Abstract
Purpose
Changes in health-related quality of life (HRQoL) were assessed in clinically obese, African-American adults after completion of a weight loss program that resulted in modest average weight loss.
Methods
Data were analyzed for 87 men and women who provided weight measurements after an initial 10-week weight loss program (Phase 1) and a subsequent clinical trial to evaluate three weight maintenance approaches (Phase 2) over an additional 8 to 18 months. HRQoL was assessed using the Short Form SF-36 questionnaire. Intra-person changes in HRQoL were assessed and analyzed for associations with weight change within each phase. Non-parametric bivariable analyses and multivariable linear regression were used in statistical analyses.
Results
Changes in HRQoL were modest; clinically significant intra-subject improvements in SF-36 domains of general health and vitality and in the mental component summary score were observed after Phase 1 but were attenuated during Phase 2. Improvements in vitality were significantly associated with greater weight loss in Phase 1, but no HRQoL change scores during Phase 2 were associated with weight change.
Conclusions
Short-term improvements in general health and vitality were observed. The vitality domain of the SF-36 appeared to be the domain of HRQoL most responsive to modest weight change.
Keywords: Health-related quality of life, Ethnic groups, Obesity, Health promotion, Weight loss, Intervention studies, African Americans
Introduction
The high prevalence of obesity, with its risks of medical comorbidities and premature mortality, is an important public health concern [1–5]. In multiple cross-sectional studies, obese persons report a lower health-related quality of life (HRQoL) than non-obese persons [6, 7]. Clinically significant weight loss, whether achieved by bariatric surgery, prescription drugs or lifestyle changes, is associated with improvements in HRQoL for obese individuals [8–12]. Lifestyle behavioral interventions have produced short-term improvements in HRQoL [11, 13, 14]. The long-term effects of such interventions are less clear, especially since the initial weight loss is often not sustained [12, 13]. In addition, whether improvements in HRQoL observed in lifestyle change programs are mediated primarily by the amount of weight loss is uncertain given that the amount of weight loss often does not correlate with the magnitude of HRQoL improvement [13].
The prevalence of obesity in African-Americans is higher than in whites, yet studies investigating the effects of obesity and its treatment in this population are few [15]. Available studies indicate that although lifestyle interventions are generally efficacious for African-Americans, weight losses in African-Americans are less than in their white counterparts [16–18]. HRQoL effects associated with minimal or modest weight loss may be of particular relevance to African-Americans.
We analyzed weight and HRQoL data for African-American women and men who completed the healthy eating and lifestyle program (HELP) study [19]. During the HELP study, we observed modest average weight loss that was well maintained regardless of weight maintenance strategies. A full description and main results of this study have been published elsewhere [19]. The primary study questions here relate to the size and direction of changes in HRQoL after the initial, intensive phase of the program and also after the subsequent, longer weight maintenance phase. Additionally, we sought to determine whether any observed changes in HRQoL were correlated with the amount of weight lost or with weight loss maintenance. We expected that participants with clinically significant weight loss during the intensive phase of the study and those who maintained their weight loss during the second phase would report the largest improvements in HRQoL.
Methods
Study design
The design and main results of the HELP study have been reported previously [19]. Briefly, African-Americans aged 25 to 70 with body mass index (BMI) between 30 and 50 kg/m2 were recruited through physician referrals and general advertisements. Participants were offered 10 weeks of weight control counseling classes (Phase 1) designed to help them begin the process of weight loss. Interested participants who completed Phase 1 were subsequently enrolled in a randomized trial that compared three approaches to weight maintenance (Phase 2) for an additional 8 to 18 months: continued group counseling classes; a self-help program facilitated by an outreach worker; or usual care (referral back to their primary care provider). Cultural adaptations included: recruiting only African-American participants to create homogeneous groups; having African-American intervention staff; using study materials (printed and video) with African-American actors, images and cultural references; featuring African-American ethnic foods; and including other content and activities designed to appeal to this audience on the basis of theory or published reports [19, 20].
Data collection procedures
Informed consent was obtained separately for Phases 1 and 2. All study procedures and protocols were approved by the University of Pennsylvania Institutional Review Board. Participants completed demographic, medical history, weight history and other health and behavior questionnaires before and after each phase.
HRQoL measurements
HRQoL was assessed using the Medical Outcomes Study Short Form Health Survey (SF-36), which was administered at the baseline visit, at the end of Phase 1 and at the end of Phase 2. The SF-36 is a 36-item, generic quality of life measure that assesses 8 domains: (1) physical functioning (PF); (2) role limitations due to physical health (RP); (3) bodily pain (BP); (4) general health perceptions (GH); (5) vitality (VT); (6) social functioning (SF); (7) role limitations due to emotional health problems (RE); and (8) mental health (MH) [21]. Each domain’s score can range from 0 to 100, with higher numbers always reflecting better function. The RP and RE domains can also be made into binary variables describing the presence (RP and RE score < 100) or absence (RP and RE score = 100) of any role limitations. These eight domains are weighted and summed to calculate the physical component summary (PCS) and the mental component summary (MCS) scores, which are standardized to a mean of 50, with scores above or below 50 representing better or worse status than the US population average, respectively [22].
Statistical methods
All statistical analyses were done with STATA/SE version 11 [23]. After preliminary analyses revealed no differences in weight change or HRQoL outcomes by randomization assignment, Phase 2 treatment groups were merged for these analyses. Only the participants who completed both phases of the study are included in these analyses. Baseline characteristics were summarized with descriptive statistics. Characteristics of participants who did or did not complete each phase were analyzed using parametric or non-parametric tests where appropriate to explore potential selection biases influencing the composition of the analysis sample relative to those who enrolled initially or at the beginning of Phase 2. The SF-36 domain scores and summary scores were explored graphically, and the normality assumption was assessed via the Shapiro–Wilk test. Medians and interquartile ranges of the baseline SF-36 domains were compared with those of the US population norms. The 95 percent (%) confidence intervals (CI) around the median of each SF-36 domain were calculated using one thousand bootstrap samples with replacement via the normal approximation method [24]; the US population medians were compared with the calculated 95% CI.
The weight change for each phase was calculated as the post-intervention weights minus pre-intervention weights. A binary variable to reflect clinically significant weight loss was created describing those who did or did not lose ≥5% of their body weight during the particular phase of the study [25]. Weight maintainers in Phase 2 were defined as participants who either lost weight or gained ≤+3% during this phase [26].
The change in HRQoL was assessed by calculating the absolute change (post-pre differences) in SF-36 domains, MCS and PCS for each phase of the study. Change scores were explored graphically and, since most were not normally distributed, non-parametric techniques were used to estimate associations between variables. The minimum clinically important difference (MCID) in HRQoL was defined as an intra-subject change of at least 5 points for each SF-36 domain or at least 2 points for the MCS or PCS [21, 22]. Bootstrap methodology was used to calculate the 95% CI around the median change scores [24]. Since we had a priori hypotheses for the eight SF-36 domain changes, we considered a Bonferoni adjusted P-value of less than 0.006 as statistically significant.
To explore the relationship between weight change and vitality change score adjusted for potential confounders, multiple linear regression analysis was used with the vitality change score as the dependent variable and the weight change variable as the independent variable. Potential confounders included the baseline vitality scores (and where appropriate the vitality scores at the beginning of Phase 2) and the baseline demographic, personal, health status and weight history variables included in Table 1 (see “Results”). Assumptions of the underlying models, including linearity, were tested and verified by using standard regression diagnostics.
Table 1.
Characteristics of 87 completers of the study
| Characteristic | Value* |
|---|---|
| Female | 87.4 |
| Age in years, mean (SD) | 46.5 (9.7) |
| Education >12 yearsa | 70.2 |
| Professional occupation | 57.5 |
| Married | 44.8 |
| Children <18 years | 54.0 |
| Current smoker | 11.8 |
| Current drinkerb | 29.2 |
| Self-rated health | |
| Excellent or very good | 23.0 |
| Good | 54.0 |
| Fair or poor | 23.0 |
| Obesity-related comorbiditiese | 77.0 |
| Weight in kg, median (IQR) | Men 118.0 (102.4–138.3) |
| Women 97.8 (87.0–109.3) | |
| BMI, median (IQR) | Men 36.4 (33.3–43.3) |
| Women 36.6 (33.2–40.9) | |
| Age first overweight by 10 + lb in yrs, mean (SD)c | 26.2 (12.6) |
| Expected weight loss in the first 3 months in lb, median (IQR)a | 23.8 (15.9–36.3) |
| # prior weight loss programs, median (IQR)d | 1 (0–2) |
| Phase 1 weight change in kg, median (IQR) | −1.27 (−3.4–0.82) |
| Phase 2 weight change in kg, median (IQR) | +0.36 (−2.5–3.0) |
BMI body mass index, IQR interquartile range, SD standard deviation
Data are given as percentages except where noted
n = 84;
n = 72;
n = 79;
n = 85
Defined as participants whose medical history information indicated diagnosis of high blood pressure, gallbladder disease, gout or elevated uric acid, obstructive sleep apnea, breathing problems, stroke, angina, heart disease, arthritis, joint pain, diabetes, hypercholesterolemia or high cholesterol
Results
Participant characteristics
Two hundred and thirty-seven individuals were enrolled in the study initially. Of these: 134 (57%) completed Phase 1, of whom 128 agreed to be randomized to the Phase 2 weight maintenance strategies; 87 of these 128 (68%) completed the Phase 2 follow-up. Some of characteristics of the 87 participants who completed both phases of the study are shown in Table 1. Participants who completed both phases of the study were similar to those who did not complete on medical, weight history and demographic characteristics except for being older (mean age ± SD of 46.5 ± 9.7 vs. 40.9 ± 10.7 years, respectively, P = 0.0005), and more likely to have a professional occupation (57.5 vs. 30.4%, respectively P < 0.001). In general, over the entire course of the study, when baseline HRQoL data were compared for the study completers versus non-completers, completers appeared to have similar median baseline SF-36 scores but with higher 25th percentile scores. Only in the PF (z = −1.65, P = 0.09) and VT, domains (z = −2.24, P = 0.03) were the differences of borderline significance, with the completers reporting a higher range of scores. Similarly, when Phase 1 completers were compared with baseline enrollees, completers tended to report a higher interquartile range of HRQoL baseline scores compared to those participants who did not complete the phase. In the RP domain, this difference reached statistical significance (binary variable, chi-squared 8.39, P = 0.004). Phase 2 completers began the phase with similar median scores but higher interquartile ranges than the non-completers of the phase; in none of the domains or summary scores were the differences statistically significant.
Table 2 shows the baseline HRQoL in our sample and compares these values to US general population norms taken from the SF-36 interpretation guide [23, 24]. Our study sample reported lower baseline vitality scores than the US population norms but was similar to the US population norms in the other HRQoL domains and in the summary scores.
Table 2.
Baseline health-related quality of life for study participants compared to the US population norm
| Study sample (n = 87) | US population norm | |
|---|---|---|
| Physical functioning | 80 (70–95) | 90 (70–100) |
| Role physical, % ceilinga | 73.6 | 70.9 |
| Bodily pain | 74 (52–100) | 74 (61–100) |
| General health | 72 (57–87) | 72 (57–85) |
| Vitalityb | 50 (40–65) | 65 (45–75) |
| Social functioning | 87.5 (75–100) | 100 (75–100) |
| Role emotional,% ceilinga | 67.8 | 71.0 |
| Mental health | 76 (68–88) | 80 (64–88) |
| Physical component score, mean (SD)c | 49.1 (7.7) | 50.0 (10.0) |
| Mental component score, mean (SD)c | 49.4 (9.4) | 50.0 (10.0) |
% percent, SD standard deviation
SF-36 domain scores for the study sample and for the US population norm are presented as median (interquartile range) except where indicated. The component scores are presented as mean (standard deviation). Given the demographics of the study sample (men and women, aged 25–70) we did not use an age-specific norm
% ceiling refers to percent with RP or RE = 100
the bootstrap calculated 95% confidence interval of the median (42.9–57.1) suggests that study population VT is significantly lower than US population norm
Physical component score and mental component score are standardized to a mean of 50 such that above or below 50 represents better or worse than the US population average (see text)
Changes in weight
Phase 1 weight change ranged from −18.8 to +4.6 kg, with a median weight change of −1.27 kg. Sixty-three percent of the participants had lost weight during Phase 1: Only 13% of participants lost at least 5% of their baseline weight; 40% lost between 1 and 5% of their baseline weight during Phase 1. Only 5 participants gained more than 3% of their baseline weight during this phase.
In Phase 2, mean weight change ranged from −9.6 kg to +13.4 kg, with a median weight change of +0.36 kg. Seventy-seven percent of the participants either lost or maintained within 3% of their weight from the beginning of Phase 2. Forty-seven percent of the participants lost weight during this phase: 11.5% lost at least 5% of their weight; 24% between 1 and 5%.
HRQoL changes
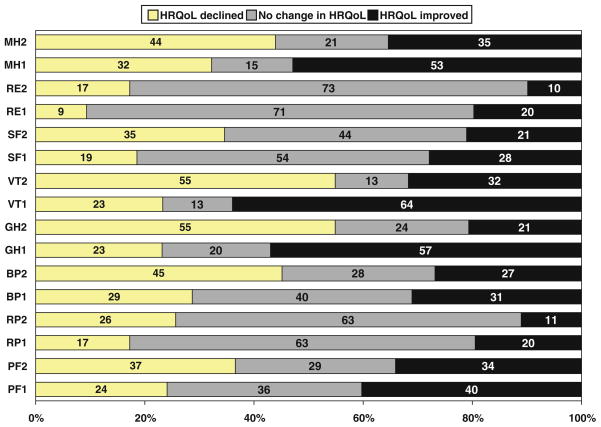
Table 3 shows the median change score and its calculated 95% CI for the eight SF-36 domains and the two component summary scores over the two phases of the study. Figure 1 shows the proportion of participants with decreased, stable or improved SF-36 scores after both phases of the study for the eight SF-36 domains.
Table 3.
Median change score and calculated 95% confidence interval for each SF-36 domain and summary score for phases 1 and 2 of the study
| SF-36 domains | Median Phase 1 change score, median (95% CI) | Median Phase 2 change scorea, median (95% CI) |
|---|---|---|
| Physical functioning | 0 (−1.62, 1.62) | 0 (−0.91, 0.91) |
| Role physical | 0 (0, 0) | 0 (0, 0) |
| Bodily pain | 0 (0, 0) | 0 (−6.44, 6.44) |
| General health | 5 (1.82, 8.18)b | −2.5 (−6.33, 1.33) |
| Vitalityb | 5 (0.11, 9.89)b | −5 (−8.82, −1.17)b |
| Social functioning | 0 (0, 0) | 0 (−1.89, 1.89) |
| Role emotional | 0 (0, 0) | 0 (0, 0) |
| Mental health | 4 (−0.13, 8.13) | 0 (−2.74, 2.74) |
| Physical component score | 0.77 (−0.30, 1.84) | −0.81 (−1.83, 0.22) |
| Mental component score | 1.9 (0.57, 3.30)b | −0.67 (−2.50,1.14) |
95% confidence interval (CI) was calculated using 1,000 bootstrap samples with replacement via the normal approximation method
Phase 2 change score uses as baseline the score at the end of Phase 1
95% confidence interval did not include zero
Fig. 1.
shows the percent of participants with decreased (change score < 0), stable (change score = 0) or improved SF-36 (change score > 0) scores after each phase of the study for the eight SF-36 domains. The eight SF-36 domains are: PF—physical functioning; RP—role limitations due physical health; BP—bodily pain; GH—general health; VT—vitality; SF—social functioning; RE –role limitations due to emotional health; MH—mental health. The number after each domain refers to the phase of the study: 1—Phase 1 (the intensive weight loss phase); 2—Phase 2 (the weight maintenance phase). Change scores for Phase 2 refer to changes in HRQoL that occurred after Phase 1, from enrollment in Phase 2 to the end of the study. See text for further details on how the change scores for each phase were calculated. In general, the RP, RE and SF domains appeared minimally responsive; most participants in either phase reported no change in these domain scores. The percentage of participants with improvements in HRQoL was higher in Phase 1 compared to Phase 2 while the percentage of participants with declines was higher in Phase 2 compared to Phase 1
Phase 1 (weight loss phase)
As shown in Table 3, the median Phase 1 HRQoL change score was zero for five of the eight SF-36 domains. A majority of participants reported no change in RP, SF or RE over Phase 1 (63, 53 and 71%, respectively). A majority reported improvements in the GH, VT and MH domains (57, 64 and 53%, respectively). Of the three domains with median change score higher than zero (GH, VT and MH), only in the VT and GH domains did the 95 percent CI of the median score not include zero. It was in these same two domains (GH, VT) that at least 50% of the study participants reported HRQoL improvements greater than or equal to the MCID of 5 points (data not shown).
The PCS and MCS scores improved for 57 and 69% of participants, respectively. The median change in MCS was about 2 points, and the 95% CI did not include zero. About 40 and 50% of the participants improved greater than the MCID for the PCS and MCS, respectively.
Association of changes in HRQoL and weight change during Phase 1
In general, greater weight loss was associated with more improvements in HRQoL scores (−0.30 > ρ < −.06). The correlation between weight change and GH change was weak and not statistically significant (ρ −0.0817, P = 0.45). More robust was the correlation between weight change and MH change (ρ −0.22, P = 0.04). The strongest correlation was seen between VT change and weight change (ρ −0.30, P = 0.005). Participants who lost at least 5% of baseline weight during Phase 1 had a median vitality change score of 30 versus a median change score of 5 in participants who did not lose at least 5% of their baseline weight during Phase 1 (z = −2.40, P = 0.0160). No other Phase 1 change score was associated with clinically significant weight loss during Phase 1.
Table 4 shows the final regression models testing the association between change in vitality and the change in weight during both phases of the study. Phase 1 vitality change was not changed by adjustment for any of the baseline characteristics in Table 1. Higher baseline vitality score was associated with less change during Phase 1. The final regression model for the vitality change score included the weight change variable with the baseline vitality score.
Table 4.
Final regression models showing the relationship between weight change and vitality change for both phases of the study
| Variables | Phase 1 vitality changea |
Phase 2 vitality changeb |
||
|---|---|---|---|---|
| Regression coefficient (95% CI) | P-value | Regression coefficient (95% CI) | P-value | |
| Phase 1 weight change (kg) | −2.05 (−2.93, −1.17) | < 0.001 | ||
| Vitality baseline scorec | −0.50 (−0.65, −0.34) | < 0.001 | ||
| Phase 2 weight change (kg) | −0.50 (−1.12, 0.12) | 0.11 | ||
| Vitality baseline scorec | 0.37 (0.20, 0.53) | < 0.001 | ||
| Vitality score at the beginning of Phase 2 | −0.61 (−0.79, −0.43) | < 0.001 | ||
The dependent variables were the Phase 1 or Phase 2 vitality change score; the independent variables were the Phase 1 weight change and the Phase 2 weight change, respectively. For both phases, potential confounders tested included age, gender, education, occupation, marital status, self-rated health, presence of obesity-related comorbidities, age when first overweight, expected weight loss, number of prior weight loss programs (see Table 1 and text for more details)
The final model included Phase 1 weight change and vitality baseline score; N = 86; R-squared = 0.41
The final model included the vitality score at the beginning of Phase 2 and the vitality baseline score; N = 81; R-squared = 0.40
Vitality baseline score refers to the vitality score at the beginning of Phase 1
Phase 2 (weight maintenance phase)
The median Phase 2 HRQoL change score for six of the eight domains was zero. Most participants reported no change in the RP and RE domains (63.4 and 72.8%, respectively). Across all domains, more participants reported declines in HRQoL than improvements. Median change scores were less than zero for the GH and VT domains. Only with the VT change score did the calculated 95% CI not include zero. About 55% of the participants reported a decrease in vitality greater than the MCID. The median change scores for both the PCS and the MCS were negative, with the 95% CI including zero. More participants reported declines than improvements in the summary component scores (57 and 51%, respectively, for the PCS and MCS).
Association of changes in HRQoL and weight change during Phase 2
As in Phase 1, Phase 2 weight change was inversely correlated with SF-36 change scores (−0.25 > ρ < −0.06, i.e., weight gain was associated with decreasing scores). Only with the VT change score was this correlation of borderline significance (ρ −0.25, P = 0.024). In general, there was no consistent relationship between weight maintenance category and direction and magnitude HRQoL changes. In a regression model with VT change score as the dependent variable, the Phase 2 weight change was not an independent predictor of change in vitality after adjusting for baseline VT scores and VT scores at the beginning of Phase 2 (see Table 4).
Discussion
In this sample of obese African-Americans with modest weight change after a 10-week lifestyle intervention, the change in HRQoL was also modest. Although weight loss was associated with improvements in HRQoL and weight gain with decreased HRQoL, the correlations were weak. The VT domain of the SF-36 appeared to be the most responsive to small changes in weight for this study population.
Subjective vitality relates to having positive energy available and within one’s control [27]. The fact that our study sample showed clinically significant improvements in VT with weight loss during Phase 1 is consistent with the growing literature on the association between vital exhaustion (the opposite of vitality) and obesity and its related comorbidities [28]. It is possible that weight loss in Phase 1 led to greater energy levels and “pep” during this phase of the intervention and/or that participants’ ability to lose weight led them to feel more motivated and in control of their lives. It is worth noting that the VT domain was also the only domain in which the study population appeared to be significantly lower at baseline than the US population norms. The distribution of VT scores in this sample may have allowed more room for improvement compared to other domains whose range of baseline scores were closer to the SF-36 ceiling.
The modest short-term improvements in GH, VT and MCS during Phase 1 of this study are consistent with earlier studies in mostly white populations on the short-term effects of lifestyle intervention for obesity on HRQoL. Rippe et al. [11] investigated the effect of a 12-week weight loss strategy that involved self-selected diet and exercise plans motivated by weekly meetings. At the end of the intervention (mean weight change of −6.1 kg compared with the −1.5 kg seen in our study), there were significant changes in the PF, VT and MH domains when compared to participants who did not receive the intervention. Fontaine et al. [14] showed in an 80% Caucasian population that after 13 weeks of behaviorally oriented treatment (mean weight change −8.6 kg) there were significant changes in the PF, RP, GH, VT and MH domains. In the one study identified that documented short-term effects of a culturally sensitive dietary intervention for obese African-Americans on HRQoL, Ard et al. [29] reported a mean weight change of −6.7 kg and significant improvements in PF, BP, RP, VT and MH domains of the SF-36. When our findings are taken together with those of these prior studies, the impression is that participants are more likely to report improvements in physical health domains of SF-36 (PF, RP, BP, GH) with relatively large weight losses but that improvements in VT and MH may emerge with only modest weight loss.
Phase 2 represents the longer-term effects of a weight loss intervention—a phase that is often associated with recidivism and weight regain. Much of the improvements in VT, GH and MCS that were observed during the first part of the study were lost during the second phase. This tendency to return to baseline in HRQoL is consistent with much of the literature on the long-term effects of weight loss interventions [30]. Blissmer et al. [31] studied the long-term effects of a 6-month intervention and showed that HRQoL effects peaked at the end of the intensive intervention, with almost all domains returning to baseline by the two-year follow-up point (only one-third of the study sample maintained at least 5% weight loss).
The lack of a consistent relationship between HRQoL changes and weight change during either phase of the intervention has several potential explanations. Changes in diet and physical activity known to occur during behavioral interventions might have led to HRQoL changes initially, independent of weight change. Insufficient data for changes in dietary intake [32] or physical activity adoption during Phase 2 precluded analyses of such effects. It is also possible that the social interaction and support offered during the behavioral intervention was responsible for some of the initial improvements in HRQoL. Our HRQoL measures may also be capturing health attributes other than those identified as part of the SF-36, e.g., the health optimism that is common among participants in behavioral interventions [33]. Assessment of health optimism might be useful in future studies of HRQoL changes.
The infeasibility of exploring, in the available data, diet and physical activity changes that might have explained the observed HRQoL changes is a limitation of this study. Following are other limitations. The drop-out rates for Phase 1 and Phase 2, although not atypical for lifestyle weight control studies [13], were high, and resulted in a relatively small sample size for these analyses. Our sample size may have been too small to detect significant changes in HRQOL. We had no untreated comparison group in Phase 1. However, in Phase 2, some participants had minimal contacts and could be considered similar to a usual care comparison group but there was no difference in HRQoL change in this treatment group compared to the others. We used a generic instrument for measuring HRQoL. While generic measures provide useful information that can be compared across populations, chronic conditions and types of weight loss interventions, they are not designed to measure specific health-related problems experienced by obese individuals. This study might have been enhanced by the complementary information an obesity-specific quality of life measure could have provided. However, to date we are unaware of any obesity-specific quality of life measure that has been validated in an African-American population. Finally, these analyses were restricted to the participants who completed the entire study and so cannot be generalized even to participants with the characteristics of those who enrolled initially. Although we explored the differences between completers and non-completers and found few statistically significant differences, we could not rule out the possibility of a clinically significant difference in HRQoL between our sample and those who enrolled but did not complete the study. Nevertheless, our exploration of these issues in an understudied population, our ability to compare the weight loss and maintenance phases, and our translation of HRQoL changes into clinically meaningful terms are strengths.
Conclusion
A lifestyle weight management program adapted for African-Americans that produced modest weight loss during the intensive phase of the study was associated with short-term improvements in general health and vitality domains. The vitality domain of the SF-36 appeared to be the most responsive HRQoL domain to modest weight change in this population. Future studies should attempt to identify aspects of the intervention other than weight change that might be important in mediating changes in HRQoL.
Acknowledgments
Funding was provided by a grant to Dr. Kumanyika from the American Heart Association National Center (9970068N). Dr. Hope conducted this study while supported by Training Grant number T32HL007891 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
Abbreviations
- BMI
Body mass index
- HELP
Healthy eating and lifestyle program study
- HRQoL
Health-related quality of life
- MCID
Minimum clinically important difference
- SF-36
The short form 36 questionnaire
- GH
General health
- PF
Physical functioning
- MH
Mental health
- RP
Role-limitations-physical
- RE
Role-limitations-emotional
- VT
Vitality
- SF
Social functioning
- BP
Bodily pain
- PCS
Physical component summary
- MCS
Mental component summary
- CI
Confidence intervals
Footnotes
Conflict of interest statement The authors declare that they have no competing interests.
Contributor Information
Aluko A. Hope, Mount Sinai School of Medicine, One Gustave L. Levy Place, New York, NY 10029-6574, USA
Shiriki K. Kumanyika, Email: skumanyi@mail.med.upenn.edu, Center for Clinical Epidemiology and Biostatistics (CCEB), University of Pennsylvania School of Medicine, 8 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104-6021, USA
Justine Shults, Center for Clinical Epidemiology and Biostatistics (CCEB), University of Pennsylvania School of Medicine, 8 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104-6021, USA.
William C. Holmes, Center for Clinical Epidemiology and Biostatistics (CCEB), University of Pennsylvania School of Medicine, 8 Blockley Hall, 423 Guardian Drive, Philadelphia, PA 19104-6021, USA
References
- 1.McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Annals of Epidemiology. 2005;15(2):87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Graubard BI. Estimates of excess deaths associated with body mass index and other anthropometric variables. American Journal of Clinical Nutrition. 2009;89(4):1213–1219. doi: 10.3945/ajcn.2008.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Fontaine KR, Barofsky I. Obesity and health-related quality of life. Obesity Reviews. 2001;2(3):173–182. doi: 10.1046/j.1467-789x.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 7.Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obesity Reviews. 2001;2(4):219–229. doi: 10.1046/j.1467-789x.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson J, Taft C, Ryden A, Sjostrom L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: The SOS intervention study. Int J Obes (Lond) 2007;31(8):1248–1261. doi: 10.1038/sj.ijo.0803573. [DOI] [PubMed] [Google Scholar]
- 9.Coakley EH, Kawachi I, Manson JE, Speizer FE, Willet WC, Colditz GA. Lower levels of physical functioning are associated with higher body weight among middle-aged and older women. International Journal of Obesity and Related Metabolic Disorders. 1998;22(10):958–965. doi: 10.1038/sj.ijo.0800698. [DOI] [PubMed] [Google Scholar]
- 10.Fine JT, Colditz GA, Coakley EH, Moseley G, Manson JE, Willett WC, et al. A prospective study of weight change and health-related quality of life in women. JAMA. 1999;282(22):2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 11.Rippe JM, Price JM, Hess SA, Kline G, DeMers KA, Damitz S, et al. Improved psychological well-being, quality of life, and health practices in moderately overweight women participating in a 12-week structured weight loss program. Obesity Research. 1998;6(3):208–218. doi: 10.1002/j.1550-8528.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine KR, Barofsky I, Bartlett SJ, Franckowiak SC, Andersen RE. Weight loss and health-related quality of life: Results at 1-year follow-up. Eating Behaviors. 2004;5(1):85–88. doi: 10.1016/S1471-0153(03)00059-X. [DOI] [PubMed] [Google Scholar]
- 13.Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. Journal of Clinical Epidemiology. 2005;58(6):568–578. doi: 10.1016/j.jclinepi.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Fontaine KR, Barofsky I, Andersen RE, Bartlett SJ, Wiersema L, Cheskin LJ, et al. Impact of weight loss on health-related quality of life. Quality of Life Research. 1999;8(3):275–277. doi: 10.1023/a:1008835602894. [DOI] [PubMed] [Google Scholar]
- 15.Kumanyika S. Ethnic minorities and weight control research priorities: Where are we now and where do we need to be? Preventive Medicine. 2008;47(6):583–586. doi: 10.1016/j.ypmed.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. American Journal of Preventive Medicine. 2008;35(2):118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumanyika SK, Espeland MA, Bahnson JL, Bottom JB, Charleston JB, Folmar S, et al. Ethnic comparison of weight loss in the trial of nonpharmacologic interventions in the elderly. Obesity Research. 2002;10(2):96–106. doi: 10.1038/oby.2002.16. [DOI] [PubMed] [Google Scholar]
- 18.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of black, white, and Hispanic men and women in the diabetes prevention program. Obesity (Silver Spring) 2008;16(6):1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 19.Kumanyika SK, Shults J, Fassbender J, Whitt MC, Brake V, Kallan MJ, et al. Outpatient weight management in African-Americans: The Healthy Eating and Lifestyle Program (HELP) study. Preventive Medicine. 2005;41(2):488–502. doi: 10.1016/j.ypmed.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 20.Kumanyika SK. Obesity treatment in minorities. In: Wadden TA, editor. Handbook of Obesity Treatment. 3. New York: Guilford Publications; 2002. pp. 416–446. [Google Scholar]
- 21.Ware JE, Snow KK, Kosinski M. SF-36 health survey Manual and interpretation guide. Boston: New England Medical Center; 1993. [Google Scholar]
- 22.Ware JE, Jr, Kosinski M, Keller SD. Massachussett’s Health Assessment Lab. Boston: New England Medical Center; 1994. SF-36 physical and mental health summary scales: A user’s manual. [Google Scholar]
- 23.Stata Corp. Stata statistical software: Release 11, college station. Texas: Stata Corp LP; 2009. [Google Scholar]
- 24.Haukoos JS, Lewis RJ. Advanced statistics: Bootstrapping confidence intervals for statistics with “difficult” distributions. Academic Emergency Medicine. 2005;12(4):360–365. doi: 10.1197/j.aem.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–The evidence report. National Institutes of Health. Obesity Research. 1998;6(Suppl 2):51S–209S. Erratum in: Obes Res 1998 Nov;6(6):464. [PubMed] [Google Scholar]
- 26.Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. International Journal of Obesity. 2006;30(3):391–399. doi: 10.1038/sj.ijo.0803175. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 28.Bryant MJ, Stevens J, Truesdale KP, Mosley T, Cham-bless L, Bryant MJ, et al. Obesity and vital exhaustion: Analysis of the Atherosclerosis Risk in the Communities study. Obesity. 2008;16(7):1545–1551. doi: 10.1038/oby.2008.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ard JD, Rosati R, Oddone EZ. Culturally-sensitive weight loss program produces significant reduction in weight, blood pressure, and cholesterol in eight weeks. Journal of the National Medical Association. 2000;92(11):515–523. [PMC free article] [PubMed] [Google Scholar]
- 30.Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification–a 2-y follow-up study. International Journal of Obesity and Related Metabolic Disorders. 2003;27(10):1233–1241. doi: 10.1038/sj.ijo.0802379. [DOI] [PubMed] [Google Scholar]
- 31.Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults intervention and 24 month follow-up effects. Health Qual Life Outcomes. 2006;4(43):43. doi: 10.1186/1477-7525-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson CAM, Kumanyika SK, Shults J, Kallan MJ, Gans KM, Risica PM. Assessing change in dietary-fat behaviors in a weight-loss program for African Americans: a potential short method. Journal of the American Dietetic Association. 2007;107(5):838–842. doi: 10.1016/j.jada.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Fontaine KR, Cheskin LJ. Optimism and obesity treatment outcomes. Journal of Clinical Psychology. 1999;55(1):141–143. doi: 10.1002/(sici)1097-4679(199901)55:1<141::aid-jclp15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]