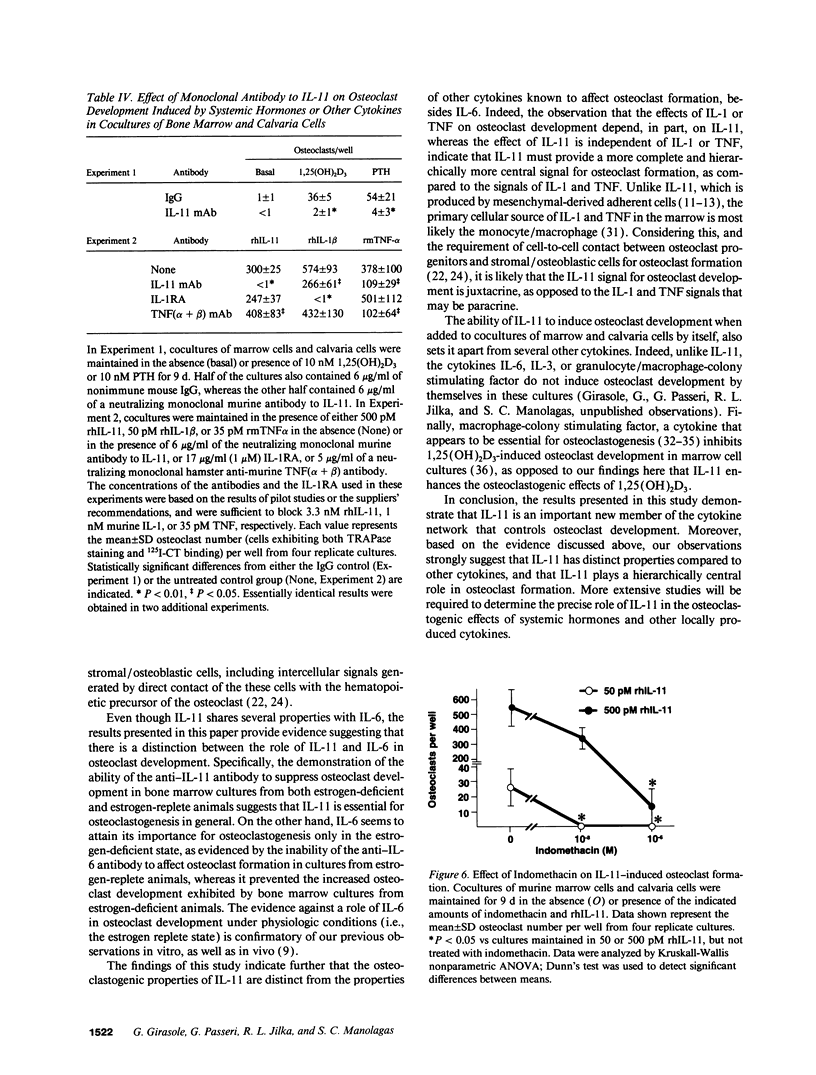
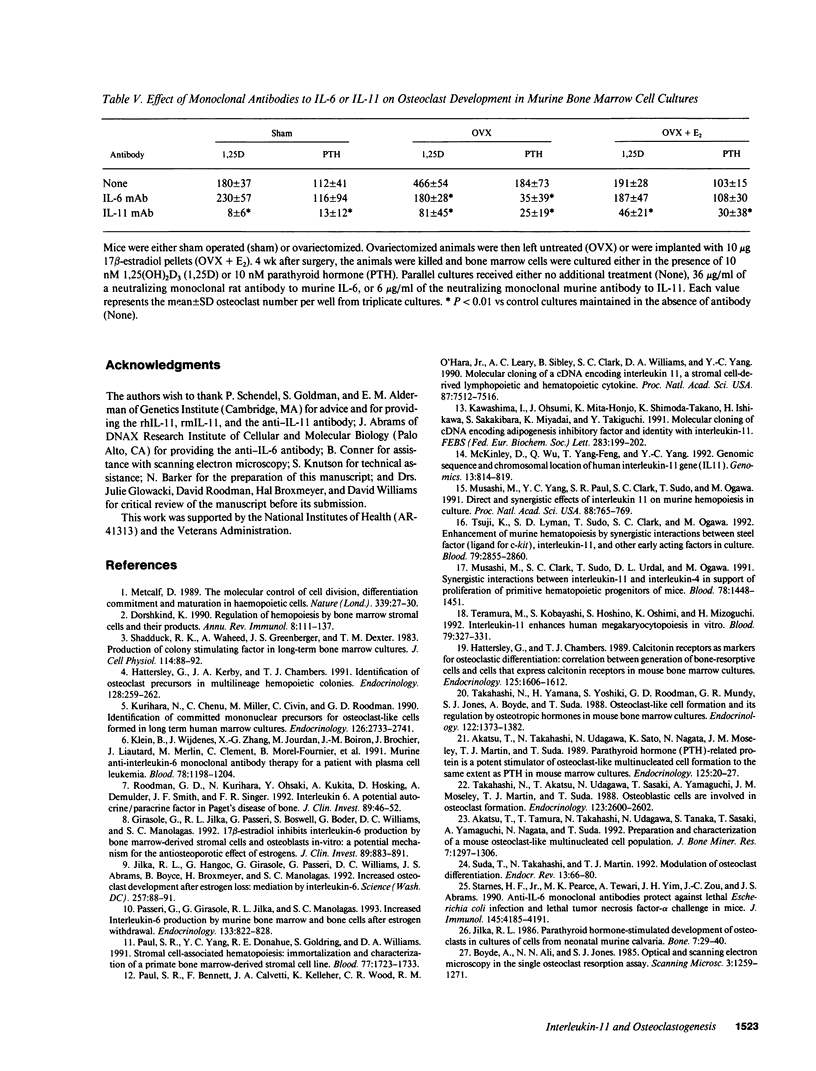
Abstract
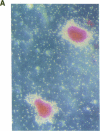
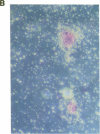
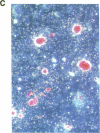
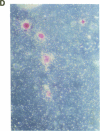
Stromal cells of the bone marrow control the development of osteoclasts through the production of cytokines capable of promoting the proliferation and differentiation of hematopoietic progenitors. Moreover, the deregulated production of the cytokine IL-6 in the bone marrow mediates an increase in osteoclastogenesis after estrogen loss. IL-6, however, does not influence osteoclastogenesis in the estrogen-replete state, suggesting that other cytokines might be responsible for osteoclast development under physiologic circumstances. We report here that IL-11, a newly discovered cytokine that is produced by marrow stromal cells, induced the formation of osteoclasts exhibiting an unusually high degree of ploidy in cocultures of murine bone marrow and calvarial cells. Osteoclasts formed in the presence of IL-11 were capable of bone resorption, as evidenced by the formation of resorption pits, as well as the release of 45Ca from prelabeled murine calvaria. Further, an antibody neutralizing IL-11 suppressed osteoclast development induced by either 1,25-dihydroxyvitamin D3, parathyroid hormone, interleukin-1, or tumor necrosis factor; whereas inhibitors of IL-1 or TNF had no effect on IL-11-stimulated osteoclast formation. The effects of IL-11 on osteoclast development were blocked by indomethacin; more important, however, they were independent of the estrogen status of the marrow donors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akatsu T., Takahashi N., Udagawa N., Imamura K., Yamaguchi A., Sato K., Nagata N., Suda T. Role of prostaglandins in interleukin-1-induced bone resorption in mice in vitro. J Bone Miner Res. 1991 Feb;6(2):183–189. doi: 10.1002/jbmr.5650060212. [DOI] [PubMed] [Google Scholar]
- Akatsu T., Takahashi N., Udagawa N., Sato K., Nagata N., Moseley J. M., Martin T. J., Suda T. Parathyroid hormone (PTH)-related protein is a potent stimulator of osteoclast-like multinucleated cell formation to the same extent as PTH in mouse marrow cultures. Endocrinology. 1989 Jul;125(1):20–27. doi: 10.1210/endo-125-1-20. [DOI] [PubMed] [Google Scholar]
- Akatsu T., Tamura T., Takahashi N., Udagawa N., Tanaka S., Sasaki T., Yamaguchi A., Nagata N., Suda T. Preparation and characterization of a mouse osteoclast-like multinucleated cell population. J Bone Miner Res. 1992 Nov;7(11):1297–1306. doi: 10.1002/jbmr.5650071109. [DOI] [PubMed] [Google Scholar]
- Boyde A., Ali N. N., Jones S. J. Optical and scanning electron microscopy in the single osteoclast resorption assay. Scan Electron Microsc. 1985;(Pt 3):1259–1271. [PubMed] [Google Scholar]
- Chambers T. J., McSheehy P. M., Thomson B. M., Fuller K. The effect of calcium-regulating hormones and prostaglandins on bone resorption by osteoclasts disaggregated from neonatal rabbit bones. Endocrinology. 1985 Jan;116(1):234–239. doi: 10.1210/endo-116-1-234. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol. 1990;8:111–137. doi: 10.1146/annurev.iy.08.040190.000551. [DOI] [PubMed] [Google Scholar]
- Felix R., Cecchini M. G., Fleisch H. Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology. 1990 Nov;127(5):2592–2594. doi: 10.1210/endo-127-5-2592. [DOI] [PubMed] [Google Scholar]
- Girasole G., Jilka R. L., Passeri G., Boswell S., Boder G., Williams D. C., Manolagas S. C. 17 beta-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992 Mar;89(3):883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley G., Chambers T. J. Calcitonin receptors as markers for osteoclastic differentiation: correlation between generation of bone-resorptive cells and cells that express calcitonin receptors in mouse bone marrow cultures. Endocrinology. 1989 Sep;125(3):1606–1612. doi: 10.1210/endo-125-3-1606. [DOI] [PubMed] [Google Scholar]
- Hattersley G., Kerby J. A., Chambers T. J. Identification of osteoclast precursors in multilineage hemopoietic colonies. Endocrinology. 1991 Jan;128(1):259–262. doi: 10.1210/endo-128-1-259. [DOI] [PubMed] [Google Scholar]
- Jilka R. L., Hangoc G., Girasole G., Passeri G., Williams D. C., Abrams J. S., Boyce B., Broxmeyer H., Manolagas S. C. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992 Jul 3;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Jilka R. L. Parathyroid hormone-stimulated development of osteoclasts in cultures of cells from neonatal murine calvaria. Bone. 1986;7(1):29–40. doi: 10.1016/8756-3282(86)90149-3. [DOI] [PubMed] [Google Scholar]
- Kawashima I., Ohsumi J., Mita-Honjo K., Shimoda-Takano K., Ishikawa H., Sakakibara S., Miyadai K., Takiguchi Y. Molecular cloning of cDNA encoding adipogenesis inhibitory factor and identity with interleukin-11. FEBS Lett. 1991 Jun 3;283(2):199–202. doi: 10.1016/0014-5793(91)80587-s. [DOI] [PubMed] [Google Scholar]
- Klein B., Wijdenes J., Zhang X. G., Jourdan M., Boiron J. M., Brochier J., Liautard J., Merlin M., Clement C., Morel-Fournier B. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood. 1991 Sep 1;78(5):1198–1204. [PubMed] [Google Scholar]
- Kodama H., Nose M., Niida S., Yamasaki A. Essential role of macrophage colony-stimulating factor in the osteoclast differentiation supported by stromal cells. J Exp Med. 1991 May 1;173(5):1291–1294. doi: 10.1084/jem.173.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara N., Chenu C., Miller M., Civin C., Roodman G. D. Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology. 1990 May;126(5):2733–2741. doi: 10.1210/endo-126-5-2733. [DOI] [PubMed] [Google Scholar]
- McKinley D., Wu Q., Yang-Feng T., Yang Y. C. Genomic sequence and chromosomal location of human interleukin-11 gene (IL11). Genomics. 1992 Jul;13(3):814–819. doi: 10.1016/0888-7543(92)90158-o. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Musashi M., Clark S. C., Sudo T., Urdal D. L., Ogawa M. Synergistic interactions between interleukin-11 and interleukin-4 in support of proliferation of primitive hematopoietic progenitors of mice. Blood. 1991 Sep 15;78(6):1448–1451. [PubMed] [Google Scholar]
- Musashi M., Yang Y. C., Paul S. R., Clark S. C., Sudo T., Ogawa M. Direct and synergistic effects of interleukin 11 on murine hemopoiesis in culture. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):765–769. doi: 10.1073/pnas.88.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passeri G., Girasole G., Jilka R. L., Manolagas S. C. Increased interleukin-6 production by murine bone marrow and bone cells after estrogen withdrawal. Endocrinology. 1993 Aug;133(2):822–828. doi: 10.1210/endo.133.2.8393776. [DOI] [PubMed] [Google Scholar]
- Paul S. R., Bennett F., Calvetti J. A., Kelleher K., Wood C. R., O'Hara R. M., Jr, Leary A. C., Sibley B., Clark S. C., Williams D. A. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7512–7516. doi: 10.1073/pnas.87.19.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S. R., Yang Y. C., Donahue R. E., Goldring S., Williams D. A. Stromal cell-associated hematopoiesis: immortalization and characterization of a primate bone marrow-derived stromal cell line. Blood. 1991 Apr 15;77(8):1723–1733. [PubMed] [Google Scholar]
- Roodman G. D., Kurihara N., Ohsaki Y., Kukita A., Hosking D., Demulder A., Smith J. F., Singer F. R. Interleukin 6. A potential autocrine/paracrine factor in Paget's disease of bone. J Clin Invest. 1992 Jan;89(1):46–52. doi: 10.1172/JCI115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadduck R. K., Waheed A., Greenberger J. S., Dexter T. M. Production of colony stimulating factor in long-term bone marrow cultures. J Cell Physiol. 1983 Jan;114(1):88–92. doi: 10.1002/jcp.1041140115. [DOI] [PubMed] [Google Scholar]
- Shinar D. M., Rodan G. A. Biphasic effects of transforming growth factor-beta on the production of osteoclast-like cells in mouse bone marrow cultures: the role of prostaglandins in the generation of these cells. Endocrinology. 1990 Jun;126(6):3153–3158. doi: 10.1210/endo-126-6-3153. [DOI] [PubMed] [Google Scholar]
- Shinar D. M., Sato M., Rodan G. A. The effect of hemopoietic growth factors on the generation of osteoclast-like cells in mouse bone marrow cultures. Endocrinology. 1990 Mar;126(3):1728–1735. doi: 10.1210/endo-126-3-1728. [DOI] [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Suda T., Takahashi N., Martin T. J. Modulation of osteoclast differentiation. Endocr Rev. 1992 Feb;13(1):66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Udagawa N., Sasaki T., Yamaguchi A., Moseley J. M., Martin T. J., Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988 Nov;123(5):2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Yamana H., Yoshiki S., Roodman G. D., Mundy G. R., Jones S. J., Boyde A., Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988 Apr;122(4):1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- Teramura M., Kobayashi S., Hoshino S., Oshimi K., Mizoguchi H. Interleukin-11 enhances human megakaryocytopoiesis in vitro. Blood. 1992 Jan 15;79(2):327–331. [PubMed] [Google Scholar]
- Tsuji K., Lyman S. D., Sudo T., Clark S. C., Ogawa M. Enhancement of murine hematopoiesis by synergistic interactions between steel factor (ligand for c-kit), interleukin-11, and other early acting factors in culture. Blood. 1992 Jun 1;79(11):2855–2860. [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]