Abstract
Objectives
The biphasic kill curve of isoniazid against Mycobacterium tuberculosis in guinea pigs is due to the presence of persisters rather than selection of isoniazid-resistant mutants. To determine whether this phenomenon is common to other bactericidal drugs, we studied the activity of streptomycin and its ability to select for streptomycin-resistant mutants in the guinea pig model of tuberculosis.
Methods
Pharmacokinetic studies were performed to establish the human-equivalent dose of streptomycin. Guinea pigs were aerosol-infected with M. tuberculosis and 2 weeks later streptomycin was given for 5 days/week via intramuscular injection. Bactericidal activity was assessed by homogenizing and plating lungs for cfu until 10 weeks of treatment. At each timepoint, cfu were isolated, suspended in normal saline and re-plated on plates containing 0.5, 1.0, 2.0 or 10.0 mg/L streptomycin.
Results
The human-equivalent dose of streptomycin was determined to be 70 mg/kg. Streptomycin showed potent activity during the first 14 days of treatment, rescuing all animals from acute tuberculosis-related death and reducing lung cfu by ∼4 log10. However, streptomycin activity was dramatically reduced thereafter, as lung cfu declined by only ∼1 log10 over the next 56 days of treatment. Although streptomycin-resistant mutants were detectable, their frequency of isolation was identical at treatment initiation and after 70 days of treatment.
Conclusions
The reduced activity of streptomycin during the second phase of monotherapy is not associated with the selection of streptomycin-resistant mutants but, rather, with the presence of phenotypically tolerant ‘persisters’.
Keywords: Mycobacterium tuberculosis, antibiotic chemotherapy, isoniazid, persistence, drug resistance
Introduction
The marked reduction in the potent early bactericidal activity (EBA) of bactericidal antituberculous drugs during the initial phase of tuberculosis (TB) treatment has been attributed to the depletion of logarithmically growing bacilli and the presence of non-replicating, phenotypically drug-tolerant ‘persisters’. This prevailing dogma was challenged by a recent study using an in vitro model to simulate concentration–time profiles of the bactericidal drug isoniazid encountered in humans, which concluded that Mycobacterium tuberculosis killing by isoniazid ceased due to the emergence of isoniazid-resistant mutants.1 To address this issue in a relevant animal model, we recently studied the bactericidal activity of isoniazid in M. tuberculosis-infected guinea pigs.2 Human-equivalent dosing of isoniazid reproduced the biphasic killing curve seen in human EBA studies of the drug.3 Interestingly, isoniazid-resistant mutants could not be isolated following isoniazid monotherapy in guinea pigs, leading us to conclude that the second-phase reduction in drug activity was related to the presence of persisters.2 However, it remains unclear whether this phenomenon may be generalized to other bactericidal antituberculous drugs.
Streptomycin has historical importance in the field of TB chemotherapy, since it was one of the first drugs reported to have antituberculous activity.4 Like isoniazid, streptomycin is a potent bactericidal drug, but, unlike isoniazid, streptomycin kills M. tuberculosis by binding tightly to 16S rRNA in the 30S ribosomal subunit, thereby inhibiting protein synthesis.4 Whereas M. tuberculosis mutants with reduced catalase–peroxidase activity, which account for the majority of isoniazid-resistant clinical isolates, have been shown to be significantly less virulent than drug-susceptible strains in the guinea pig TB model,2 streptomycin-resistant mutants appear to retain full virulence in guinea pigs.4 Therefore, in the current study, we studied the bactericidal activity of streptomycin in the guinea pig model of TB and determined whether reduction in bacillary killing was associated with selection of streptomycin-resistant mutants or, as in the case of isoniazid, with the presence of drug-tolerant persisters.
Materials and methods
M. tuberculosis strain
A wild-type M. tuberculosis H37Rv strain,2 kindly provided by Dr David McMurray (College of Medicine, Texas A&M Health Science Center) and subsequently passaged in mice and guinea pigs, was used in this study. The MIC of streptomycin for this strain was determined to be 2.0 mg/L.
Animals
Female outbred Hartley guinea pigs with or without jugular vein vascular catheters (250–300 g) were purchased from Charles River Laboratories. Animals were maintained under pathogen-free conditions and fed water and chow ad libitum. All procedures followed protocols approved by the Institutional Animal Care and Use Committee at Johns Hopkins.
Pharmacokinetic experiments
Separate groups of three guinea pigs each were given a single dose of streptomycin sulphate (Sigma) at 70 or 275 mg/kg. Doses were prepared in water in a final volume of 0.2 mL and injected into the thigh muscle. Blood (0.3 mL) was drawn through the intravenous catheter at various timepoints after antibiotic dosing. Plasma was separated, stored at −70°C and analysed for streptomycin concentration using a validated HPLC method, whose performance characteristics have been described previously.5
Aerosol infections
Guinea pigs were aerosol-infected with log-phase M. tuberculosis cultures using an Inhalation Exposure System (Glas-Col) calibrated to deliver an inoculum resulting in ∼105 cfu per lung on the day after infection. High-dose infection was used to achieve a peak lung bacillary burden approximating that of a human TB cavity.6
Antibiotic therapy
Intramuscular injection of streptomycin sulphate 70 mg/kg given five times weekly was initiated 14 days after aerosol infection. Antibiotic dosing was adjusted throughout the study based on mean weekly body weights. Three animals were sacrificed on days 0, 7, 14 and 70 following treatment initiation. Lungs were examined for gross lesions and homogenized, as described previously.2,7 Undiluted and diluted lung homogenates were plated on selective 7H11 plates (Becton-Dickinson, Sparks, MD, USA) for cfu enumeration. Log-transformed cfu values were used to calculate means and standard deviations for graphing purposes.
Assessment of streptomycin-resistant mutants
Colonies were scraped from 7H11 plates at all timepoints and suspended in 10 mL of PBS. Colony suspensions were diluted and plated on 7H11 agar (final inoculum, 7.5–8.5 log10 cfu/plate) containing streptomycin at the following concentrations: 0.0, 0.5, 1.0, 2.0 and 10.0 mg/L. Colonies were counted 28 days after incubation at 37°C. The breakpoint used to define streptomycin resistance was 2.0 mg/L.
Results
Identification of the human-equivalent dose of streptomycin in guinea pigs
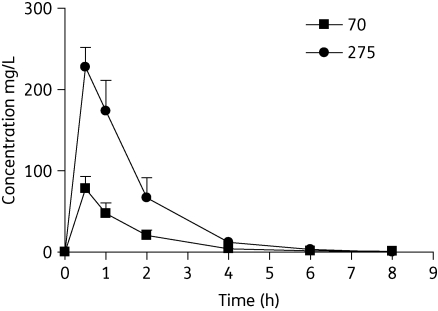
Figure 1 shows streptomycin plasma concentrations following single intramuscular doses. The maximum concentration (Cmax) was a median (range) of 69.39 mg/L (60.79–87.43) and 237.49 mg/L (199.95–245.43) following dosing at 70 and 275 mg/kg, respectively. Corresponding AUC0–∞ observed values were 108.64 mg·h/L (89.51–148.64) and 377.12 mg·h/L (294.24–456.10). The Cmax/dose ratios were 0.99 and 0.86 and the AUC0–∞ observed/dose ratios were 1.55 and 1.37, indicating reasonable dose proportionality. All apparent times of Cmax (Tmax) were the first sampling time, 0.5 ± 0.0 h. The median elimination half-life values were 1.13 h (0.70–2.02) and 0.85 h (0.84–0.90) for the 70 and 275 mg/kg doses, respectively.
Figure 1.
Plasma concentration profile of streptomycin in guinea pigs after single doses of 70 mg/kg and 275 mg/kg. n = 3 per timepoint; error bars represent standard deviation of the mean.
Like other aminoglycosides, streptomycin has concentration-dependent bactericidal activity, which has been demonstrated clearly in animal models8 and in humans.9 Typical human doses are 15 mg/kg daily or 25 mg/kg thrice weekly administered via intramuscular or intravenous injection.5,10 The intermittent dosing regimen produces a Cmax of 65–80 mg/L, and proportionally higher Cmax/MIC, AUC/MIC and AUC > MIC values.10 The 70 mg/kg dose in guinea pigs produces a Cmax similar to 25 mg/kg in humans, and was used to assess the antituberculous activity of streptomycin in guinea pigs. Given the considerably shorter half-life in guinea pigs compared with humans, this equivalent dose was given five times weekly rather than three times weekly, which may have yielded a slightly higher AUC than the corresponding average value in humans.10
Assessment of bactericidal activity of streptomycin in heavily infected guinea pigs
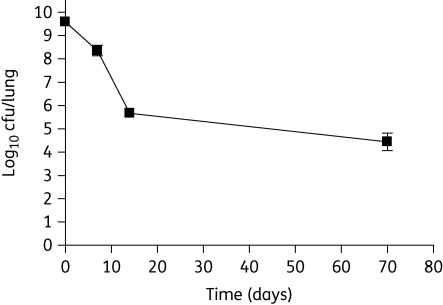
Streptomycin 70 mg/kg given five times weekly rescued animals from death and significantly improved gross lung pathology (data not shown). Streptomycin showed strong bactericidal activity during the first 14 days, reducing lung cfu by ∼4 log10 from 9.59 ± 0.06 log10 to 5.66 ± 0.11 log10; however, its activity was greatly diminished thereafter, causing a further reduction of only ∼1.0 log10 cfu/lung over the next 56 days of treatment (Figure 2).
Figure 2.
Biphasic killing of M. tuberculosis in guinea pig lungs by streptomycin treatment (70 mg/kg given five times/week), beginning 14 days after infection. n = 3 per timepoint; error bars represent standard deviation of log-transformed cfu values.
Lack of selection of streptomycin-resistant mutants in guinea pig lungs
The frequencies of streptomycin-resistant mutants for tubercle bacilli harvested from guinea pig lungs using streptomycin concentrations of 0.5, 1.0, 2.0 and 10.0 mg/L were 1.2 × 10−2–1.1 × 10−3, 1.9 × 10−5–6.2 × 10−5, 1.1 × 10−5–1.9 × 10−5 and <3.2 × 10−8, respectively, remaining constant after 0, 7, 14 and 70 days of treatment. The lack of enrichment in streptomycin-resistant mutants with streptomycin monotherapy indicates that the markedly reduced activity of the drug after the first 14 days of treatment is attributable to phenotypic drug tolerance.
Discussion
In this study, we demonstrate that streptomycin monotherapy in guinea pigs infected with M. tuberculosis produces a biphasic kill curve, as observed in humans with TB.9 Interestingly, streptomycin showed more potent antituberculous activity in the first 14 days of treatment in guinea pigs than did similar drug exposures in humans, in whom cfu declined by 0.133 log10 per day during the first 2 days of treatment, and in mice, in which lung cfu were reduced by ∼1.0 log10 after 4 weeks of treatment.11 The frequency of streptomycin-resistant mutants recovered from guinea pig lungs did not increase with continued streptomycin treatment, accounting for ∼0.0001% of cultivable bacilli after 70 days of treatment, indicating that the marked reduction in bactericidal activity of streptomycin during the second phase of the bacillary kill curve is due to the presence of drug-tolerant, persistent bacilli. An alternative explanation is that the remaining bacilli were inaccessible to the drug; however, streptomycin appears to readily penetrate TB lesions, including cavities,12 and penetration of the drug into guinea pig lung lesions would be expected to improve with continued treatment due to resolving inflammation.
In contrast to our findings, the selection of streptomycin-resistant mutants during streptomycin monotherapy has been well described in humans12 and mice.11 Lack of selection of streptomycin-resistant mutants in our study may have been related to higher drug exposures than those commonly observed in humans or because of the relatively short duration of therapy. Feldman et al.13 found that streptomycin-resistant mutants could be isolated from guinea pig lungs only after prolonged treatment (>200 days) with relatively low doses of streptomycin (6 mg/day). Crofton and Mitchison12 reported a mean time interval to emergence of streptomycin resistance exceeding 50 days among patients treated with streptomycin 2 g/day (given intramuscularly as 0.5 g doses four times daily), which would be expected to yield a lower Cmax/MIC than that experienced by the guinea pigs in our study. Finally, our inability to select for streptomycin-resistant mutants may potentially be explained by reduced virulence of these mutants in guinea pigs, as previous studies have shown that guinea pigs infected with strains of streptomycin-resistant tubercle bacilli survived longer than those infected with a similar inoculum of drug-susceptible organisms.14
The current study assists in defining the potential role of the guinea pig model of TB in future drug screening programmes. The lack of selection of drug-resistant mutants following monotherapy with streptomycin or isoniazid2 and the long-term persistence of fully drug-susceptible organisms suggest that the guinea pig may be the model of choice to evaluate the sterilizing activity of novel drugs against persisters. Recent studies have shown that combination regimens containing bactericidal and sterilizing antibiotics exhibit greater activity in guinea pigs relative to mice.7 Further characterization of the guinea pig as a preclinical model of TB chemotherapy will require comparison of the activity of combination regimens containing two or more bactericidal drugs with that of combinations containing multiple sterilizing drugs.
Funding
This work was supported by The Bill and Melinda Gates Foundation (TB Accelerator grant #42851 to J. H. G., E. L. N. and P. C. K.) and the National Institutes of Health (AI064229 and AI083125 to P. C. K.).
Transparency declarations
None to declare.
Acknowledgements
Portions of this work were presented as a poster at the American Thoracic Society International Conference, New Orleans, LA, USA, 2010 (#E25, Session B48).
References
- 1.Gumbo T, Louie A, Liu W, et al. Isoniazid's bactericidal activity ceases because of the emergence of resistance, not depletion of Mycobacterium tuberculosis in the log phase of growth. J Infect Dis. 2007;195:194–201. doi: 10.1086/510247. doi:10.1086/510247. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad Z, Klinkenberg LG, Pinn ML, et al. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis. 2009;200:1136–43. doi: 10.1086/605605. doi:10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 3.Jindani A, Aber VR, Edwards EA, et al. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–49. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 4.Karakousis PC. Mechanisms of action and resistance of antimycobacterial agents. In: Mayers D, editor. Antimicrobial Drug Resistance. New York: Humana Press; 2009. pp. 271–91. [Google Scholar]
- 5.Zhu M, Burman WJ, Jaresko GS, et al. Population pharmacokinetics of intravenous and intramuscular streptomycin in patients with tuberculosis. Pharmacotherapy. 2001;21:1037–45. doi: 10.1592/phco.21.13.1037.34625. doi:10.1592/phco.21.13.1037.34625. [DOI] [PubMed] [Google Scholar]
- 6.Canetti G. Present aspects of bacterial resistance in tuberculosis. Am Rev Respir Dis. 1965;92:687–703. doi: 10.1164/arrd.1965.92.5.687. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad Z, Nuermberger EL, Tasneen R, et al. Comparison of the ‘Denver regimen' against acute tuberculosis in the mouse and guinea pig. J Antimicrob Chemother. 2010;65:729–34. doi: 10.1093/jac/dkq007. doi:10.1093/jac/dkq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhillon J, Fielding R, Adler-Moore J, et al. The activity of low-clearance liposomal amikacin in experimental murine tuberculosis. J Antimicrob Chemother. 2001;48:869–76. doi: 10.1093/jac/48.6.869. doi:10.1093/jac/48.6.869. [DOI] [PubMed] [Google Scholar]
- 9.Donald PR, Sirgel FA, Venter A, et al. The early bactericidal activity of streptomycin. Int J Tuberc Lung Dis. 2002;6:693–8. [PubMed] [Google Scholar]
- 10.Peloquin CA, Berning SE, Nitta AT, et al. Aminoglycoside toxicity: daily versus thrice-weekly dosing for treatment of mycobacterial diseases. Clin Infect Dis. 2004;38:1538–44. doi: 10.1086/420742. doi:10.1086/420742. [DOI] [PubMed] [Google Scholar]
- 11.Lounis N, Ji B, Truffot-Pernot C, et al. Which aminoglycoside or fluoroquinolone is more active against Mycobacterium tuberculosis in mice? Antimicrob Agents Chemother. 1997;41:607–10. doi: 10.1128/aac.41.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crofton J, Mitchison DA. Streptomycin resistance in pulmonary tuberculosis. Br Med J. 1948;2:1009–15. doi: 10.1136/bmj.2.4588.1009. doi:10.1136/bmj.2.4588.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman WH, Karlson AG, Hinshaw HC. Streptomycin in experimental tuberculosis: the effects in guinea pigs following infection in intravenous inoculation. Am Rev Tuberc. 1947;56:346–59. [PubMed] [Google Scholar]
- 14.Karlson AG, Feldman WH, Hinshaw HC. Persistence of resistance of tubercle bacilli to streptomycin during passage through guinea pigs. Proc Soc Exp Biol Med. 1947;64:6. doi: 10.3181/00379727-64-15681. [DOI] [PubMed] [Google Scholar]