Abstract
Objectives
Free ritonavir, lopinavir and efavirenz injected intraperitoneally were compared with antiretroviral (AR) nanoparticles (NPs).
Methods
This is a prospective study in BALB/c mice comparing the pharmacokinetics of free drugs with AR NPs. All animals received free drugs or AR NPs (20 mg/kg) in PBS. In vitro replication assays were used for determination of the anti-HIV efficacy of NP formulations. At specific times (free drugs 0.08, 0.125, 0.25, 0.33, 1, 2 and 3 days; AR NPs 0.125, 0.33, 1, 2, 4, 7, 14, 21, 28, 35 and 42 days) mice were euthanized and serum and organs were harvested for determination of AR concentrations by HPLC. Single treatment of monocyte-derived macrophages (MDMs) infected with HIV-1ada compared AR NPs (0.005–0.05 mg/mL) with free efavirenz or lopinavir/ritonavir (0.01–0.1 mg/mL), blank NPs and controls. Results are presented as means ± SEM.
Results
Serum free AR drug concentrations peaked 4 h post-injection (ritonavir 3.9 ± 3.05, lopinavir 3.4 ± 2.5 and efavirenz 1.8 ± 0.63 µg/mL) and were eliminated by 72 h. Poly(dl-lactide-co-glycolide) NP animals had detectable ritonavir, lopinavir and efavirenz concentrations in all tissues for 28 days. Treatment of MDMs with AR NPs resulted in sustained inhibition of HIV-1ada replication.
Conclusions
AR drug concentrations from NPs are sustained for 28 days in vivo and anti-HIV inhibition was comparable to that of free drugs in vitro and could be a sustained treatment for delivery of AR drugs.
Keywords: HIV-1, ritonavir, lopinavir, efavirenz, PLGA
Introduction
Combination antiretroviral (AR) therapy (ART) has significantly reduced HIV-1 disease morbidity and lengthened life expectancy. Combinations of AR drugs from different classes have been proved to offer sustained efficacy, durability and long-term safety as long as the patient maintains a high adherence rate. The nucleoside-class-sparing regimen of ritonavir, lopinavir and efavirenz has been evaluated in several small trials.1,2 The results of these trials demonstrated that this regimen containing lopinavir/ritonavir and efavirenz can reduce viral load to non-detectable levels in >80% of patients and should be considered a treatment option for treatment-naive patients.
Controlling viral replication allows at least partial reconstitution of the immune system. However, despite sustained viral suppression for prolonged periods, eradication of HIV-1 from patients has not been achieved. A number of factors make eradication of HIV-1 by ART difficult, including: long-term adherence to complex AR regimens of drugs with low margins of pharmacokinetic deviation; viral reservoirs that survive in the presence of ART; and the potential existence of sanctuary sites within the body where AR drug concentrations are not optimal.3 Additionally, treatment failure due to non-adherence, emerging resistance to ARs and limited global access has prevented worldwide suppression of HIV infection using ART.4,5
Here we present initial data that AR nanoparticles (NPs) containing lopinavir/ritonavir and efavirenz deliver sustained concentrations of ARs in the serum and organs of non-infected BALB/c mice. Additionally, we studied the in vitro efficacy of the AR NPs using single-dose replication assays. This is the first report of the in vivo pharmacokinetics and in vitro efficacy for triple-drug AR NPs.
Methods
AR NPs containing ritonavir, lopinavir and efavirenz were fabricated using a homogenization solvent extraction procedure as previously described.6 Briefly, a water-in-oil-in-water emulsion of poly(dl-lactide-co-glycolide) (PLGA) polymer in methylene chloride containing 20 mg of each of the AR drug powders (Sequoia Research Products Ltd, UK) was used to fabricate the NPs. This was homogenized in 0.1% polyvinyl alcohol and added to 1% Pluronic F-127 at 100 W. The methylene chloride was evaporated from the emulsion over 4 h; the emulsion was rinsed with double-distilled water, centrifuged at 15 000 rpm (4°C) for 30 min and lyophilized for 24–48 h. Drug loading and entrapment efficiency were determined as previously described.6
The lyophilized NP powder was weighed and the equivalent of 20 mg/kg was dissolved in PBS and injected into BALB/c male mice (25–28 g) intraperitoneally. Free drug powders (20 mg/kg) of ritonavir, lopinavir and efavirenz were dissolved in ethyl alcohol (10 µL/mouse) and then further dissolved in PBS and injected intraperitoneally. Animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC #0833) of Creighton University. Animals were treated in accordance with relevant care guidelines. At specified times (0.08, 0.125, 0.25, 0.33, 1, 2 and 3 days for free drugs only; 0.125, 0.33, 1, 2, 4, 7, 14, 21, 28, 35 and 42 days for AR NPs), mice (n = 3/timepoint) were euthanized using a CO2 chamber and organs (spleen, liver, kidneys, brain and testes) and blood (100–150 µL) were removed from each mouse. Blood was allowed to clot, clarified by centrifugation (1000 g) and the serum harvested. The organs were harvested, immediately placed on ice and frozen (−80°C) until analysed by HPLC. For HPLC analysis, an aliquot of the organ tissue was weighed and placed in a microfuge tube where 500 µL of 100% methanol was added to tissue and serum samples. The tissue was homogenized using a pellet homogenizer, equilibrated at 4°C for 30 min and then centrifuged (11 400 rpm for 15 min at 4°C). An aliquot of the supernatant (20 µL/injection) was added to autosampler vials with glass inserts. Lopinavir, ritonavir and efavirenz concentrations were determined using an HPLC instrument (Shimadzu Corp., Columbia, MD, USA) using a previously published HPLC method.7 Tissues and serum samples were assayed in duplicate and standards assayed in triplicate for each experiment. The organ/serum sample concentrations were calculated using EZ-Chrom software (Shimadzu Corp.) using the peak area of the standard curve (2.25–50 µg/mL) that was performed on the same day. Intra-day and inter-day variability of the standards was calculated as <10%.
Frozen elutriated monocytes were obtained from AllCells, LLC (Emeryville, CA, USA). Cells were thawed in a water bath at 37°C and counted using Trypan Blue exclusion. Cells (5 × 105/well) were placed into 4-well chamber-tek slides (Nunc, Lab-tek; Fisher, St Louis, MO, USA) with filtered sterilized Dulbecco's modified Eagle's medium containing 10% human serum, 2 mM l-glutamine, 1% penicillin/streptomycin and 10 µg/mL ciprofloxacin. Medium was supplemented with 1000 U/mL macrophage colony stimulating factor (R & D Systems, Inc. Minneapolis, MN, USA) to differentiate the cells into macrophages after 7 days of incubation.8 Medium was half-exchanged every 2–3 days throughout the experiments. On day 7, medium was removed and fresh medium (medium as above without macrophage colony stimulating factor; medium B) was added to the cells. Monocyte-derived macrophages (MDMs) were infected with HIV-1ada (AIDS Research Resources, NIH, Bethesda, MD, USA) overnight at a multiplicity of infection (moi) of 0.01. The next morning, the medium was removed, the cells were washed with warm PBS and fresh medium B was added to the cells. Cells were inoculated with 0.01 and 0.1 mg/mL blank NPs, free efavirenz (0.01 and 0.1 mg/mL), free lopinavir/ritonavir (0.01 and 0.1 mg/mL each drug, respectively) or 0.005 and 0.05 mg/mL AR NPs for 4 h in triplicate. After 4 h the medium was removed, the cells were washed with warm PBS and fresh medium B was added to the cells for the remainder of the experiment. On days 5, 7 and 10, medium was collected, passed through a 0.22 µm filter and stored at −80°C. p24 antigen ELISA assays were performed according to the manufacturer's instructions (Advanced Bioscience Laboratories, Inc., Kensington, MD, USA). All samples were assayed in duplicate for each experiment. The ELISA data are presented as means ± SEM of triplicate experiments.
The area under the serum concentration–time curve to the last concentration (AUC0−last) was calculated by the trapezoid rule using pharmacokinetic software (PK Solutions version 2.0; Summit Research Services, Montrose, CO, USA). Comparison of AUC0−last and peak serum concentrations was performed between free drugs and AR NPs using Student's t-test (SPSS, Chicago, IL, USA). The a priori level of significance was 0.05. Data are presented as means ± SEM.
Results
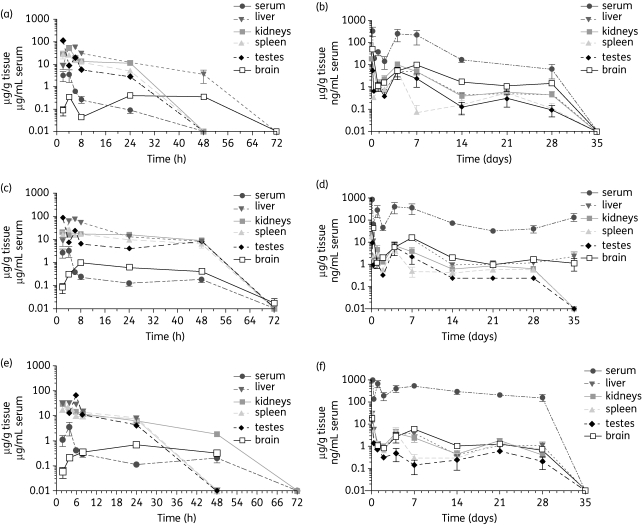
The delivery and sustainability of AR-containing NPs was assessed by comparing the serum and organ concentrations of AR over time with free injected ARs. The serum and organ concentrations of the free (20 mg/kg) lopinavir/ritonavir/efavirenz combination drug treatments are shown in Figure 1(a, c and e). The peak free AR drug concentrations in the serum were found at 4 h post-injection (ritonavir 3.9 ± 3.1, lopinavir 3.4 ± 2.5, efavirenz 1.8 ± 0.63 µg/mL). The free AR drugs were eliminated to non-detectable concentrations in all tissues and serum by day 3.
Figure 1.
Serum and organ concentration versus time curves for AR drugs after 20 mg/kg intraperitoneal injection into mice. (a, c and e) Free drug injections. (b, d and f) AR NP injections. (a) Free ritonavir concentrations. (b) Ritonavir concentrations from NP formulation. (c) Free lopinavir concentrations. (d) Lopinavir concentrations from NP formulation. (e) Free efavirenz concentrations. (f) Efavirenz concentrations from NP formulation. Error bars represent SEM of three mice measurements by HPLC.
AR NPs were synthesized using a homogenization solvent extraction procedure. The size of the AR NPs averaged 331.2 ± 77.2 nm with an average surface charge of −13.8 ± 1.9 (n = 4 batches of NPs). AR NPs were injected into mice intraperitoneally and samples were collected for a total of 42 days. The concentrations of ARs found in the serum and organs for the mice treated with 20 mg/kg of the combination drugs in PLGA NPs are shown in Figure 1(b, d and f). In stark contrast to the free injected ARs, the delivery of AR in NPs resulted in a substantially longer presence of ARs in all tested organs and serum. Detectable concentrations of ARs were found in serum and tissue up to 28 days post-injection. Compared with the free injected ARs the peak AR drug concentrations in serum were lower (ritonavir 0.72 ± 0.02, lopinavir 1.0 ± 0.1 and efavirenz 1.0 ± 0.15 µg/mL; P > 0.05). The AUC0−last was significantly higher (P < 0.05) for efavirenz from AR NPs (ritonavir 97.9 ± 137.2, lopinavir 183.4 ± 169.3 and efavirenz 230.6 ± 80.6 µg h/mL) compared with free drugs (ritonavir 17.2 ± 9.7, lopinavir 22.6 ± 22.5 and efavirenz 20.4 ± 21.1 µg h/mL). Lopinavir and ritonavir AUC0−last were not significantly different, averaging >8 and >5 times higher than the free drugs, respectively.
To determine whether AR NPs would penetrate the blood–brain barrier, we determined brain tissue concentrations of the AR drugs. For all three of the ARs, the peak concentrations delivered to the brains of mice injected with NP ARs were significantly higher (P < 0.03) than for the mice injected with soluble ARs. None of the three freely injected ARs reached a concentration of >1 µg/g in brain tissue during the experiment. In sharp contrast, the concentrations of ARs delivered by NPs peaked at concentrations of >5 µg/g in the brain. Indeed, lopinavir concentrations in the brain were still detectable (averaging 1.2 µg/g) 35 days post-injection of the single dose.
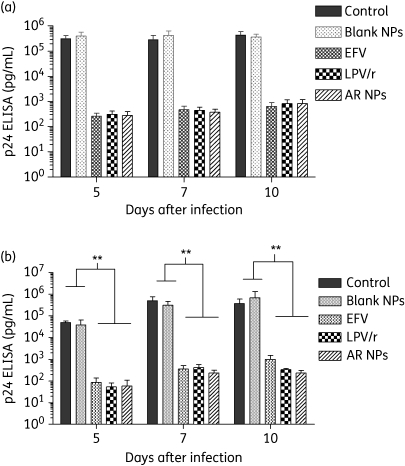
To determine whether sustainable inhibition of virus replication was achievable with single AR NP delivery, we assayed the replication of HIV-1ada in MDMs given a single 4 h treatment with either free ARs or AR NP suspension. Virus replication was monitored by p24 antigen ELISA of supernatants at 5, 7 and 10 days post-infection. Assays were performed with two different concentrations of AR NPs (0.005 and 0.05 mg/mL; Figure 2a and b, respectively). A single 4 h treatment of efavirenz and lopinavir/ritonavir as free drugs (0.1 mg/mL, respectively) or AR NPs (0.05 mg/mL) resulted in a significant (>90%; P < 0.01) inhibition of HIV-1 compared with MDMs treated with either blank NPs or controls. When 0.01 mg/mL free efavirenz or lopinavir/ritonavir, respectively, or 0.005 mg/mL AR NPs was added to the cell cultures the replication assays approached significance (P = 0.073).
Figure 2.
AR NPs inhibit HIV-1 replication in MDMs in vitro. Virus replication was measured by the level of capsid protein (p24) released into the medium at the days indicated. Cells were treated as indicated for 4 h at 1 day post-infection. Control represents HIV-1-infected MDMs. (a) Data from an experiment in which free drugs were given at 0.01 mg/mL and AR NP was given at 0.005 mg/mL. (b) Data from an experiment in which MDMs were treated with 0.1 mg/mL free drugs or 0.05 mg/mL AR NPs. Error bars represent SEM. Data are representative of three independent experiments. **A significant difference between blank NPs or control MDMs and free AR drugs or AR NPs (P < 0.01). EFV, efavirenz; LPV/r, lopinavir/ritonavir.
Discussion
The results of these experiments show that AR NPs can be fabricated to include three AR drugs using PLGA polymer. The AR NPs released detectable concentrations of the three ARs for a minimum of 28 days in vivo, significantly longer than freely injected drugs. The AUC0−last for the three AR drugs was larger for the AR NPs, but was only significant for efavirenz. These results show that the AR drugs associated with the NPs are able to maintain drug concentrations for a prolonged period. Nanotechnology is a drug delivery method that attempts to maintain similar drug concentrations to a slow continuous infusion, without large fluctuations in the peak and trough concentrations. Additionally, the AR NPs were able to interact with MDMs and inhibit virus replication up to 1000-fold for 10 days. These data provide evidence that NPs may be able to offer sustainable highly active ART with a single intramuscular/subcutaneous injection. There are many patient populations that would find this dosage form useful by offering a less intensive treatment option, including patients in some low-socio-economic countries affected by HIV and non-adherent patients.
NP drug delivery systems have been fabricated with a single AR drug.9–18 However, this is the first demonstration of NP delivery with a combination of three AR drugs in the same NP. Examination of the AR time curves show that the AR NPs display a longer residence time within serum and tissues as compared with the free drugs when administered in the same equivalent dose in an animal model. This may be due to a number of factors including the slow breakdown of the AR polymer and/or the cellular uptake of the particles followed by the slow release of the ARs from intracellular breakdown of the polymer. We previously demonstrated efficient uptake by and sustained presence of AR NPs in MDMs.6 Fluorescent NPs could be visualized in MDMs for up to 42 days (data not shown). Coupled with the in vivo data presented here, a potential mechanism of the movement of the AR drugs to distal organs of the body could be the phagocytosis and transport of AR NPs by peritoneal macrophages via the circulatory system. More research is necessary to track the exact transport of the NPs over time.
The ability of the AR NPs to sustain prolonged concentrations of ARs in tissues may help to eradicate HIV-1 from sequestered sites where free drug concentrations are sub-therapeutic.19–21 Moreover, monocytes and macrophages are able to survive the cytopathic effect of HIV-1 replication and support long-term production of HIV-1 virions without significant alteration in their homeostasis.8,22,23 HIV-1-infected MDMs have also been shown to fuse with autologous and heterologous CD4+ T cells and efficiently transmit virus.24,25 The AR NP drug delivery strategy may be useful to prevent HIV-1 transmission via this mechanism. Additionally, since NPs release AR drugs for up to 30 days after a single 20 mg/kg dose in mice, this may inhibit HIV-1 in MDMs functioning as a reservoir of HIV-1 infection. However, more AR NP research is necessary to determine whether this could be a viable treatment option. A non-human primate model using RT-SHIV, a chimera of simian immunodeficiency virus containing the HIV-1 reverse transcriptase, may be necessary to fully evaluate AR NPs as a treatment modality. Finally, the replication studies demonstrated that single treatments of AR NPs are capable of inhibiting HIV-1 up to 10 days post-infection. A single dose of AR NPs at 5 µg/mL produced a similar level of inhibition to free ARs at 10 µg/mL.
This combination of AR drugs is not typically considered as a first-line treatment regimen for HIV-infected patients; however, several reports demonstrate its utility. In a comparative trial in treatment-naive patients, Riddler et al.2 showed that lopinavir/ritonavir + efavirenz as a nucleoside reverse transcriptase inhibitor (NRTI)-sparing treatment option had similar efficacy to efavirenz + two NRTIs after 96 weeks of follow-up (83% and 89%, respectively). In studies of AR-experienced patients, Calmy et al.26 showed that the percentage of patients who had plasma HIV RNA < 500 copies/mL rose from 25% at baseline to 68% at 2 years using NRTI-sparing regimens. This report also showed that CD4+ cells increased by an average of 111 cells/µL during the same time period.
Currently, PLGA is not considered a mainstay drug delivery system in humans. In an animal model there is one report of the use of PLGA NPs as a sustained delivery option for prophylaxis against HIV. Woodrow et al.27 reported PLGA NPs loaded with small-interfering RNA (siRNA) as a topical microbicide. The PLGA NPs incorporated siRNA targeted against enhanced green fluorescent protein (EGFP) and were delivered by vaginal instillation into transgenic GFP mice. Mice were monitored over 2 weeks for GFP expression. NPs significantly reduced GFP expression near the cervix as well as in the uterine horns after 10 days. Additionally, it appeared that the PLGA NPs did not produce inflammation when delivered by vaginal instillation. PLGA could be used as a topical microbicide delivery vehicle.
The experiments reported here demonstrate the in vivo feasibility of using PLGA-fabricated AR NPs as a monthly drug delivery system for multiple ARs. A single injection of AR NPs provided serum and tissue concentrations of ritonavir, lopinavir and efavirenz above the IC50 for wild-type virus for a minimum of 28 days. The efficacy of the AR NPs is shown by their ability to inhibit HIV-1 in vitro comparably to free drugs.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (grant number 1R15AI076039-01A1 to C. J. D.). The Animal Facility has been supported by G20RR024001 from the National Center for Research Resources.
Transparency declarations
None of the authors has any financial or other disclosures associated with the production of this manuscript.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Acknowledgements
The following reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID: NIH HIV-1ada (from Dr Howard Gendelman).
References
- 1.Allavena C, Ferre V, Brunet-Francois C, et al. Efficacy and tolerability of a nucleoside reverse transcriptase inhibitor-sparing combination of lopinavir/ritonavir and efavirenz in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39:300–6. doi: 10.1097/01.qai.0000165914.42827.bb. doi:10.1097/01.qai.0000165914.42827.bb. [DOI] [PubMed] [Google Scholar]
- 2.Riddler SA, Haubrich R, DiRenzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. New Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. doi:10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crowe S, Zhu T, Muller WE. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–41. doi: 10.1189/jlb.0503204. doi:10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- 4.Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutrition. 2005;21:96–9. doi: 10.1016/j.nut.2004.09.013. doi:10.1016/j.nut.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Plot P, Bartos M, Ghys PD, et al. The global impact of HIV/AIDS. Nature. 2001;410:968–73. doi: 10.1038/35073639. doi:10.1038/35073639. [DOI] [PubMed] [Google Scholar]
- 6.Destache CJ, Belgum T, Christensen K, et al. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC Infect Dis. 2009;9:198. doi: 10.1186/1471-2334-9-198. doi:10.1186/1471-2334-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weller DR, Brundage RC, Balfour HH, Jr, et al. An isocratic liquid chromatography method for determining HIV non-nucleoside reverse transcriptase inhibitor and protease inhibitor concentrations in human plasma. J Chromatogr B Anal Technol Biomed Life Sci. 2007;848:369–73. doi: 10.1016/j.jchromb.2006.10.022. doi:10.1016/j.jchromb.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Gendelman HE, Orenstein JM, Martin MA, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–41. doi: 10.1084/jem.167.4.1428. doi:10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dou H, Destache CJ, Morehead JR, et al. Development of a macrophage-based nanoparticle platform for anti-retroviral drug delivery. Blood. 2006;108:2827–35. doi: 10.1182/blood-2006-03-012534. doi:10.1182/blood-2006-03-012534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dou H, Morehead J, Destache CJ, et al. Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocytes-derived macrophages. Virology. 2007;358:148–58. doi: 10.1016/j.virol.2006.08.012. doi:10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Gagne J-F, Desormeaux A, Perron S, et al. Targeted delivery of indinavir to HIV-1 primary reservoirs with immunoliposomes. Biochim Biophys Acta. 2002;1558:198–210. doi: 10.1016/s0005-2736(01)00432-1. doi:10.1016/S0005-2736(01)00432-1. [DOI] [PubMed] [Google Scholar]
- 12.Gorantla S, Dou H, Boska M, et al. Quantitative magnetic resonance and SPECT imaging for macrophage tissue migration and nanoformulated drug delivery. J Leukoc Biol. 2006;80:1165–74. doi: 10.1189/jlb.0206110. doi:10.1189/jlb.0206110. [DOI] [PubMed] [Google Scholar]
- 13.Kuo Y-C. Loading efficiency of stavudine on polybutylcyanoacrylate and methmethacrylate-sulfopropylmethacrylate copolymer nanoparticles. Int J Pharmaceut. 2005;290:161–72. doi: 10.1016/j.ijpharm.2004.11.025. doi:10.1016/j.ijpharm.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Mainardes RM, Gremiao MP, Brunetti IL, et al. Zidovudine-loaded PLA and PLA-PEG blend nanoparticles: influence of polymer type on phagocytic uptake by polymorphonuclear cells. J Pharm Sci. 2009;98:257–67. doi: 10.1002/jps.21406. doi:10.1002/jps.21406. [DOI] [PubMed] [Google Scholar]
- 15.Nowacek AS, Miller RL, McMillan J, et al. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (Lond) 2009;4:903–17. doi: 10.2217/nnm.09.71. doi:10.2217/nnm.09.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah LK, Amiji MM. Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS. Pharm Res. 2006;23:2638–45. doi: 10.1007/s11095-006-9101-7. doi:10.1007/s11095-006-9101-7. [DOI] [PubMed] [Google Scholar]
- 17.Bender AR, von Briesen H, Kreuter J, et al. Efficiency of nanoparticles as a carrier system for antiretroviral agents in human immunodeficiency virus-infected human monocytes/macrophages in vitro. Antimicrob Agents Chemother. 1996;40:1467–71. doi: 10.1128/aac.40.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chattopadhyay N, Zastre J, Wong HL, et al. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res. 2008;25:2262–71. doi: 10.1007/s11095-008-9615-2. doi:10.1007/s11095-008-9615-2. [DOI] [PubMed] [Google Scholar]
- 19.Dybul M, Chun TW, Ward DJ, et al. Evaluation of lymph node virus burden in human immunodeficiency virus-infected patients receiving efavirenz-based protease inhibitor-sparing highly active antiretroviral therapy. J Infect Dis. 2000;181:1273–9. doi: 10.1086/315407. doi:10.1086/315407. [DOI] [PubMed] [Google Scholar]
- 20.Wong JK, Gunthard HF, Havlir DV, et al. Reduction of HIV-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proc Natl Acad Sci USA. 1997;94:12574–9. doi: 10.1073/pnas.94.23.12574. doi:10.1073/pnas.94.23.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Mascio M, Srinivasula S, Bhattacharjee A, et al. Antiretroviral tissue kinetics: in vivo imaging using positron emission tomography. Antimicrob Agents Chemother. 2009;53:4086–95. doi: 10.1128/AAC.00419-09. doi:10.1128/AAC.00419-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schrager LK, D'Souza P. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. J Am Med Assoc. 1998;280:67–71. doi: 10.1001/jama.280.1.67. doi:10.1001/jama.280.1.67. [DOI] [PubMed] [Google Scholar]
- 23.Solas C, Lafeuillade A, Halfon P, et al. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47:238–43. doi: 10.1128/AAC.47.1.238-243.2003. doi:10.1128/AAC.47.1.238-243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe SM, Mills J, Elbeik T, et al. Human immunodeficiency virus-infected monocyte-derived macrophages express surface gp120 and fuse with CD4 lymphoid cells in vitro: a possible mechanism of T lymphocyte depletion in vivo. Clin Immunol Immunopathol. 1992;65:143–51. doi: 10.1016/0090-1229(92)90217-c. doi:10.1016/0090-1229(92)90217-C. [DOI] [PubMed] [Google Scholar]
- 25.Crowe SM, Mills J, Kirihara J, et al. Full-length recombinant CD4 and recombinant gp120 inhibit fusion between HIV infected macrophages and uninfected CD4-expressing T-lymphoblastoid cells. AIDS Res Hum Retroviruses. 1990;6:1031–7. doi: 10.1089/aid.1990.6.1031. [DOI] [PubMed] [Google Scholar]
- 26.Calmy A, Petoumenos K, Lewden C, et al. Combination antiretroviral therapy without a nucleoside reverse transcriptase inhibitor: experience from 334 patients in three cohorts. HIV Med. 2007;8:171–80. doi: 10.1111/j.1468-1293.2007.00448.x. doi:10.1111/j.1468-1293.2007.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woodrow KA, Cu Y, Booth CJ, et al. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526. doi: 10.1038/nmat2444. 33 doi:10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]