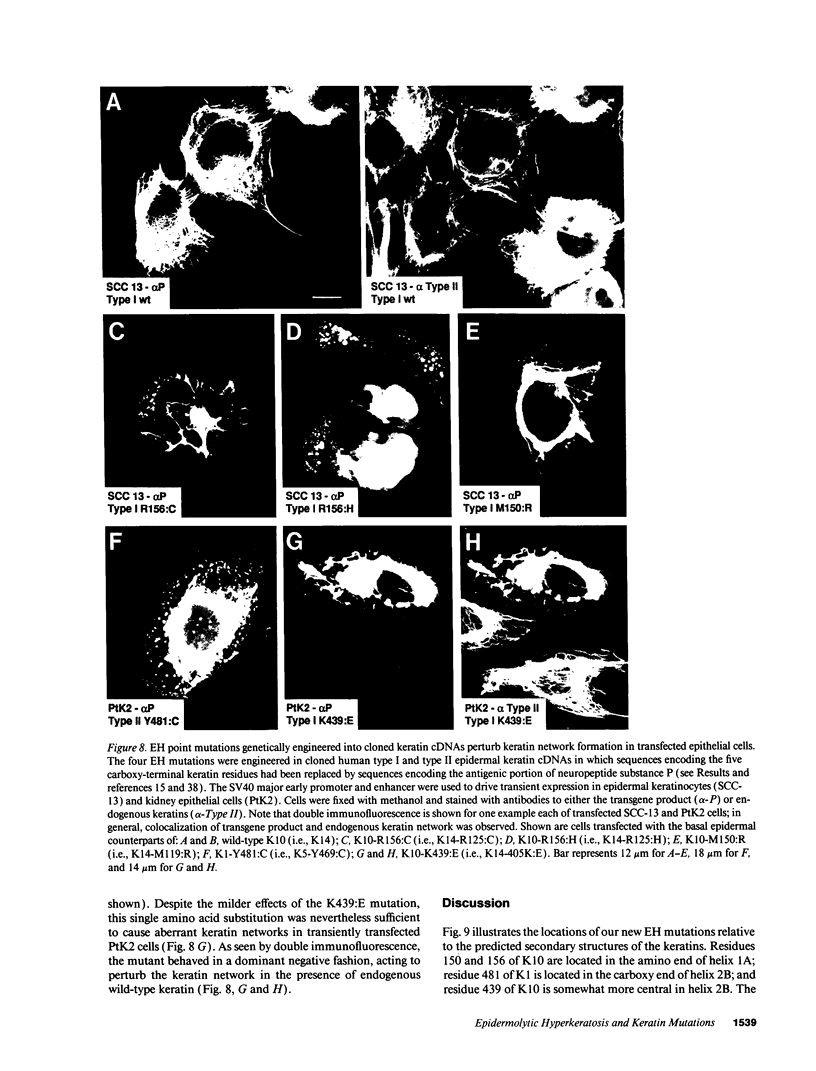
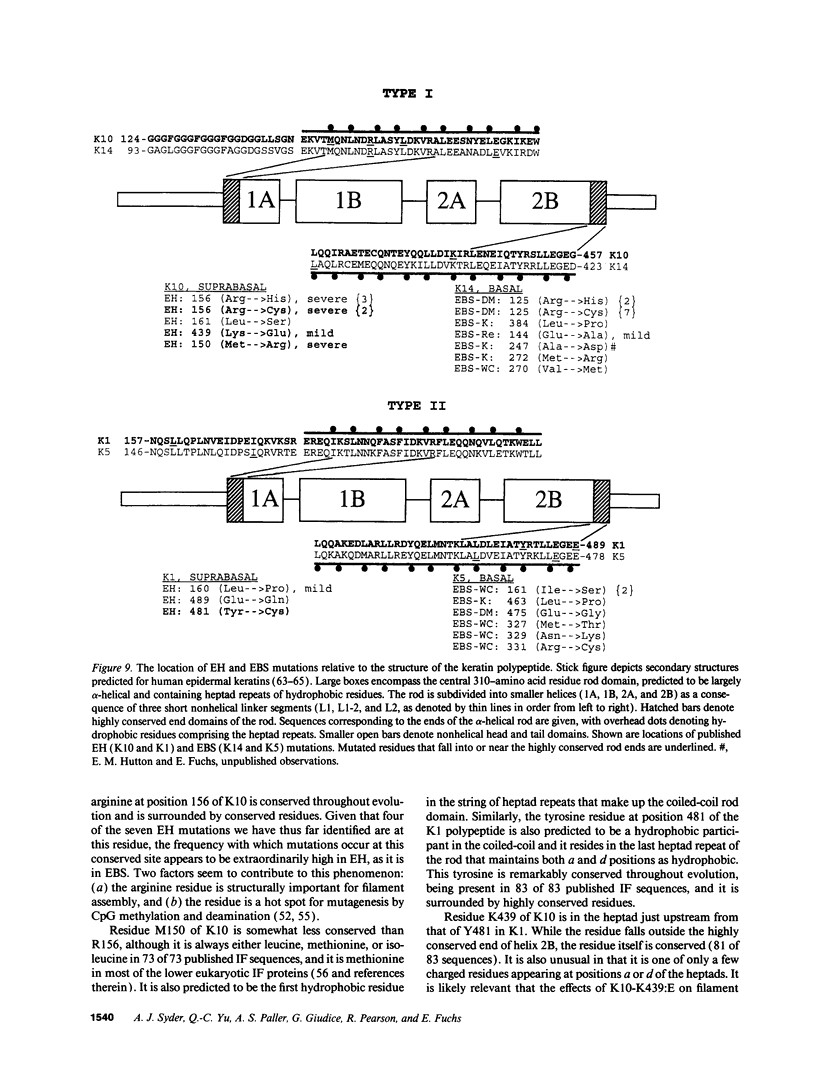
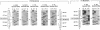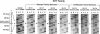Abstract
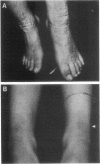
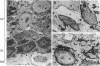
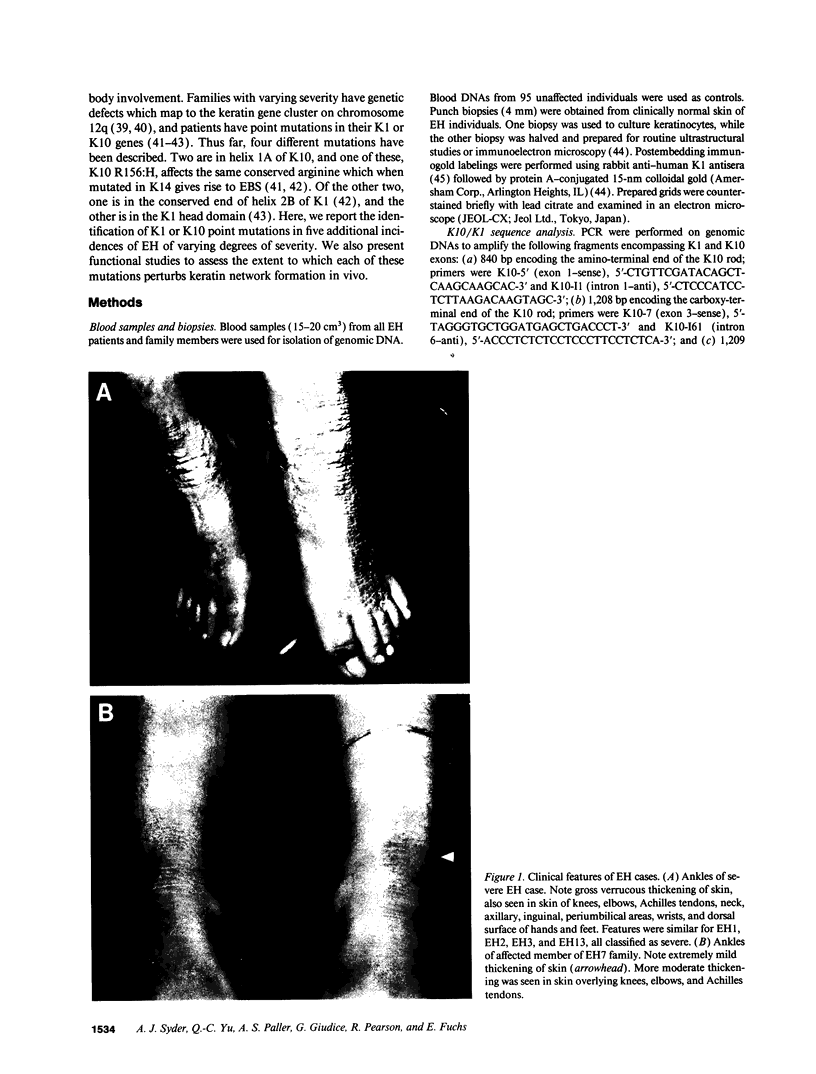
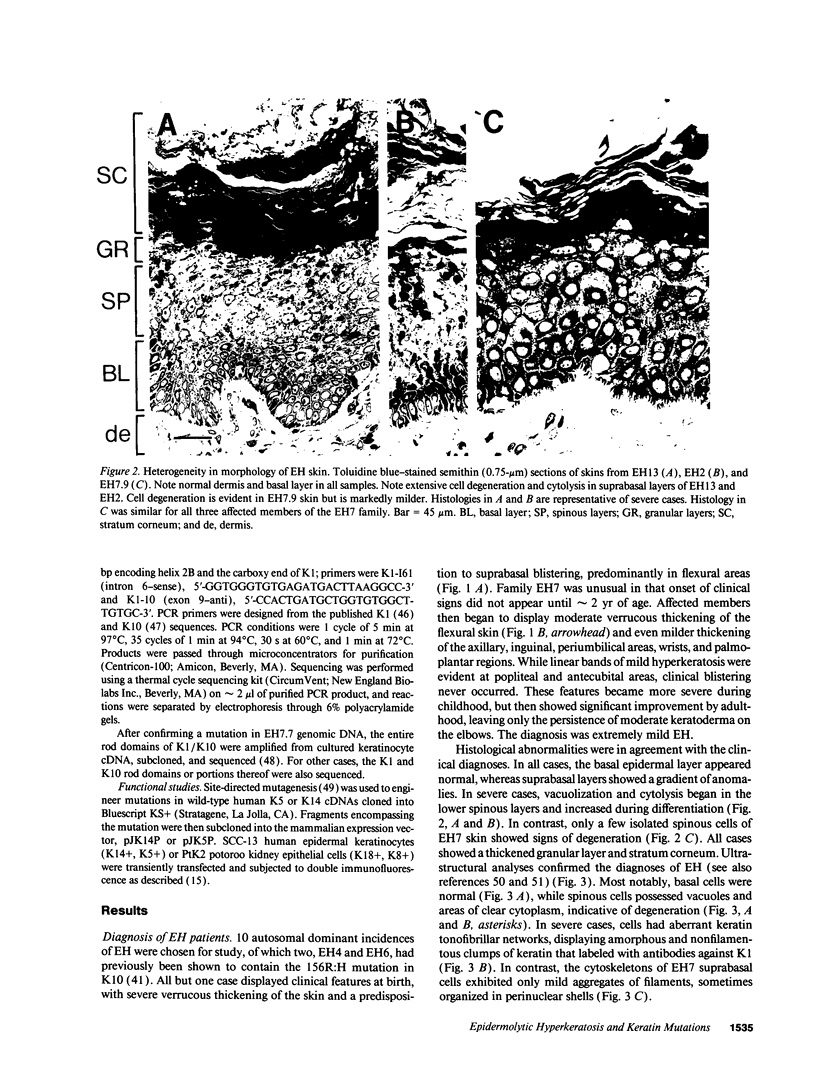
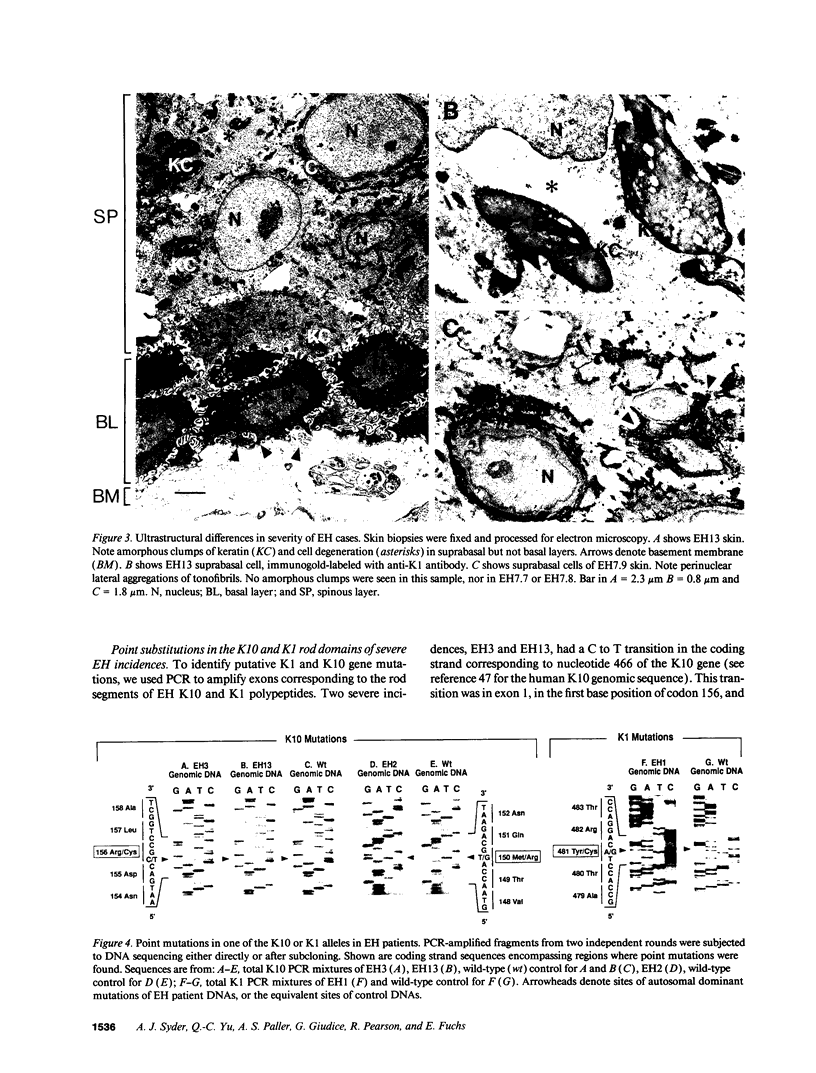
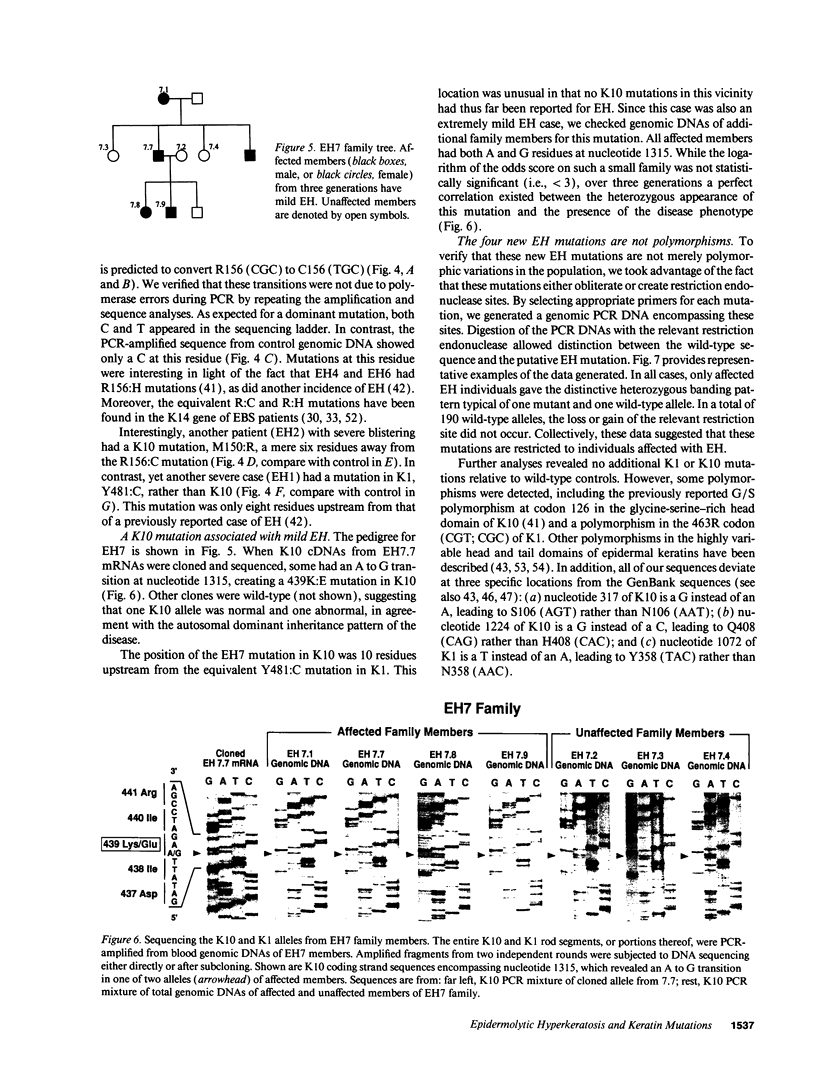
Epidermolytic hyperkeratosis (EH) is a skin disease caused by mutations in the genes encoding K1 and K10, the differentiation-specific keratins of epidermis. To explore the heterogeneity of mutations and to assess whether a correlation exists between disease severity and the extent to which a mutation interferes with keratin network formation, we determined the genetic bases of four severe incidences of EH and one unusually mild case. Two severe cases have the same mutation, K10-R156:C, at a conserved arginine that we previously showed was mutated to a histidine in two unrelated EH families. An additional severe case has a mutation six residues away, still within the amino end of the alpha-helical rod domain of K10. The other severe case has a mutation in the conserved carboxy end of the K1 rod. In contrast, affected members of the atypically mild family have a mutation just proximal to the conserved carboxy end of the K10 rod. By genetic engineering and gene transfection, we demonstrate that each mutation is functionally responsible for the keratin filament aberrations that are typical of keratinocytes cultured from these patients. Moreover, we show that the mild EH mutation less severely affects filament network formation. Taken together, our studies strengthen the link between filament perturbations, cell fragility, and degeneration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Fowler W. E., Rew P., Sun T. T. The fibrillar substructure of keratin filaments unraveled. J Cell Biol. 1983 Oct;97(4):1131–1143. doi: 10.1083/jcb.97.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers K., Fuchs E. Expression of mutant keratin cDNAs in epithelial cells reveals possible mechanisms for initiation and assembly of intermediate filaments. J Cell Biol. 1989 Apr;108(4):1477–1493. doi: 10.1083/jcb.108.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers K., Fuchs E. The expression of mutant epidermal keratin cDNAs transfected in simple epithelial and squamous cell carcinoma lines. J Cell Biol. 1987 Aug;105(2):791–806. doi: 10.1083/jcb.105.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers K., Fuchs E. The molecular biology of intermediate filament proteins. Int Rev Cytol. 1992;134:243–279. doi: 10.1016/s0074-7696(08)62030-6. [DOI] [PubMed] [Google Scholar]
- Anton-Lamprecht I., Schnyder U. W. Ultrastructure of inborn errors of keratinization. VI. Inherited ichthyoses--a model system for heterogeneities in keratinization disturbances. Arch Dermatol Forsch. 1974;250(3):207–227. [PubMed] [Google Scholar]
- Bonifas J. M., Rothman A. L., Epstein E. H., Jr Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1991 Nov 22;254(5035):1202–1205. doi: 10.1126/science.1720261. [DOI] [PubMed] [Google Scholar]
- Chan Y. M., Yu Q. C., Fine J. D., Fuchs E. The genetic basis of Weber-Cockayne epidermolysis bullosa simplex. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7414–7418. doi: 10.1073/pnas.90.15.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Syder A. J., Yu Q. C., Letai A., Paller A. S., Fuchs E. The genetic basis of epidermolytic hyperkeratosis: a disorder of differentiation-specific epidermal keratin genes. Cell. 1992 Sep 4;70(5):811–819. doi: 10.1016/0092-8674(92)90314-3. [DOI] [PubMed] [Google Scholar]
- Chipev C. C., Korge B. P., Markova N., Bale S. J., DiGiovanna J. J., Compton J. G., Steinert P. M. A leucine----proline mutation in the H1 subdomain of keratin 1 causes epidermolytic hyperkeratosis. Cell. 1992 Sep 4;70(5):821–828. doi: 10.1016/0092-8674(92)90315-4. [DOI] [PubMed] [Google Scholar]
- Chipev C. C., Yang J. M., DiGiovanna J. J., Steinert P. M., Marekov L., Compton J. G., Bale S. J. Preferential sites in keratin 10 that are mutated in epidermolytic hyperkeratosis. Am J Hum Genet. 1994 Feb;54(2):179–190. [PMC free article] [PubMed] [Google Scholar]
- Compton J. G., DiGiovanna J. J., Santucci S. K., Kearns K. S., Amos C. I., Abangan D. L., Korge B. P., McBride O. W., Steinert P. M., Bale S. J. Linkage of epidermolytic hyperkeratosis to the type II keratin gene cluster on chromosome 12q. Nat Genet. 1992 Jul;1(4):301–305. doi: 10.1038/ng0792-301. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Chan Y. M., Albers K., Fuchs E. Deletions in epidermal keratins leading to alterations in filament organization in vivo and in intermediate filament assembly in vitro. J Cell Biol. 1990 Dec;111(6 Pt 2):3049–3064. doi: 10.1083/jcb.111.6.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Fuchs E. Elucidating the early stages of keratin filament assembly. J Cell Biol. 1990 Jul;111(1):153–169. doi: 10.1083/jcb.111.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S., Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991 Sep 20;66(6):1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Vassar R., Fuchs E. A function for keratins and a common thread among different types of epidermolysis bullosa simplex diseases. J Cell Biol. 1991 Dec;115(6):1661–1674. doi: 10.1083/jcb.115.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Kopan R., Fuchs E. Expression of keratin K14 in the epidermis and hair follicle: insights into complex programs of differentiation. J Cell Biol. 1989 Nov;109(5):2295–2312. doi: 10.1083/jcb.109.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Ryynänen M., Uitto J. Identification of a leucine-to-proline mutation in the keratin 5 gene in a family with the generalized Köbner type of epidermolysis bullosa simplex. Hum Mutat. 1993;2(2):94–102. doi: 10.1002/humu.1380020206. [DOI] [PubMed] [Google Scholar]
- Engel A., Eichner R., Aebi U. Polymorphism of reconstituted human epidermal keratin filaments: determination of their mass-per-length and width by scanning transmission electron microscopy (STEM). J Ultrastruct Res. 1985 Mar;90(3):323–335. doi: 10.1016/s0022-5320(85)80010-1. [DOI] [PubMed] [Google Scholar]
- Fuchs E. V., Coppock S. M., Green H., Cleveland D. W. Two distinct classes of keratin genes and their evolutionary significance. Cell. 1981 Nov;27(1 Pt 2):75–84. doi: 10.1016/0092-8674(81)90362-7. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Coulombe P. A. Of mice and men: genetic skin diseases of keratin. Cell. 1992 Jun 12;69(6):899–902. doi: 10.1016/0092-8674(92)90607-e. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Esteves R. A., Coulombe P. A. Transgenic mice expressing a mutant keratin 10 gene reveal the likely genetic basis for epidermolytic hyperkeratosis. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6906–6910. doi: 10.1073/pnas.89.15.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Geisler N., Schünemann J., Weber K. Chemical cross-linking indicates a staggered and antiparallel protofilament of desmin intermediate filaments and characterizes one higher-level complex between protofilaments. Eur J Biochem. 1992 Jun 15;206(3):841–852. doi: 10.1111/j.1432-1033.1992.tb16992.x. [DOI] [PubMed] [Google Scholar]
- Gill S. R., Wong P. C., Monteiro M. J., Cleveland D. W. Assembly properties of dominant and recessive mutations in the small mouse neurofilament (NF-L) subunit. J Cell Biol. 1990 Nov;111(5 Pt 1):2005–2019. doi: 10.1083/jcb.111.5.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. Cultured cells for the treatment of disease. Sci Am. 1991 Nov;265(5):96–102. doi: 10.1038/scientificamerican1191-96. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a Type II cytoskeletal keratin reveals constant and variable structural domains among keratins. Cell. 1983 Jul;33(3):915–924. doi: 10.1016/0092-8674(83)90034-x. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Fuchs E. The cDNA sequence of a human epidermal keratin: divergence of sequence but conservation of structure among intermediate filament proteins. Cell. 1982 Nov;31(1):243–252. doi: 10.1016/0092-8674(82)90424-x. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M., Weber K. Modulation of keratin intermediate filament assembly by single amino acid exchanges in the consensus sequence at the C-terminal end of the rod domain. J Cell Sci. 1991 Jun;99(Pt 2):351–362. doi: 10.1242/jcs.99.2.351. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M., Weber K. The coiled coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratins: use of site-specific mutagenesis and recombinant protein expression. J Cell Biol. 1990 Apr;110(4):1199–1210. doi: 10.1083/jcb.110.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990 May 18;61(4):579–589. doi: 10.1016/0092-8674(90)90470-y. [DOI] [PubMed] [Google Scholar]
- Johnson L. D., Idler W. W., Zhou X. M., Roop D. R., Steinert P. M. Structure of a gene for the human epidermal 67-kDa keratin. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1896–1900. doi: 10.1073/pnas.82.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korge B. P., Gan S. Q., McBride O. W., Mischke D., Steinert P. M. Extensive size polymorphism of the human keratin 10 chain resides in the C-terminal V2 subdomain due to variable numbers and sizes of glycine loops. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):910–914. doi: 10.1073/pnas.89.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane E. B., Rugg E. L., Navsaria H., Leigh I. M., Heagerty A. H., Ishida-Yamamoto A., Eady R. A. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992 Mar 19;356(6366):244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- Letai A., Coulombe P. A., Fuchs E. Do the ends justify the mean? Proline mutations at the ends of the keratin coiled-coil rod segment are more disruptive than internal mutations. J Cell Biol. 1992 Mar;116(5):1181–1195. doi: 10.1083/jcb.116.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A., Coulombe P. A., McCormick M. B., Yu Q. C., Hutton E., Fuchs E. Disease severity correlates with position of keratin point mutations in patients with epidermolysis bullosa simplex. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3197–3201. doi: 10.1073/pnas.90.8.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Lane E. B. Retrovirus-mediated transgenic keratin expression in cultured fibroblasts: specific domain functions in keratin stabilization and filament formation. Cell. 1990 Aug 24;62(4):681–696. doi: 10.1016/0092-8674(90)90114-t. [DOI] [PubMed] [Google Scholar]
- McLean W. H., Eady R. A., Dopping-Hepenstal P. J., McMillan J. R., Leigh I. M., Navsaria H. A., Higgins C., Harper J. I., Paige D. G., Morley S. M. Mutations in the rod 1A domain of keratins 1 and 10 in bullous congenital ichthyosiform erythroderma (BCIE). J Invest Dermatol. 1994 Jan;102(1):24–30. doi: 10.1111/1523-1747.ep12371726. [DOI] [PubMed] [Google Scholar]
- Mischke D., Wild G. Polymorphic keratins in human epidermis. J Invest Dermatol. 1987 Feb;88(2):191–197. doi: 10.1111/1523-1747.ep12525329. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Sun T. T. The 50- and 58-kdalton keratin classes as molecular markers for stratified squamous epithelia: cell culture studies. J Cell Biol. 1983 Jul;97(1):244–251. doi: 10.1083/jcb.97.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen L., Christiano A. M., Knowlton R. G., Uitto J. Epidermolytic hyperkeratosis (bullous congenital ichthyosiform erythroderma). Genetic linkage to chromosome 12q in the region of the type II keratin gene cluster. J Clin Invest. 1993 Jan;91(1):357–361. doi: 10.1172/JCI116193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan R. A., Hatzfeld M., Franke W. W., Lustig A., Schulthess T., Engel J. Characterization of dimer subunits of intermediate filament proteins. J Mol Biol. 1986 Nov 20;192(2):337–349. doi: 10.1016/0022-2836(86)90369-4. [DOI] [PubMed] [Google Scholar]
- Raats J. M., Henderik J. B., Verdijk M., van Oort F. L., Gerards W. L., Ramaekers F. C., Bloemendal H. Assembly of carboxy-terminally deleted desmin in vimentin-free cells. Eur J Cell Biol. 1991 Oct;56(1):84–103. [PubMed] [Google Scholar]
- Raats J. M., Pieper F. R., Vree Egberts W. T., Verrijp K. N., Ramaekers F. C., Bloemendal H. Assembly of amino-terminally deleted desmin in vimentin-free cells. J Cell Biol. 1990 Nov;111(5 Pt 1):1971–1985. doi: 10.1083/jcb.111.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M., Franke W. W. Identification of an orthologous mammalian cytokeratin gene. High degree of intron sequence conservation during evolution of human cytokeratin 10. J Mol Biol. 1988 Dec 20;204(4):841–856. doi: 10.1016/0022-2836(88)90045-9. [DOI] [PubMed] [Google Scholar]
- Riemer D., Dodemont H., Weber K. Analysis of the cDNA and gene encoding a cytoplasmic intermediate filament (IF) protein from the cephalochordate Branchiostoma lanceolatum; implications for the evolution of the IF protein family. Eur J Cell Biol. 1992 Jun;58(1):128–135. [PubMed] [Google Scholar]
- Rothnagel J. A., Dominey A. M., Dempsey L. D., Longley M. A., Greenhalgh D. A., Gagne T. A., Huber M., Frenk E., Hohl D., Roop D. R. Mutations in the rod domains of keratins 1 and 10 in epidermolytic hyperkeratosis. Science. 1992 Aug 21;257(5073):1128–1130. doi: 10.1126/science.257.5073.1128. [DOI] [PubMed] [Google Scholar]
- Rugg E. L., Morley S. M., Smith F. J., Boxer M., Tidman M. J., Navsaria H., Leigh I. M., Lane E. B. Missing links: Weber-Cockayne keratin mutations implicate the L12 linker domain in effective cytoskeleton function. Nat Genet. 1993 Nov;5(3):294–300. doi: 10.1038/ng1193-294. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Bale S. J. Genetic skin diseases caused by mutations in keratin intermediate filaments. Trends Genet. 1993 Aug;9(8):280–284. doi: 10.1016/0168-9525(93)90014-9. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Marekov L. N., Fraser R. D., Parry D. A. Keratin intermediate filament structure. Crosslinking studies yield quantitative information on molecular dimensions and mechanism of assembly. J Mol Biol. 1993 Mar 20;230(2):436–452. doi: 10.1006/jmbi.1993.1161. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Rice R. H., Roop D. R., Trus B. L., Steven A. C. Complete amino acid sequence of a mouse epidermal keratin subunit and implications for the structure of intermediate filaments. Nature. 1983 Apr 28;302(5911):794–800. doi: 10.1038/302794a0. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. The two-chain coiled-coil molecule of native epidermal keratin intermediate filaments is a type I-type II heterodimer. J Biol Chem. 1990 May 25;265(15):8766–8774. [PubMed] [Google Scholar]
- Stephens K., Sybert V. P., Wijsman E. M., Ehrlich P., Spencer A. A keratin 14 mutational hot spot for epidermolysis bullosa simplex, Dowling-Meara: implications for diagnosis. J Invest Dermatol. 1993 Aug;101(2):240–243. doi: 10.1111/1523-1747.ep12365079. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Hainfeld J. F., Trus B. L., Wall J. S., Steinert P. M. Epidermal keratin filaments assembled in vitro have masses-per-unit-length that scale according to average subunit mass: structural basis for homologous packing of subunits in intermediate filaments. J Cell Biol. 1983 Dec;97(6):1939–1944. doi: 10.1083/jcb.97.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler A., Kopan R., Duvic M., Fuchs E. Use of monospecific antisera and cRNA probes to localize the major changes in keratin expression during normal and abnormal epidermal differentiation. J Cell Biol. 1988 Aug;107(2):427–446. doi: 10.1083/jcb.107.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybert V. P., Holbrook K. A., Levy M. Prenatal diagnosis of severe dermatologic diseases. Adv Dermatol. 1992;7:179–210. [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991 Jan 25;64(2):365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- Wilgram G. F., Caulfield J. B. An electron microscopic study of epidermolytic hyperkeratosis. With a special note on the keratinosome as the "fourth" structural factor in the formation of the horny layer. Arch Dermatol. 1966 Aug;94(2):127–143. doi: 10.1001/archderm.94.2.127. [DOI] [PubMed] [Google Scholar]
- Wilson A. K., Coulombe P. A., Fuchs E. The roles of K5 and K14 head, tail, and R/K L L E G E domains in keratin filament assembly in vitro. J Cell Biol. 1992 Oct;119(2):401–414. doi: 10.1083/jcb.119.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. C., Cleveland D. W. Characterization of dominant and recessive assembly-defective mutations in mouse neurofilament NF-M. J Cell Biol. 1990 Nov;111(5 Pt 1):1987–2003. doi: 10.1083/jcb.111.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. M., Chipev C. C., DiGiovanna J. J., Bale S. J., Marekov L. N., Steinert P. M., Compton J. G. Mutations in the H1 and 1A domains in the keratin 1 gene in epidermolytic hyperkeratosis. J Invest Dermatol. 1994 Jan;102(1):17–23. doi: 10.1111/1523-1747.ep12371725. [DOI] [PubMed] [Google Scholar]