Abstract
Introduction
RTOG 97-14 concluded a single fraction of radiation was as effective in relieving pain as multiple fractions in the treatment of patients with bone metastases. A statistically significant higher re-treatment rate, however, was noted in patients undergoing a single fraction treatment. The purpose of the analysis was to determine if multiple fraction treatment is cost-effective in treating patients with bone metastasis by preventing further re-treatment.
Methods & Material
A Markov model was used to evaluate the cost-effectiveness of 30 Gy in 10 fractions compared to 8 Gy in 1 fraction. Transition probabilities, cost and utilities were obtained from the clinical trial. Costs and outcomes were not discounted because of the short-time line for the study.
Results
The expected mean cost and quality adjusted survival in months for patients receiving 8 Gy in 1 fraction and 30 Gy in 10 fractions was $998 and 7.26 months and $2,316 and 9.53 months, respectively. The incremental cost-effectiveness ratio (ICER) was $6973/quality-adjusted life year. The results were sensitive to the utility of the post-treatment state for both single and multiple fraction treatment.
Conclusion
Single fraction treatment was the less expensive treatment in the treatment of patients with bone metastasis treated on RTOG 97-14.
Keywords: Cost-effectiveness, Radiation, Bone Metastases
Introduction
A single fraction of radiation is as effective as multiple fractions in the palliation of painful bone metastasis.(1-5) Re-treatment rates, however, are higher in patients undergoing a single fraction but overall pain control is similar between groups. Radiation Therapy Oncology Group (RTOG) 97-14 evaluated the ability of a single fraction of radiation therapy to relieve painful bone metastases from breast and prostate cancer compared to multiple fractions.(6) Three-year updated results have confirmed the earlier report of similar overall pain relief between fractionation schedules but with higher re-treatment rates in the 8 Gy fraction treatment,18% re-treatment rate, compared to 9% for patients treated with 30 Gy.(7)
Single fraction treatment is less expensive and time consuming for patients and families compared to multiple fraction treatment. But would the higher re-treatment rate experienced by patients counter the initial cost savings? Single fraction treatment resulted in comparable palliation and quality of life with lower medical and societal costs compared to multiple fraction treatment in a subset of 166 patients treated on the Dutch Bone Metastasis Study in The Netherlands.(8) This study randomized patients to either a single fraction of 8Gy or 6 fractions of 4 Gy.(1) The decision for retreatment was at the discretion of the physician and was found to be higher in the single fraction arm of the Dutch Bone Metastasis Study. Reasons given for higher rates of retreatment in the single fraction arm included less concern over radiation tolerance of adjacent normal structures.
The specific aim of this study was to perform an economic analysis comparing 8 Gy in 1 fraction to 30 Gy in 10 fractions using a Markov model using data from RTOG 97-14. We hypothesize that, although more costly initially, multiple fraction radiation treatments will be cost-effective within the range of acceptability of $50,000/QALY.
Methods and Materials
A Markov model was developed and informed with data from RTOG 97-14. The health states and transition probabilities for this clinical scenario have been previously published.(9) The Markov model was created for patients to spend one month in each state before transitioning to the next allowable state. The transition states differed by treatment and the Markov termination condition was 36 months. Monthly transition probability estimates for state transitions, assuming constant rates, were calculated from rates obtained from RTOG 97-14 by the following equation; Monthly rate= [-ln (1-P)/n] where P is the probability of the occurrence of interest and n is the number of months the rate is measured.(7, 10) The monthly probability of the event is calculated using the following formula: Monthly probability= 1-exp(-Monthly rate). Treeage Pro 2006, Healthcare decision analysis software (TreeAge Software, Inc. Williamstown, MA) was used to analyze the Markov Model. Sensitivity analyses were performed on the cost, transition probabilities, and utility values.
The occurrence of pain and the need for re-treatment were assumed to be at the original site of treatment. Only the results of palliative treatment were included in the generation of the model.
Single and Multi-fraction External Beam Radiation Therapy Model
The models for SFX and MFX were the same except for the initial cost of the treatment and are depicted in Figures 1 and 2. Upon entering the model at either SFX or MFX patients could either die or be alive. Death was the absorbing state in all models. If the patients were alive they either had pain relief or had pain and were retreated with the same fractionation schedule. Patients having no pain after the initial treatment entered No Pain 1 state where they could either remain pain free or have pain and be retreated. Patients being retreated could become pain free and enter No Pain 2 state or still have pain and enter Pain Meds staying until death. Patients in No Pain 2 could remain pain free or have return of pain and entered the Pain Meds state.
Figure 1.
This figure depicts the flow of patients in the model for a single fraction treatment. Patients start out at SRT or single radiation treatment and then move through the model as determined by the transition probabilities. Each cyle through the model was 1 month in length.
Figure 2.
This figure depicts the flow of patients in the model for multiple fractions. Patients start out in MRT or multiple radiation treatments and move through the model as determined by the transitions probabilities.
Radiotherapy Cost
Costs were estimated based upon usual and reasonable consumptions of resources for treatment of bone metastases. Global fees were used to account for both the technical aspect of treatment, such as the radiation treatment, and the professional or physician work aspect of treatment. A simple level of complexity was assumed for treatment planning, simulation, and treatment. No treatment devices were used. Table 1 outlines the Current Procedural Terminology (CPT) codes used to derive the unit costs were then matched to the Resource Value Units (RVU’s). The RVU’s were multiplied by the frequency of use, totaled and multiplied by the 2008 national Medicare conversion factor of $38.087/RVU to arrive at a total cost in dollars. Visits to the doctor and other health care professionals were assumed to be equal across all treatment arms and were not included in the incremental analysis. The modeled costs were sampled using a range of costs listed in Table 2. Costs were calculated from a payer’s perspective using Medicare as the model. The costs would have been different had a different perspective been adopted for the trial. Time away from home, travel costs and lost productivity costs would have added additional costs to treatment had a patient perspective been utilized. Treatment cost, however, in this model may have been non-existent if the patient had insurance which covered the treatment as only patient out-of-pocket expenses are covered in this model.
Table 1.
Radiation Resource Use Calculation
| CPT | Frequency | Single Fraction |
Multi-fraction | |
|---|---|---|---|---|
| Treatment Plan | 77263 | 1 | 4.33 | 4.33 |
| Simple Simulation |
77280 | 1 | 5.35 | 5.35 |
| Basic Calculation |
77300 | 1 | 1.9 | 1.9 |
| Port Films | 77417 | 1 | ..4 | .8 |
| Treatments | 77416 | 1 | 7.86 | 78.6 |
| Weekly Management |
77427 | 2 | 10.52 | |
| Single Management |
77431 | 1 | 2.68 | |
| Weekly Physics | 77336 | 1 | 1.3 | 2.6 (×2) |
| Total RVU’s | 23.82 | 104.1 | ||
| Total Cost ($) | $907 | $3965 |
RVU’s-Relative Value Units CPT-Current Procedural Terminology
Table 2.
Values used and tested in sensitivity analysis
| Variable | Value used in Mode | Value used in Sensitivity Analysis |
|---|---|---|
| Cost of Single Fraction Radiation ( From unit cost estimate) |
$907 | $500-1250 |
| Cost of Multiple Fraction Radiation (From unit cost estimate) |
$3965 | $1250-4000 |
| Cost of Single Fraction Radiation (From trial participates) |
$887 | $570-1224 |
| Cost of Multiple Fraction Radiation (From trial participates) |
$2206 | $1703-2516 |
| Cost of Pain Medication | $200 | $100-400 |
| Utility of No Pain Multiple Fraction Radiation |
.56 | .2-.8 |
| Utility of No Pain Single Fraction Radiation |
.51 | .2-.8 |
| Utility of Pain Meds | .2 | .2-.8 |
| Probability of 3 year survival Multiple Fraction Radiation |
16% | 0-99% |
| Probability of 3 year survival Single Fraction Radiation |
9% | 0-99% |
| Probability of Re-treatment at 3-years Multiple Fraction Radiation |
9% | 0-99% |
| Probability of Re-treatment at 3-years Single Fraction Radiation |
18% | 0-99% |
RVU utilization was available for the initial treatment from patients treated at a minority of institutions that volunteered to collect these data. The cost of care was calculated by totaling the RVU’s for the primary treatment and multiplying by the above conversion factor. Thirty-four patients had RVU data collected, 17 from each arm. Five patients in the multiple fraction arm were excluded because they had missing data; treatment management codes, etc, leaving 12 eligible and analyzable patients. One patient received only 1 fraction and was excluded as well. Six patients in the single fraction arm had missing data or received either 10 or 5 radiotherapy fractions and were excluded from the analysis leaving 11 eligible and analyzable patients.
The mean cost of treatment for patients receiving a single fraction for actual trial participants was $1381 (range:682-4271; std. dev. $1001). The mean cost of treatment for participants receiving multiple treatments was $3493 (range:1,316-4657; std. dev.1065).
The incremental cost-effectiveness ratio was calculated by comparing 8 Gy in 1 fraction as the standard treatment to 30 Gy in 10 fractions as the experimental treatment.
Utilities
Utilities for each transition state were obtained from utilities collected from patients randomized on 97-14 and from van den Hout et al (8). Utilities were collected with the Health Utilities Index III and were .43 prior to the initiation of the trial and .56 and .51 3 months after completing radiation for patients receiving 30 Gy and 8 Gy respectively. The utility for the re-treatment state was assumed to be less than the initial utility of treatment (0.5) and the utility for patients receiving pain medications after failing re-treatment was estimated at 0.2. A sensitivity analysis was used to test the utility assumptions.
Transition Probabilities
Overall survival at 3 years obtained from the trial was 5% and 17% for male and female patients receiving 8 Gy and 10% and 23% respectively for male and female patients receiving 30 Gy. The decision for retreatment was determined by the physician. Retreatment rates were 9% for the multi-fraction group and 18% for the single fraction group despite equal rates of analgesic use, totaling 68% and 67%, respectively, 3 months after completion of radiation therapy.(7)
Results
The expected mean cost for patients receiving 8 Gy was $996.77 with a quality-adjusted survival of 7.26 months. The expected mean cost for patients receiving 30 Gy was $2,315.82 with a quality-adjusted survival of 9.53 months. The incremental cost-effectiveness ratio (ICER) was $6972.95/Quality-adjusted life year (QALY).
Sensitivity analysis was performed testing the impact of the treatment assumptions, both cost and effect, on the overall outcome. Only post treatment utility had an effect on which treatment was the preferred. The 30 Gy in 10 fractions was favored over single fraction treatment as long as the utility for the post-treatment state was > 0.43. That is, the longer treatment course is associated with a higher quality-adjusted life expectancy as long as the post-treatment quality of life is improved over the pre-treatment quality of life (the pre-trial utility is 0.43). The 8Gy in 1 fraction arm would be favored if the utility for the post-treatment state was >0.67. In other words, the post-treatment utility after single fraction treatment would need to be higher than that after multiple fraction treatment for it to become the preferred strategy. The cost-effective acceptability curve is depicted in Figure 3. The probability of being cost-effective at $4,166/quality adjusted life month is 100%. $4,166/quality adjusted life month is equivalent to $50,000/QALY which is the normally accepted standard of cost-effectiveness. There was no difference in the outcome when another sensitivity analysis was performed using cost as calculated using the Hospital Outpatient Prospective Payment System (HOPPS) Ambulatory Payment Classification (APC) system.
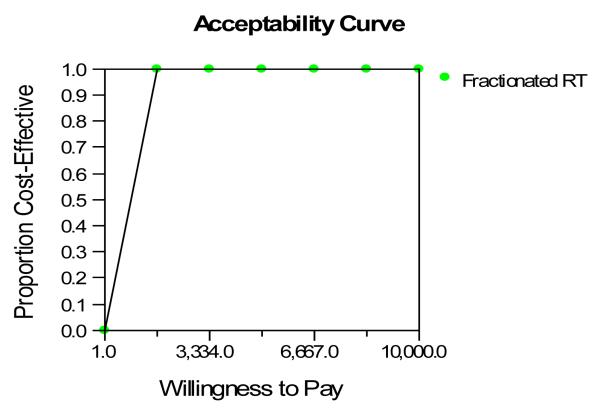
Figure 3.
This figure shows the cost-effective acceptability curve. The probability that multiple fractions of radiation would be cost-effective compared to a single fraction at $3,334/Quality-Adjusted Life Month was 100%.
Another way of reporting the results would be the cost in dollars per re-treatment avoided. Ten patients receiving 30 Gy in 10 fractions had re-treatment compared to 21 patients receiving 8 Gy in 1 fraction. The results in an incremental cost ratio of $131/re-treatment avoided. Health policy decision makers, however, cannot use this ratio, however, because it cannot be compared to other related health-care interventions like breast cancer screening or diabetes care.
The expected mean cost for patients was $1009 and $2322 for patients treated with 8 Gy and 30 Gy respectively when actual cost data was used with the same QALY for each treatment arm. This resulted in an ICER of $6,956/QALY.
Discussion
The results of this economic analysis found 30 Gy in 10 fractions was cost-effective compared to a single fraction because it prevented the need for additional re-treatment. There was however, a very small difference in quality of life, an approximate 2 month increase in quality-adjusted survival, with an approximate doubling of cost. The increase in marginal expected cost, however, was also very small at $1,319. The small difference in cost between single and multiple radiation fractionation schedules was also reported by van den Hout.(8) They reported only a small difference in cost of $873 favoring the single fraction arm. The difference was larger, $1,753, when viewed from a societal viewpoint. The method they used in calculating the radiotherapy cost, however, differed from our method.
Cost-benefit analysis, cost-effectiveness analysis, cost-utility analysis and cost-minimization analysis are the 4 different types of economic analyses. RTOG 97-14 was designed and powered as an equivalency trial with the primary endpoint being 1 fraction being the same as 10 in relieving pain. With the primary endpoint being equivalence and with the end-result no difference in pain control, a cost-minimization analysis would be the economic analysis of choice. In cost-minimization analysis, the treatment with the least cost would be the preferred treatment. But was there a difference between the two arms? The clinical result of 97-14 found no difference in pain control comparing 8 Gy in 1 fraction to 30 Gy in 10 fractions. Alternatively, the re-treatment rate was significantly higher in the single fraction arm resulting inthe ICER being very small, well below the usual threshold of $50,000/LY or QALY as commonly quoted and the probability of cost-effectiveness was 100%. Johnston et al. have recommended calculating cost-effectiveness ratios based upon final end points even in situations were non-significant differences exists to avoid publication bias with estimation of the uncertainty in the ICER.(11) In this situation, the ICER reveals a favorable determination of cost-effectiveness even though the clinical trial result is one of 8 Gy being the preferred treatment. This analysis also found the $/re-treatment avoided was also low but unfortunately this value cannot be compared to any other metric of cost-effectiveness as it is specific for this intervention and could not be readily compared to other health-care interventions.
Sensitivity analysis found only if the utility of patients in the post-treatment state receiving a single fraction of radiation was greater than 0.67 would multiple fraction treatment not be considered “cost-effective”. It is very hard to imagine a clinical situation were this would be possible. Unfortunately, utilities of patients in this trial were not collected past the 3 month period so we cannot look to patients in this trial to determine if this would be clinically realistic expectation. van den Hout reported the utilities of patients treated with either fraction of radiation did not increase over 0.6 in the post-treatment period in the Dutch Bone Metastasis Study, although they used a different method to determine utility.(8)
A number of clinical trials have been performed comparing a single fraction to various multiple fractions in the treatment of patients with metastatic bone disease. All have found a single fraction equivalent to the many multiple fraction schedules used in relieving pain. The re-treatment rates have been uniformly higher in the single fraction arm but the overall relief of pain has been equivalent regardless of the fractionation schedule. The decision for retreatment was determine by the physician in both the RTOG, the Dutch Bone Metastases Trial, and other clinical trials evaluating a single fraction of palliative radiation. Higher rates of retreatment in single fraction arms of these studies have often been related to physician concerns for normal tissue tolerance with retreatment after multifraction palliative radiation. This also may be the case in the RTOG trial since narcotic use at 3 months follow-up was equivalent for single and multifraction arms.
It could be argued then that a single fraction should be the standard of care given the lower cost, both initial and overall. We have found the use of multiple fractions, however, when required, is a cost-effective alternative to single fraction treatment. We have previously reported that un-partnered males receiving 8 Gy in 1 fraction had the same re-treatment rate compared to un-partnered males receiving 30 Gy in 10 fractions.(7) The re-treatment rate for all of the other gender/partner subgroups, partnered males and both females groups, receiving 8 Gy in 1 fraction was higher compared to 30 Gy in 10 fractions. It may be hypothesized un-partnered males may not have the same social support for additional treatment even though they may be in pain. Multiple radiation fractions would be considered cost effective in this patient subgroup if it were felt they would not return for additional treatment if only given one treatment. The opportunity cost in time and lost productivity of patients and family for multiple fractions of radiation, however, is often difficult to assess. This is particularly important since partnered males and females had higher rates of retreatment.
The Dutch Bone Metastasis Study Group performed an economic analysis on a subgroup of patients that completed a cost questionnaire. Eighteen percent of patients in the single fraction group required re-treatment compared to 5% in the multiple fraction group. A similar $/re-treatment avoidance analysis results in $87/retreatment avoided if only medical costs are included and $175/re-treatment avoided if societal costs are included. These numbers compare favorably to our analysis.
This study points to the difficulties adding an economic endpoint to a clinical trial. Outcome interpretation will become more difficult as treatments become more expensive with potentially small incremental gains in the primary endpoints. An example of this is the National Cancer Institute of Canada Clinical Trials Group comparison of Erlotinib plus gemcitabine to gemcitabine alone in patients with locally advanced unresectable or metastatic pancreatic cancer. Overall survival based upon an intention to treat analysis was significantly prolonged with the combination therapy arm, although the median survival was 6.24 months for the combination treatment compared to 5.91 months for the gemcitabine only arm, a difference of only .33 months.(12) The relatively small increase in survival comes at a high incremental cost. In the current study, there was no difference in the primary endpoint of pain relief with patients receiving a single fraction having a statistically higher re-treatment rate. They still, however, had a lower expected mean cost even including the re-treatment and increased pain medicine usage. Despite this, the economic analysis found the ICER to be in the range of cost-effectiveness and the $/re-treatment avoided was also very low.
Despite different economic methods used to analyze the respective clinical trials, this study confirms the results of the Dutch Bone Metastasis Study Group that single fraction radiation treatment is the most cost-effective in the treatment of bone metastasis. This study points to the difficulty that may be encountered in the future when economic analyses are incorporated or performed after the completion of clinical trials.
References
- 1.Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen OS, Bentzen SM, Sandberg E, et al. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol. 1998;47:233–240. doi: 10.1016/s0167-8140(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 3.Niewald M, Tkocz HJ, Abel U, et al. Rapid course radiation therapy vs. more standard treatment: a randomized trial for bone metastases. Int J Radiat Oncol Biol Phys. 1996;36:1085–1089. doi: 10.1016/s0360-3016(96)00388-4. [DOI] [PubMed] [Google Scholar]
- 4.Price P, Hoskin PJ, Easton D, et al. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol. 1986;6:247–255. doi: 10.1016/s0167-8140(86)80191-8. [DOI] [PubMed] [Google Scholar]
- 5.Rasmusson B, Vejborg I, Jensen AB, et al. Irradiation of bone metastases in breast cancer patients: a randomized study with 1 year follow-up. Radiother Oncol. 1995;34:179–184. doi: 10.1016/0167-8140(95)01520-q. [DOI] [PubMed] [Google Scholar]
- 6.Hartsell WF, Scott C, Bruner DW, et al. Phase III randomized trial of 8 Gy in 1 fraction vs. 30 Gy in 10 fractions for palliation of painful bone metastases: preliminary results of RTOG 97-14. Int J Radiat Oncol Biol Phys. 2003;57:S124. [Google Scholar]
- 7.Konski A, Desilvio M, Hartsell W, et al. Continuing evidence for poorer treatment outcomes for single male patients: Retreatment data from RTOG 97-14. Int J Radiat Oncol Biol Phys. 2006 doi: 10.1016/j.ijrobp.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95:222–229. doi: 10.1093/jnci/95.3.222. [DOI] [PubMed] [Google Scholar]
- 9.Konski A. Radiotherapy is a cost-effective palliative treatment for patients with bone metastasis from prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60:1373–1378. doi: 10.1016/j.ijrobp.2004.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 11.Johnston K, Gray A, Moher M, et al. Reporting the cost-effectiveness of interventions with nonsignificant effect differences: example from a trial of secondary prevention of coronary heart disease. Int J Technol Assess Health Care. 2003;19:476–489. doi: 10.1017/s0266462303000412. [DOI] [PubMed] [Google Scholar]
- 12.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]