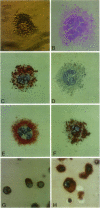
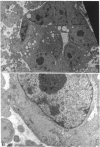
Abstract
Fibroblast heterogeneity is known to exist in chronically inflamed tissue such as pulmonary fibrosis (IPF) and scleroderma. We have previously shown differences in proliferation rates in primary lines and cloned lines of fibroblasts derived from IPF tissue compared with normal lung. In this study, we report that cell lines derived from fibrotic tissue demonstrate anchorage-independent growth in soft agarose culture whereas normal lung fibroblast lines do not. We also show that fibroblast lines derived from neonatal lung tissue form colonies at about the same frequency as the fibrotic cells. Colonies from both fibrotic and neonatal lines were shown to be positive for vimentin, laminin, fibronectin, fibronectin receptor, beta-actin, and tropomyosin by immunohistochemistry but were negative for desmin, keratin, Factor VIII, alpha-smooth muscle cell actin, and tenascin. Treatment with cytokines TGF-beta and PDGF or with corticosteroid modified the colony-forming capacity of fibrotic and neonatal cell lines, however, none of these treatments induced normal lung cell lines to form colonies. The presence of cells in adult fibrotic tissue with growth characteristics similar to those exhibited by neonatal cells is further evidence of fibroblast heterogeneity and suggests newly differentiated fibroblasts may be prevalent in fibrotic tissue and contribute directly to the matrix disorder seen in this disease.
Full text
PDF


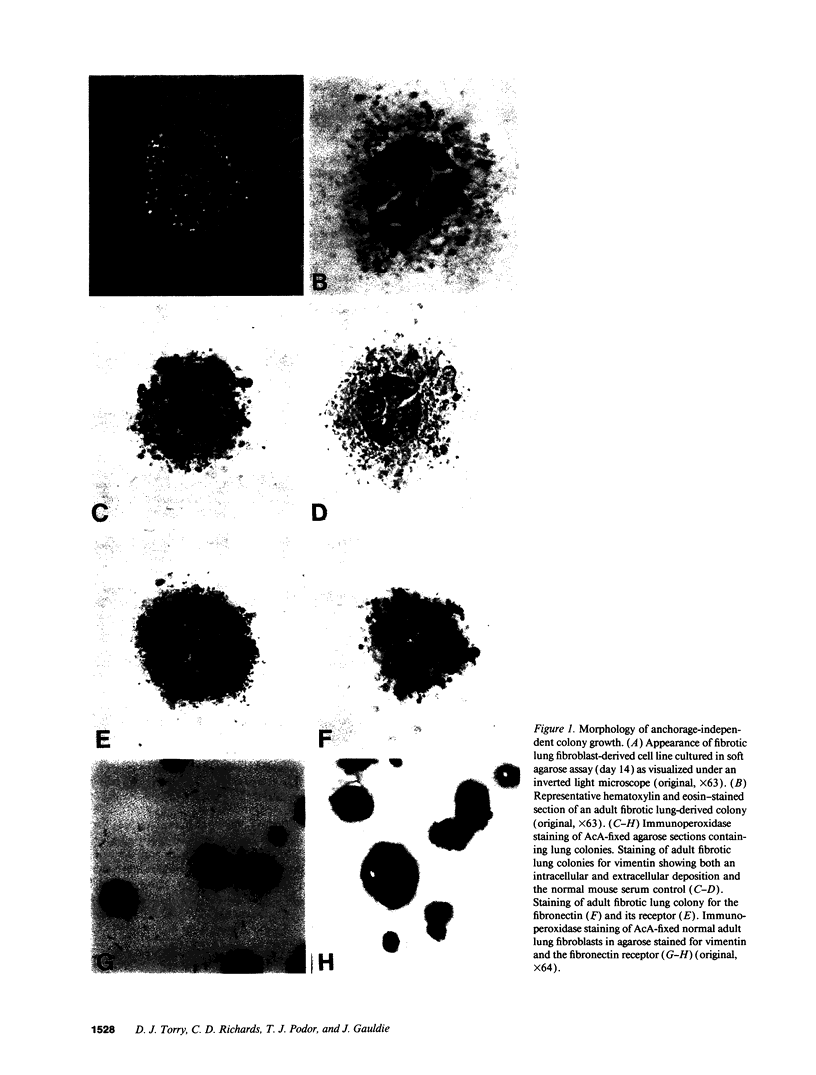
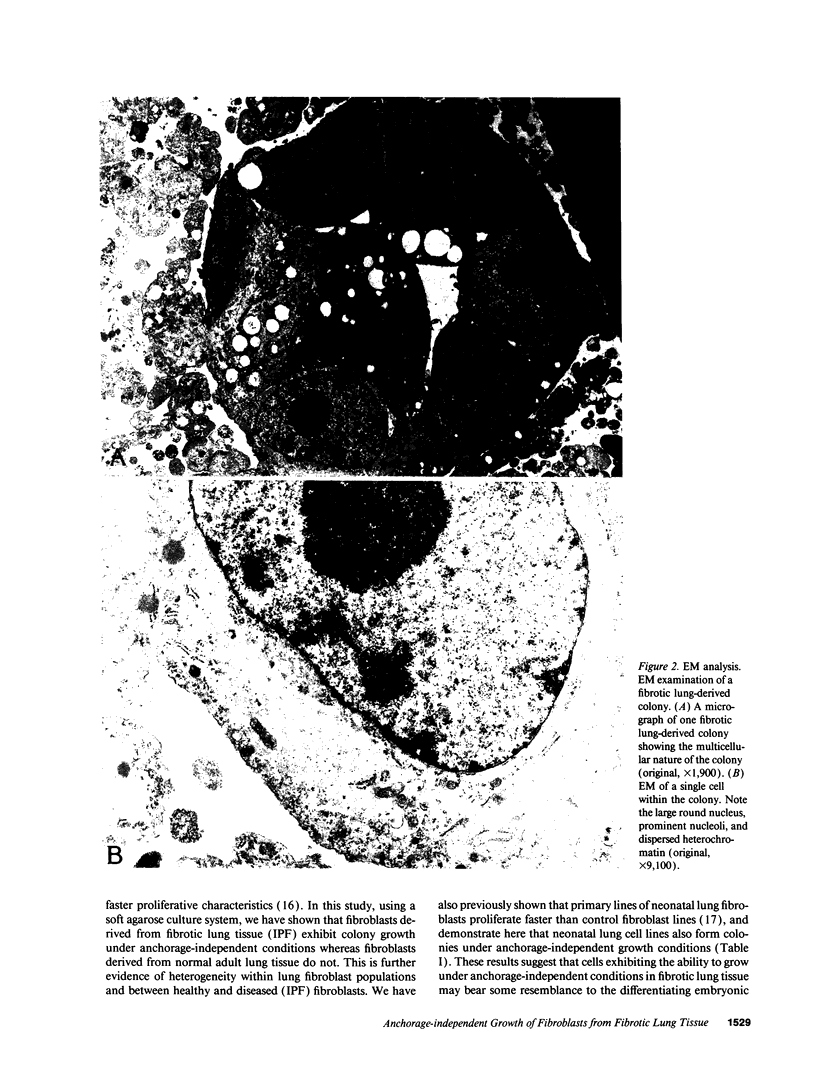



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Bravo M. A., Avila R. E., Galanopoulos T., Neville-Golden J., Maxwell M., Selman M. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990 Oct;86(4):1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basset F., Ferrans V. J., Soler P., Takemura T., Fukuda Y., Crystal R. G. Intraluminal fibrosis in interstitial lung disorders. Am J Pathol. 1986 Mar;122(3):443–461. [PMC free article] [PubMed] [Google Scholar]
- Bateman E. D., Turner-Warwick M., Adelmann-Grill B. C. Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax. 1981 Sep;36(9):645–653. doi: 10.1136/thx.36.9.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordin S., Page R. C., Narayanan A. S. Heterogeneity of normal human diploid fibroblasts: isolation and characterization of one phenotype. Science. 1984 Jan 13;223(4632):171–173. doi: 10.1126/science.6691142. [DOI] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Clark R. A. Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol. 1990 Jun;94(6 Suppl):128S–134S. doi: 10.1111/1523-1747.ep12876104. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract (first of two parts). N Engl J Med. 1984 Jan 19;310(3):154–166. doi: 10.1056/NEJM198401193100304. [DOI] [PubMed] [Google Scholar]
- Darby I., Skalli O., Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990 Jul;63(1):21–29. [PubMed] [Google Scholar]
- Derdak S., Penney D. P., Keng P., Felch M. E., Brown D., Phipps R. P. Differential collagen and fibronectin production by Thy 1+ and Thy 1- lung fibroblast subpopulations. Am J Physiol. 1992 Aug;263(2 Pt 1):L283–L290. doi: 10.1152/ajplung.1992.263.2.L283. [DOI] [PubMed] [Google Scholar]
- Dunnill M. S. Pulmonary fibrosis. Histopathology. 1990 Apr;16(4):321–329. doi: 10.1111/j.1365-2559.1990.tb01135.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Appelhans B., Schmid E., Freudenstein C., Osborn M., Weber K. Identification and characterization of epithelial cells in mammalian tissues by immunofluorescence microscopy using antibodies to prekeratin. Differentiation. 1979;15(1):7–25. doi: 10.1111/j.1432-0436.1979.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Kuhn C., Jackson B. W., Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. III. Primary mesenchymal cells and the first appearance of vimentin filaments. Differentiation. 1982;23(1):43–59. doi: 10.1111/j.1432-0436.1982.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Jordana M., Cox G., Ohtoshi T., Dolovich J., Denburg J. Fibroblasts and other structural cells in airway inflammation. Am Rev Respir Dis. 1992 Feb;145(2 Pt 2):S14–S17. doi: 10.1164/ajrccm/145.2_Pt_2.S14. [DOI] [PubMed] [Google Scholar]
- Goddard D. H. Regulation of synovial cell growth by polypeptide growth factors. DNA Cell Biol. 1992 Apr;11(3):259–263. doi: 10.1089/dna.1992.11.259. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Toda K., Takashima A. Activation of keratinocyte fibronectin receptor function during cutaneous wound healing. J Cell Sci Suppl. 1987;8:199–209. doi: 10.1242/jcs.1987.supplement_8.11. [DOI] [PubMed] [Google Scholar]
- Jones R. B., Smith J. R. A stochastic model of cellular senescence. II. Concordance with experimental data. J Theor Biol. 1982 Jun 7;96(3):443–460. doi: 10.1016/0022-5193(82)90120-5. [DOI] [PubMed] [Google Scholar]
- Jordana M., Schulman J., McSharry C., Irving L. B., Newhouse M. T., Jordana G., Gauldie J. Heterogeneous proliferative characteristics of human adult lung fibroblast lines and clonally derived fibroblasts from control and fibrotic tissue. Am Rev Respir Dis. 1988 Mar;137(3):579–584. doi: 10.1164/ajrccm/137.3.579. [DOI] [PubMed] [Google Scholar]
- Katzenstein A. L. Pathogenesis of "fibrosis" in interstitial pneumonia: an electron microscopic study. Hum Pathol. 1985 Oct;16(10):1015–1024. doi: 10.1016/s0046-8177(85)80279-3. [DOI] [PubMed] [Google Scholar]
- Khalil N., O'Connor R. N., Unruh H. W., Warren P. W., Flanders K. C., Kemp A., Bereznay O. H., Greenberg A. H. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991 Aug;5(2):155–162. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- Kirkwood T. B., Holliday R. Commitment to senescence: a model for the finite and infinite growth of diploid and transformed human fibroblasts in culture. J Theor Biol. 1975 Sep;53(2):481–496. doi: 10.1016/s0022-5193(75)80018-x. [DOI] [PubMed] [Google Scholar]
- Kuhn C., 3rd, Boldt J., King T. E., Jr, Crouch E., Vartio T., McDonald J. A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989 Dec;140(6):1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- Kuhn C., McDonald J. A. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991 May;138(5):1257–1265. [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R., Remmers E. F., Roberts A. B., Yocum D. E., Sporn M. B., Wilder R. L. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J Clin Invest. 1989 Apr;83(4):1267–1276. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Halfter W., Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988 Dec;107(6 Pt 2):2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Furthmayr H. Collagen polymorphism in the lung. An immunochemical study of pulmonary fibrosis. Hum Pathol. 1980 Jul;11(4):353–366. doi: 10.1016/s0046-8177(80)80031-1. [DOI] [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Raghu G., Chen Y. Y., Rusch V., Rabinovitch P. S. Differential proliferation of fibroblasts cultured from normal and fibrotic human lungs. Am Rev Respir Dis. 1988 Sep;138(3):703–708. doi: 10.1164/ajrccm/138.3.703. [DOI] [PubMed] [Google Scholar]
- Remmers E. F., Sano H., Lafyatis R., Case J. P., Kumkumian G. K., Hla T., Maciag T., Wilder R. L. Production of platelet derived growth factor B chain (PDGF-B/c-sis) mRNA and immunoreactive PDGF B-like polypeptide by rheumatoid synovium: coexpression with heparin binding acidic fibroblast growth factor-1. J Rheumatol. 1991 Jan;18(1):7–13. [PubMed] [Google Scholar]
- Roche W. R., Beasley R., Williams J. H., Holgate S. T. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989 Mar 11;1(8637):520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Shepro D., Morel N. M. Pericyte physiology. FASEB J. 1993 Aug;7(11):1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- Sims D. E. Recent advances in pericyte biology--implications for health and disease. Can J Cardiol. 1991 Dec;7(10):431–443. [PubMed] [Google Scholar]
- Skalli O., Pelte M. F., Peclet M. C., Gabbiani G., Gugliotta P., Bussolati G., Ravazzola M., Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989 Mar;37(3):315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- Starcher B. C., Kuhn C., Overton J. E. Increased elastin and collagen content in the lungs of hamsters receiving an intratracheal injection of bleomycin. Am Rev Respir Dis. 1978 Feb;117(2):299–305. doi: 10.1164/arrd.1978.117.2.299. [DOI] [PubMed] [Google Scholar]
- Uitto J., Bauer E. A., Eisen A. Z. Scleroderma: increased biosynthesis of triple-helical type I and type III procollagens associated with unaltered expression of collagenase by skin fibroblasts in culture. J Clin Invest. 1979 Oct;64(4):921–930. doi: 10.1172/JCI109558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Lafyatis R., Roberts A. B., Case J. P., Kumkumian G. K., Sano H., Sporn M. B., Remmers E. F. Transforming growth factor-beta in rheumatoid arthritis. Ann N Y Acad Sci. 1990;593:197–207. doi: 10.1111/j.1749-6632.1990.tb16112.x. [DOI] [PubMed] [Google Scholar]