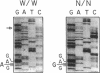
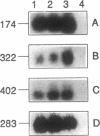
Abstract
The effect of nonsense mutations on mRNA levels is variable. The levels of some mRNAs are not affected and truncated proteins are produced, while the levels of others are severely decreased and null phenotypes are observed. The effect on mRNA levels is important for the understanding of phenotype-genotype association. Cystic fibrosis (CF) is a lethal autosomal recessive disease with variable clinical presentation. Recently, two CF patients with mild pulmonary disease carrying nonsense mutations (R553X, W1316X) were found to have severe deficiency of mRNA. In the Jewish Ashkenazi CF patient population, 60% of the chromosomes carry a nonsense mutation, W1282X. Patients homozygous for this mutation have severe disease presentation with variable pulmonary disease. The presence of CF transcripts in a group of patients homozygous and heterozygous for this mutation was studied by reverse transcriptase PCR of various regions of the gene. Subsequent hybridization to specific CF PCR probes and densitometry analysis indicated that the CF mRNA levels in patients homozygous for the W1282X mutation are not significantly decreased by the mutation. mRNA levels were compared for patients heterozygous for the W1282X mutation. The relative levels of mRNA with the W1282X, and the delta F508 or the normal alleles, were similar in each patient. These results indicate that the severe clinical phenotype of patients carrying the W1282X mutation is not due to a severe deficiency of mRNA. In addition, the severity, progression, and variability of the pulmonary disease are affected by other, as yet unknown factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwater J. A., Wisdom R., Verma I. M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Atweh G. F., Brickner H. E., Zhu X. X., Kazazian H. H., Jr, Forget B. G. New amber mutation in a beta-thalassemic gene with nonmeasurable levels of mutant messenger RNA in vivo. J Clin Invest. 1988 Aug;82(2):557–561. doi: 10.1172/JCI113632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga S. J., Benz E. J., Jr Nonsense mutations in the human beta-globin gene affect mRNA metabolism. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2056–2060. doi: 10.1073/pnas.85.7.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkley G., Harris A. Lymphocyte mRNA as a resource for detection of mutations and polymorphisms in the CF gene. J Med Genet. 1991 Nov;28(11):777–780. doi: 10.1136/jmg.28.11.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz H. C., Valle D., Francomano C. A., Kendzior R. J., Jr, Pyeritz R. E., Cutting G. R. The skipping of constitutive exons in vivo induced by nonsense mutations. Science. 1993 Jan 29;259(5095):680–683. doi: 10.1126/science.8430317. [DOI] [PubMed] [Google Scholar]
- Fojo S. S., Lohse P., Parrott C., Baggio G., Gabelli C., Thomas F., Hoffman J., Brewer H. B., Jr A nonsense mutation in the apolipoprotein C-IIPadova gene in a patient with apolipoprotein C-II deficiency. J Clin Invest. 1989 Oct;84(4):1215–1219. doi: 10.1172/JCI114287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globerman H., Amor M., Parker K. L., New M. I., White P. C. Nonsense mutation causing steroid 21-hydroxylase deficiency. J Clin Invest. 1988 Jul;82(1):139–144. doi: 10.1172/JCI113562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamosh A., Rosenstein B. J., Cutting G. R. CFTR nonsense mutations G542X and W1282X associated with severe reduction of CFTR mRNA in nasal epithelial cells. Hum Mol Genet. 1992 Oct;1(7):542–544. doi: 10.1093/hmg/1.7.542. [DOI] [PubMed] [Google Scholar]
- Hamosh A., Trapnell B. C., Zeitlin P. L., Montrose-Rafizadeh C., Rosenstein B. J., Crystal R. G., Cutting G. R. Severe deficiency of cystic fibrosis transmembrane conductance regulator messenger RNA carrying nonsense mutations R553X and W1316X in respiratory epithelial cells of patients with cystic fibrosis. J Clin Invest. 1991 Dec;88(6):1880–1885. doi: 10.1172/JCI115510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Ley T. J., Anagnou N. P., Baur A. W., Nienhuis A. W. Beta O-39 thalassemia gene: a premature termination codon causes beta-mRNA deficiency without affecting cytoplasmic beta-mRNA stability. Blood. 1984 Jul;64(1):23–32. [PubMed] [Google Scholar]
- Kerem B. S., Buchanan J. A., Durie P., Corey M. L., Levison H., Rommens J. M., Buchwald M., Tsui L. C. DNA marker haplotype association with pancreatic sufficiency in cystic fibrosis. Am J Hum Genet. 1989 Jun;44(6):827–834. [PMC free article] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kerem E., Corey M., Kerem B. S., Rommens J., Markiewicz D., Levison H., Tsui L. C., Durie P. The relation between genotype and phenotype in cystic fibrosis--analysis of the most common mutation (delta F508). N Engl J Med. 1990 Nov 29;323(22):1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- Kristidis P., Bozon D., Corey M., Markiewicz D., Rommens J., Tsui L. C., Durie P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am J Hum Genet. 1992 Jun;50(6):1178–1184. [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Brown M. S., Davis C. G., Elhammer A., Russell D. W., Goldstein J. L. The Lebanese allele at the low density lipoprotein receptor locus. Nonsense mutation produces truncated receptor that is retained in endoplasmic reticulum. J Biol Chem. 1987 Jan 5;262(1):401–410. [PubMed] [Google Scholar]
- Losson R., Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Rachmilewitz E. A., Ross J. Unstable beta-globin mRNA in mRNA-deficient beta o thalassemia. Cell. 1981 Dec;27(3 Pt 2):543–553. doi: 10.1016/0092-8674(81)90396-2. [DOI] [PubMed] [Google Scholar]
- Matsuo M., Masumura T., Nishio H., Nakajima T., Kitoh Y., Takumi T., Koga J., Nakamura H. Exon skipping during splicing of dystrophin mRNA precursor due to an intraexon deletion in the dystrophin gene of Duchenne muscular dystrophy kobe. J Clin Invest. 1991 Jun;87(6):2127–2131. doi: 10.1172/JCI115244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschonas N., de Boer E., Grosveld F. G., Dahl H. H., Wright S., Shewmaker C. K., Flavell R. A. Structure and expression of a cloned beta o thalassaemic globin gene. Nucleic Acids Res. 1981 Sep 11;9(17):4391–4401. doi: 10.1093/nar/9.17.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Belasco J. G., Cohen S. N., von Gabain A. Effect of premature termination of translation on mRNA stability depends on the site of ribosome release. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4890–4894. doi: 10.1073/pnas.84.14.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goff S. C. Nonsense and frameshift mutations in beta 0-thalassemia detected in cloned beta-globin genes. J Biol Chem. 1981 Oct 10;256(19):9782–9784. [PubMed] [Google Scholar]
- Ramirez F., Starkman D., Bank A., Kerem H., Cividalli G., Rachmilewitz E. A. Absence of beta mRNA in beta0-thalassemia in Kurdish Jews. Blood. 1978 Oct;52(4):735–739. [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Santis G., Osborne L., Knight R. A., Hodson M. E. Linked marker haplotypes and the delta F508 mutation in adults with mild pulmonary disease and cystic fibrosis. Lancet. 1990 Jun 16;335(8703):1426–1429. doi: 10.1016/0140-6736(90)91448-j. [DOI] [PubMed] [Google Scholar]
- Shoshani T., Augarten A., Gazit E., Bashan N., Yahav Y., Rivlin Y., Tal A., Seret H., Yaar L., Kerem E. Association of a nonsense mutation (W1282X), the most common mutation in the Ashkenazi Jewish cystic fibrosis patients in Israel, with presentation of severe disease. Am J Hum Genet. 1992 Jan;50(1):222–228. [PMC free article] [PubMed] [Google Scholar]
- Takeshita K., Forget B. G., Scarpa A., Benz E. J., Jr Intranuclear defect in beta-globin mRNA accumulation due to a premature translation termination codon. Blood. 1984 Jul;64(1):13–22. [PubMed] [Google Scholar]
- Tsui L. C. The spectrum of cystic fibrosis mutations. Trends Genet. 1992 Nov;8(11):392–398. doi: 10.1016/0168-9525(92)90301-j. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu N., Kobayashi H., Miyatake T., Tsuji S. A novel exon mutation in the human beta-hexosaminidase beta subunit gene affects 3' splice site selection. J Biol Chem. 1992 Feb 5;267(4):2406–2413. [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]