertain. A frequently reported activity of ceruloplasmin is its ability to suppress oxidation of lipids. In light of the intense recent interest in the oxidation of plasma LDL, we investigated the effects of ceruloplasmin on the oxidation of this lipoprotein. In contrast to our expectations, highly purified, undegraded human ceruloplasmin enhanced rather than suppressed copper ion-mediated oxidation of LDL. Ceruloplasmin increased the oxidative modification of LDL as measured by thiobarbituric acid-reacting substances by at least 25-fold in 20 h, and increased electrophoretic mobility, conjugated dienes, and total lipid peroxides. In contrast, ceruloplasmin that was degraded to a complex containing 115- and 19-kD fragments inhibited cupric ion oxidation of LDL, as did commercial preparations, which were also degraded. However, the antioxidant capability of degraded ceruloplasmin in this system was similar to that of other proteins, including albumin. The copper in ceruloplasmin responsible for oxidant activity was not removed by ultrafiltration, indicating a tight association. Treatment of ceruloplasmin with Chelex-100 removed one of seven copper atoms per molecule and completely blocked oxidant activity. Restoration of the copper to ceruloplasmin also restored oxidant activity. These data indicate that ceruloplasmin, depending on the integrity of its structure and its bound copper, can exert a potent oxidant rather than antioxidant action on LDL. Our results invite speculation that ceruloplasmin may be in part responsible for oxidation of LDL in blood or in the arterial wall and may thus have a physiological role that is quite distinct from what is commonly believed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Timimi D. J., Dormandy T. L. The inhibition of lipid autoxidation by human caeruloplasmin. Biochem J. 1977 Nov 15;168(2):283–288. doi: 10.1042/bj1680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcain F., Löw H., Crane F. L. Ceruloplasmin stimulates thymidine incorporation by CCL-39 cells in the absence of serum or growth factors. Biochem Biophys Res Commun. 1991 Oct 31;180(2):790–796. doi: 10.1016/s0006-291x(05)81134-9. [DOI] [PubMed] [Google Scholar]
- Alessandri G., Raju K., Gullino P. M. Mobilization of capillary endothelium in vitro induced by effectors of angiogenesis in vivo. Cancer Res. 1983 Apr;43(4):1790–1797. [PubMed] [Google Scholar]
- Avogaro P., Bon G. B., Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis. 1988 Jan-Feb;8(1):79–87. [PubMed] [Google Scholar]
- BROMAN L. Separation and characterization of two coeruloplasmins from human serum. Nature. 1958 Dec 13;182(4650):1655–1657. doi: 10.1038/1821655a0. [DOI] [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Eaton J. W., Belcher J. D., Vercellotti G. M. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb. 1991 Nov-Dec;11(6):1700–1711. doi: 10.1161/01.atv.11.6.1700. [DOI] [PubMed] [Google Scholar]
- Belch J. J., Chopra M., Hutchison S., Lorimer R., Sturrock R. D., Forbes C. D., Smith W. E. Free radical pathology in chronic arterial disease. Free Radic Biol Med. 1989;6(4):375–378. doi: 10.1016/0891-5849(89)90082-8. [DOI] [PubMed] [Google Scholar]
- Bowry V. W., Stanley K. K., Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Morel D. W., Chisolm G. M., 3rd Superoxide anion participation in human monocyte-mediated oxidation of low-density lipoprotein and conversion of low-density lipoprotein to a cytotoxin. J Immunol. 1989 Mar 15;142(6):1963–1969. [PubMed] [Google Scholar]
- Chisolm G. M., Irwin K. C., Penn M. S. Lipoprotein oxidation and lipoprotein-induced cell injury in diabetes. Diabetes. 1992 Oct;41 (Suppl 2):61–66. doi: 10.2337/diab.41.2.s61. [DOI] [PubMed] [Google Scholar]
- Church W. R., Jernigan R. L., Toole J., Hewick R. M., Knopf J., Knutson G. J., Nesheim M. E., Mann K. G., Fass D. N. Coagulation factors V and VIII and ceruloplasmin constitute a family of structurally related proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6934–6937. doi: 10.1073/pnas.81.22.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormandy T. L. Caeruloplasmin: acute-phase antioxidant. Agents Actions Suppl. 1981;8:185–197. [PubMed] [Google Scholar]
- Esterbauer H., Striegl G., Puhl H., Rotheneder M. Continuous monitoring of in vitro oxidation of human low density lipoprotein. Free Radic Res Commun. 1989;6(1):67–75. doi: 10.3109/10715768909073429. [DOI] [PubMed] [Google Scholar]
- Fleming R. E., Whitman I. P., Gitlin J. D. Induction of ceruloplasmin gene expression in rat lung during inflammation and hyperoxia. Am J Physiol. 1991 Feb;260(2 Pt 1):L68–L74. doi: 10.1152/ajplung.1991.260.2.L68. [DOI] [PubMed] [Google Scholar]
- Fox P. L., Chisolm G. M., DiCorleto P. E. Lipoprotein-mediated inhibition of endothelial cell production of platelet-derived growth factor-like protein depends on free radical lipid peroxidation. J Biol Chem. 1987 May 5;262(13):6046–6054. [PubMed] [Google Scholar]
- Frieden E. Perspectives on copper biochemistry. Clin Physiol Biochem. 1986;4(1):11–19. [PubMed] [Google Scholar]
- Goldstein I. M., Kaplan H. B., Edelson H. S., Weissmann G. Ceruloplasmin. A scavenger of superoxide anion radicals. J Biol Chem. 1979 May 25;254(10):4040–4045. [PubMed] [Google Scholar]
- Gutteridge J. M. Antioxidant properties of caeruloplasmin towards iron- and copper-dependent oxygen radical formation. FEBS Lett. 1983 Jun 27;157(1):37–40. doi: 10.1016/0014-5793(83)81111-9. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Inhibition of the Fenton reaction by the protein caeruloplasmin and other copper complexes. Assessment of ferroxidase and radical scavenging activities. Chem Biol Interact. 1985 Dec 17;56(1):113–120. doi: 10.1016/0009-2797(85)90043-2. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J. Caeruloplasmin: physiological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14(4):257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- Hallbök T., Hedelin H. Changes in serum copper and serum ceruloplasmin concentration induced by surgical trauma. Acta Chir Scand. 1980;146(6):371–373. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Suzuki L. A., Chait A. The role of sulfur-containing amino acids in superoxide production and modification of low density lipoprotein by arterial smooth muscle cells. J Biol Chem. 1987 Jul 25;262(21):10098–10103. [PubMed] [Google Scholar]
- Huber C. T., Frieden E. Substrate activation and the kinetics of ferroxidase. J Biol Chem. 1970 Aug 10;245(15):3973–3978. [PubMed] [Google Scholar]
- Kalant N., McCormick S. Inhibition by serum components of oxidation and collagen-binding of low-density lipoprotein. Biochim Biophys Acta. 1992 Oct 30;1128(2-3):211–219. doi: 10.1016/0005-2760(92)90310-r. [DOI] [PubMed] [Google Scholar]
- Kataoka M., Tavassoli M. The role of liver endothelium in the binding and uptake of ceruloplasmin: studies with colloidal gold probe. J Ultrastruct Res. 1985 Feb;90(2):194–202. doi: 10.1016/0889-1605(85)90109-0. [DOI] [PubMed] [Google Scholar]
- Kingston I. B., Kingston B. L., Putnam F. W. Chemical evidence that proteolytic cleavage causes the heterogeneity present in human ceruloplasmin preparations. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5377–5381. doi: 10.1073/pnas.74.12.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Bactericidal effect of Fe2+, ceruloplasmin, and phosphate. Arch Biochem Biophys. 1992 Jun;295(2):302–308. doi: 10.1016/0003-9861(92)90522-x. [DOI] [PubMed] [Google Scholar]
- Kok F. J., Van Duijn C. M., Hofman A., Van der Voet G. B., De Wolff F. A., Paays C. H., Valkenburg H. A. Serum copper and zinc and the risk of death from cancer and cardiovascular disease. Am J Epidemiol. 1988 Aug;128(2):352–359. doi: 10.1093/oxfordjournals.aje.a114975. [DOI] [PubMed] [Google Scholar]
- Kosugi K., Morel D. W., DiCorleto P. E., Chisolm G. M. Toxicity of oxidized low-density lipoprotein to cultured fibroblasts is selective for S phase of the cell cycle. J Cell Physiol. 1987 Mar;130(3):311–320. doi: 10.1002/jcp.1041300302. [DOI] [PubMed] [Google Scholar]
- Krsek-Staples J. A., Webster R. O. Ceruloplasmin inhibits carbonyl formation in endogenous cell proteins. Free Radic Biol Med. 1993 Feb;14(2):115–125. doi: 10.1016/0891-5849(93)90002-c. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lykens M. G., Davis W. B., Pacht E. R. Antioxidant activity of bronchoalveolar lavage fluid in the adult respiratory distress syndrome. Am J Physiol. 1992 Feb;262(2 Pt 1):L169–L175. doi: 10.1152/ajplung.1992.262.2.L169. [DOI] [PubMed] [Google Scholar]
- Løvstad R. A. The protective action of ceruloplasmin on copper ion stimulated lysis of rat erythrocytes. Int J Biochem. 1982;14(7):585–589. doi: 10.1016/0020-711x(82)90041-6. [DOI] [PubMed] [Google Scholar]
- Matsuda I., Pearson T., Holtzman N. A. Determination of apoceruloplasmin by radioimmunoassay in nutritional copper deficiency, Menkes' kinky hair syndrome, Wilson's disease, and umbilical cord blood. Pediatr Res. 1974 Oct;8(10):821–824. doi: 10.1203/00006450-197410000-00001. [DOI] [PubMed] [Google Scholar]
- McNally A. K., Chisolm G. M., 3rd, Morel D. W., Cathcart M. K. Activated human monocytes oxidize low-density lipoprotein by a lipoxygenase-dependent pathway. J Immunol. 1990 Jul 1;145(1):254–259. [PubMed] [Google Scholar]
- Morel D. W., Chisolm G. M. Antioxidant treatment of diabetic rats inhibits lipoprotein oxidation and cytotoxicity. J Lipid Res. 1989 Dec;30(12):1827–1834. [PubMed] [Google Scholar]
- Morel D. W., DiCorleto P. E., Chisolm G. M. Endothelial and smooth muscle cells alter low density lipoprotein in vitro by free radical oxidation. Arteriosclerosis. 1984 Jul-Aug;4(4):357–364. doi: 10.1161/01.atv.4.4.357. [DOI] [PubMed] [Google Scholar]
- Musci G., Bonaccorsi di Patti M. C., Fagiolo U., Calabrese L. Age-related changes in human ceruloplasmin. Evidence for oxidative modifications. J Biol Chem. 1993 Jun 25;268(18):13388–13395. [PubMed] [Google Scholar]
- Nakano H., Ogita K., Gutteridge J. M., Nakano M. Inhibition by the protein ceruloplasmin of lipid peroxidation stimulated by an Fe3+-ADP-adriamycin complex. FEBS Lett. 1984 Jan 30;166(2):232–236. doi: 10.1016/0014-5793(84)80086-1. [DOI] [PubMed] [Google Scholar]
- Noyer M., Dwulet F. E., Hao Y. L., Putnam F. W. Purification and characterization of undegraded human ceruloplasmin. Anal Biochem. 1980 Mar 1;102(2):450–458. doi: 10.1016/0003-2697(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Ortel T. L., Takahashi N., Putnam F. W. Structural model of human ceruloplasmin based on internal triplication, hydrophilic/hydrophobic character, and secondary structure of domains. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4761–4765. doi: 10.1073/pnas.81.15.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reunanen A., Knekt P., Aaran R. K. Serum ceruloplasmin level and the risk of myocardial infarction and stroke. Am J Epidemiol. 1992 Nov 1;136(9):1082–1090. doi: 10.1093/oxfordjournals.aje.a116573. [DOI] [PubMed] [Google Scholar]
- Rydén L., Björk I. Reinvestigation of some physicochemical and chemical properties of human ceruloplasmin (ferroxidase). Biochemistry. 1976 Aug 10;15(16):3411–3417. doi: 10.1021/bi00661a003. [DOI] [PubMed] [Google Scholar]
- Salmon S., Maziere C., Theron L., Beucler I., Ayrault-Jarrier M., Goldstein S., Polonovski J. Immunological detection of low-density lipoproteins modified by malondialdehyde in vitro or in vivo. Biochim Biophys Acta. 1987 Aug 15;920(3):215–220. doi: 10.1016/0005-2760(87)90097-x. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Salonen R., Korpela H., Suntioinen S., Tuomilehto J. Serum copper and the risk of acute myocardial infarction: a prospective population study in men in eastern Finland. Am J Epidemiol. 1991 Aug 1;134(3):268–276. doi: 10.1093/oxfordjournals.aje.a116080. [DOI] [PubMed] [Google Scholar]
- Samokyszyn V. M., Miller D. M., Reif D. W., Aust S. D. Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J Biol Chem. 1989 Jan 5;264(1):21–26. [PubMed] [Google Scholar]
- Sato M., Gitlin J. D. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J Biol Chem. 1991 Mar 15;266(8):5128–5134. [PubMed] [Google Scholar]
- Sato M., Schilsky M. L., Stockert R. J., Morell A. G., Sternlieb I. Detection of multiple forms of human ceruloplasmin. A novel Mr 200,000 form. J Biol Chem. 1990 Feb 15;265(5):2533–2537. [PubMed] [Google Scholar]
- Schilsky M. L., Stockert R. J., Pollard J. W. Caeruloplasmin biosynthesis by the human uterus. Biochem J. 1992 Dec 1;288(Pt 2):657–661. doi: 10.1042/bj2880657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh J., Fairclough G. F., Jr, Haschemeyer R. H. Oxygen-mediated heterogeneity of apo-low-density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3173–3177. doi: 10.1073/pnas.75.7.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M. J., Hennekens C. H., Manson J. E., Colditz G. A., Rosner B., Willett W. C. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993 May 20;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Antioxidants in the prevention of human atherosclerosis. Summary of the proceedings of a National Heart, Lung, and Blood Institute Workshop: September 5-6, 1991, Bethesda, Maryland. Circulation. 1992 Jun;85(6):2337–2344. doi: 10.1161/01.cir.85.6.2337. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M. D., DiSilvestro R. A., Harris E. D. Specific receptor for ceruloplasmin in membrane fragments from aortic and heart tissues. Biochemistry. 1984 Jan 17;23(2):261–266. doi: 10.1021/bi00297a014. [DOI] [PubMed] [Google Scholar]
- Stocks J., Gutteridge J. M., Sharp R. J., Dormandy T. L. The inhibition of lipid autoxidation by human serum and its relation to serum proteins and alpha-tocopherol. Clin Sci Mol Med. 1974 Sep;47(3):223–233. doi: 10.1042/cs0470223. [DOI] [PubMed] [Google Scholar]
- Sunderman F. W., Jr, Nomoto S. Measurement of human serum ceruloplasmin by its p-phenylenediamine oxidase activity. Clin Chem. 1970 Nov;16(11):903–910. [PubMed] [Google Scholar]
- Takahashi N., Ortel T. L., Putnam F. W. Single-chain structure of human ceruloplasmin: the complete amino acid sequence of the whole molecule. Proc Natl Acad Sci U S A. 1984 Jan;81(2):390–394. doi: 10.1073/pnas.81.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Berkel T. J., De Rijke Y. B., Kruijt J. K. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991 Feb 5;266(4):2282–2289. [PubMed] [Google Scholar]
- Vulpe C., Levinson B., Whitney S., Packman S., Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993 Jan;3(1):7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Yamashoji S., Kajimoto G. Antioxidant effect of caeruloplasmin on microsomal lipid peroxidation. FEBS Lett. 1983 Feb 21;152(2):168–170. doi: 10.1016/0014-5793(83)80371-8. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S. Macrophages and oxidized low density lipoproteins in the pathogenesis of atherosclerosis. Ann Med. 1991;23(5):561–567. doi: 10.3109/07853899109150518. [DOI] [PubMed] [Google Scholar]
- el-Saadani M., Esterbauer H., el-Sayed M., Goher M., Nassar A. Y., Jürgens G. A spectrophotometric assay for lipid peroxides in serum lipoproteins using a commercially available reagent. J Lipid Res. 1989 Apr;30(4):627–630. [PubMed] [Google Scholar]