SUMMARY
Purpose
To describe the trial design for the multicenter Early Randomized Surgical Epilepsy Trial (ERSET). Patients with pharmacoresistant epilepsy are generally referred for surgical treatment an average of two decades after onset of seizures, often too late to avoid irreversible disability. ERSET was designed to assess the safety and efficacy of early surgical intervention compared to continued pharmacotherapy.
Methods
ERSET is a randomized controlled, parallel group clinical trial with blinded outcome adjudication. Participants are patients with mesial temporal lobe epilepsy (MTLE) over the age of 12 who have had pharmacoresistant seizures for not more than two years and are determined by detailed evaluation to be surgical candidates prior to randomization. The primary outcome measure is seizure freedom in the second year of a two-year follow-up period. Health-related quality of life (HRQOL), neurocognitive function, ancillary outcomes, and adverse events were also measured.
Results
Significant methodological problems addressed by the study design included: recruitment of participants early in the course of epilepsy, establishment of operational definitions for “pharmacoresistant” and “early,” and standardization of diagnostic testing, medical treatment and surgical interventions across multiple centers.
Discussion
Rigorous trial designs to assess surgical interventions in epilepsy are necessary to provide evidence to guide treatment. This paper is the first of a series; trial results will be reported in subsequent publications.
INTRODUCTION
Following a randomized controlled trial (RCT) demonstrating the effectiveness of surgery for mesial temporal lobe epilepsy (MTLE) (Wiebe et al., 2001), an American Academy of Neurology (AAN) practice parameter recommended surgery as the treatment of choice for pharmacoresistant MTLE, and called for a multicenter RCT to address the efficacy of early surgical intervention (Engel et al., 2003). In 2003, NIH funded the multicenter Early Randomized Surgical Epilepsy Trial (ERSET) to compare surgical intervention for MTLE with continued medical therapy no more than 2 years after failure of two antiepileptic drug (AED) trials (Engel, 2004). A unique feature of ERSET was the intention to randomize patients only after a definitive diagnosis of MTLE appropriate for surgical treatment, i.e., to investigate as pure a population with surgically remediable pharmacoresistant MTLE as possible. ERSET presented several major difficulties, including recruitment of patients with pharmacoresistant MTLE who are not usually referred to epilepsy surgery centers early (Berg et al., 2003), as well as the need for: clear definitions of “pharmacoresistance” and “early” intervention; an unequivocal diagnosis of surgically remediable MTLE; rigorously defined medical and surgical therapies; and blinded outcome assessment. The detailed methodology designed to overcome these difficulties is reported here.
METHODS
General study design
ERSET is a multicenter, randomized, controlled, parallel group clinical trial comparing surgery with continued pharmacotherapy in patients with MTLE. Patients had pharmacoresistant seizures and were determined by detailed evaluation to be surgical candidates prior to randomization. Participants were followed for two years after randomization. The primary outcome variable was seizure freedom in the second year of the two-year follow-up period. Secondary outcome variables included changes from baseline in HRQOL, neurocognitive function, and ancillary outcomes, as well as adverse events.
Subject selection
Participants were male or female, age 12 or older with MTLE and disabling seizures that persisted for no more than two consecutive years following adequate trials of two brand-name AEDs. In addition, participants had to be considered candidates for anteromesial temporal resection based on a standardized presurgical evaluation protocol.
Definitions of “pharmacoresistant” and “early” intervention
Patients were considered “early” in their course if pharmacoresistance had been established for the first time within two years prior to enrollment. “Pharmacoresistance” was defined as persistent disabling seizures despite prior trials of at least two AEDs, one of which had to have had a monotherapy indication at the time of the study. “Persistent” was defined as a) having (on average) at least one day every two months when at least one seizure occurred and b) there were no more than 6 months between seizures during the two years prior to enrollment. Patients could be included if they had had active epilepsy prior to this two year period, as long as a prior period of pharmacoresistance did not exceed two consecutive years. “Disabling” seizures were defined as seizures that resulted in loss of awareness (e.g., complex partial or generalized tonic-clonic) or a change in behavior that interfered with ability to carry out usual activities or were noticeable by others. Simple partial seizures (e.g., sensory, autonomic, psychic) that were only noticeable by the patient and during which they could carry out usual activities (auras) did not meet this criterion.
The following brand name drugs were indicated for monotherapy in this RCT: Dilantin, Tegretol, Carbatrol, Trileptal, Depakote/Depakote ER, Lamictal, or Topamax. In adults, secondary failure could include any of the above drugs used as monotherapy or polytherapy, as well as Keppra, Neurontin, and Zonegran as adjunctive therapy with one of the primary AEDs listed above. In the adolescent population (age 12–16), adjunctive Neurontin therapy could not be counted as a secondary failure. Appropriate AED treatment meant that maximum tolerable dosage levels had been used at correct dosing intervals and that failure was due to inefficacy, not intolerance.
Other inclusion and exclusion criteria
Febrile seizures, simple partial seizures, complex partial seizures, and secondarily generalized seizures were acceptable by history. If auras occurred, they had to consist of autonomic, psychic, olfactory, gustatory, or nonspecific somatosensory, but not other primary sensory symptoms. Patients were excluded if seizures began with focal motor movements (other than automatisms or dystonic posturing), bilateral massive movements, or aphasia. Postictal confusion following complex partial seizures was required. Patients were also excluded for a history of serious cerebral insult after the age of five; a progressive neurological disorder; nonepileptic psychogenic seizures; focal neurologic deficits other than memory disturbances; greater than four secondarily generalized seizures per year for more than three years; or more than one incident of status epilepticus, other than febrile status. The latter two exclusionary factors were felt to stretch the concept of “early.”
Patients with vagus nerve stimulators (VNS) in place were excluded if: 1) the stimulator could not be turned off; 2) the presence of the stimulator interfered with performance of the ERSET-required diagnostic testing; or 3) there were enduring adverse effects of the stimulator that would unduly complicate collection of outcome measures. The VNS device did not need to be removed, but was turned off at least a month prior to initiation of the ERSET treatment protocol and kept off for the duration of the study.
Recruitment
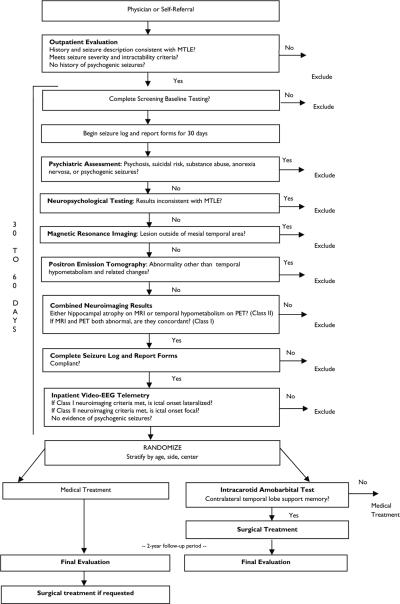
The ERSET protocol flow chart is shown in Figure 1. Patients were solicited based on a history and seizure semiology compatible with that of MTLE (Engel et al., 2008; Wieser et al., 2004). Patient and physician focus groups were held in order to assess barriers and attitudes to participation in an early surgical randomized trial (Swarztrauber et al., 2003) and to develop material for media and public relations, as well as direct mailings to physicians. Prospective participants referred to study sites were initially seen as outpatients. After obtaining informed consent, a complete history, detailed description of seizures, neurologic examination, and review of past records were performed.
Figure 1.
ERSET protocol flow chart
Presurgical screening
All testing was standardized across sites and consisted of the following: Psychiatric assessment: Participants were excluded if this evaluation revealed psychosis, suicidal risk, recent or current substance abuse, anorexia nervosa, or psychogenic nonepileptic seizures. Neuropsychological testing: Participants were excluded if IQ was below 70 or there was strong evidence of material-specific memory asymmetry that was inconsistent with other lateralizing information (e.g., significant non-verbal memory deficit and intact verbal memory in subject with left MTLE). Neuroimaging: Positive findings required for inclusion consisted of either: 1) hippocampal atrophy on MRI T1 imaging with either increased ipsilateral mesial signal on T2 imaging or ipsilateral hypometabolism on PET (Class I), or 2) either hippocampal atrophy on MRI only or temporal hypometabolism on PET only (Class II). Participants were excluded if there was evidence of temporal neocortical or extratemporal epileptogenic lesions on neuroimaging, but were not excluded for incidental asymptomatic MRI lesions if: 1) There was only one lesion which was distant from the suspected epileptogenic mesial temporal area, and a definitive diagnosis could be made of a specific nonprogressive defect that is often an incidental finding associated with epilepsy. Lesions that could be epileptogenic, such as calcification indicative of neurocysticercosis, or cavernous angioma, had to be contralateral to the suspected epileptogenic mesial temporal area, but lesions that were highly unlikely to be epileptogenic, such as a subarachnoid cyst, or venous angioma, could be ipsilateral; 2) Seizure semiology by history, and confirmed by video-EEG monitoring, indicated that all ictal events were coming from one mesial temporal area, and there was no evidence of a second seizure type that might be coming from the area of the incidental lesion; and 3) No interictal EEG abnormalities were identified at the site of the incidental lesion. Participants were excluded if PET imaging revealed diffuse unilateral or bilateral hypometabolism. Neurophysiology: Ictal EEG onsets recorded during inpatient video-EEG monitoring needed only to be lateralized to the ipsilateral side if neuroimaging findings were Class I, but a focal temporal ipsilateral ictal EEG onset (Risinger et al., 1989) was required if neuroimaging findings were Class II. Participants were excluded if there were contralateral or extratemporal ictal onsets, persistence of extratemporal or strongly (> 90%) contralateral focal interictal spikes or slowing, generalized interictal spikes, or nonepileptic psychogenic seizures.
Participants meeting the above presurgical evaluation criteria completed a baseline daily seizure log and report forms for one month to confirm their reported seizure frequencies. All seizures were classified into 4 types: 1) Simple partial seizures without impairment (without alteration in consciousness, that are not noticeable by an observer, and do not interfere with activities of daily living, also referred to as auras); 2) Simple partial seizures with impairment (without alteration in consciousness, that are noticeable by an observer, or do interfere with activities of daily living); 3) Complex partial seizures (with alteration in consciousness, that do not progress to involve bilateral tonic and clonic motor movements); and 4) Secondarily generalized seizures (with alteration in consciousness, that do progress to involve bilateral tonic and clonic movements). All seizures were considered disabling seizures except auras. Baseline HRQOL batteries and ancillary psychosocial data were also obtained. Participants unable to comply with these tasks were excluded.
Randomization
Participants were randomly assigned with equal allocation to either the medical or surgical treatment arms. Randomization was stratified by center, the subject's age (12–16 or ≥ 17), and side of ictal onset and included blocking within each stratum. The randomization plan was prepared by a programmer in the Biostatistics Center (Rochester, NY) whose only other involvement was to prepare data reports for the Data and Safety Monitoring Board (DSMB). No other study personnel had access to treatment assignments prior to the enrollment of a participant.
The ERSET site coordinator called the Clinical Trials Coordination Center (CTCC; Rochester, NY) within 60 days of the initial consent to verify that the participant met eligibility criteria and to obtain a randomization assignment. After randomization, medical arm participants began the medical management protocol immediately and were followed for a 24 month period. Participants randomized to the surgical arm began follow-up immediately after randomization, although the timing of subsequent evaluations was anchored to the date of surgery, which was expected to occur within 30 days of randomization. All patients randomized to the surgical arm had to pass a bilateral intracarotid amobarbital procedure (IAP) before surgery could be performed.
Follow-up
During two years of follow-up, participants were seen at the study site every three months, and the site coordinator called participants every other week for the first twelve weeks to ensure compliance with completing seizure logs.
Standardized Evaluations
Clinical neurophysiology
Video-EEG monitoring was performed on inpatient monitoring units using standard 10–20 electrode placements, sphenoidal electrodes, in some cases additional basal electrode placements (T1/T2, AF 7/8, F9/10), and EKG. At least 32 channels of digital EEG samples included routine segments of EEG collected on admission, timed samples taken throughout the course of the inpatient study, and ictal events. Sample ictal EEG recordings were reviewed by a central EEG committee to ensure that all site investigators were interpreting recordings in the same manner. Further details are provided in the accompanying supporting information. Ictal semiology was classified as consistent with an ipsilateral onset temporal lobe seizure, suggestive of an extratemporal onset seizure, or suggestive of a contralateral onset temporal lobe seizure according to standardized criteria. Adjudication by the central EEG committee was required if ictal onset patterns were unclear, or ictal semiology suggested extratemporal or contralateral temporal onsets.
Neuroimaging
MRI and PET studies were performed at the study sites and interpreted by site investigators for purposes of participant enrollment. All imaging data were also reviewed by a central neuroimaging committee, who confirmed the findings reported by the site. Further details are provided in the accompanying supporting information.
Intracarotid amobarbital procedure (IAP)
A standardized bilateral IAP protocol (Loring et al., 1993;1994;1995) was performed preoperatively at all sites for participants randomized to receive surgical treatment. Each center performed a “mock” IAP, which was videotaped and reviewed by a central IAP committee, to ensure that this test was carried out in an identical fashion at each site. Further details are provided in the accompanying supporting information.
Neuropsychological evaluation
A standard, brief neuropsychological battery was performed at baseline and at 1 and 2 years post-randomization (or post-surgery for participants in the surgical arm). Primary cognitive domains of interest were memory (Rey Auditory Verbal Learning Test (Schmidt, 1996), Wechsler Memory Scale-Revised Logical Memory Subtest (Wechsler, 1987), Brief Visuo-spatial Memory Test-Revised (Benedict, 1997)), word-finding (Boston Naming Test (Kaplan et al., 2001)), attention (Digit Span (Wechsler, 1997)), Trail Making Test (Strauss et al., 2006), and motor speed (Grooved Pegboard (Strauss et al., 2006)).
Psychiatric evaluation
The following assessments of diagnosis and severity of psychopathology were performed: The Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1994) for participants age 16 and over, and Kiddie Schedule of Affective Disorders and Schizophrenia (K-SADS) (Kaufman et al., 1997) for participants under age 16. The K-SADS DSM-IV summary diagnoses were derived from both child and parent responses to identical questions. Participants meeting criteria for any psychiatric diagnosis on the MINI then underwent a semi-structured psychiatry interview (MINI Addendum). In addition, adults completed the Brief Symptom Inventory (BSI) (Derogatis, 1994) self-report scale and children underwent the Child Behavior Checklist (CBCL) (Achenbach and Edelbrock, 1991). Treatment for psychiatric disorders that developed during the course of follow-up was standardized with respect to the order of medications used for specific psychiatric disturbances.
Interventions
Participants were randomized to receive surgical treatment or medical treatment. Participants allocated to receive surgical treatment also received medical treatment as described below.
Surgical treatment
Participating epilepsy centers had to be Level 4 as defined by the National Association of Epilepsy Centers (National Association of Epilepsy Centers, 2001). Surgical programs and neurosurgeons were required to meet minimal criteria with respect to years of experience and volume of anteromesial temporal resections (at least 12 patients a year for 5 years, with the majority not requiring invasive evaluation). All sites utilized the same en bloc anteromesial temporal lobe resection (AMTR) (Spencer et al.,1984), which provided mesial temporal tissue for histopathological analyses. Surgeons completed a surgical report form specifying the extent of the resection, noting gross pathology and specimens submitted. Postoperative management was also standardized with respect to steroid therapy and antiepileptic drug administration. Postoperative neurological deficits were evaluated at routine follow-up visits and documented on the adverse event case report forms, and an MRI scan (TI only) was obtained at three months to document the extent of resection. Operative report forms, MRIs, and pathology reports were reviewed by a central neurosurgical committee to ensure consistent surgical practices.
Medical treatment
The objectives of pharmacotherapy in this study were: 1) to achieve and maintain a seizure free state, first through monotherapy trials, then through polytherapy when necessary, and 2) to minimize adverse effects because they, like seizures, could affect HRQOL. The pharmacotherapy protocol reflected current practice and followed criteria that are widely adopted in most epilepsy centers throughout the country. After assessment of previous medication history, the four-stage protocol consisted of: 1) monotherapy; 2) ditherapy; 3) (optional) treatment with rarely used AEDs; and 4) treatment with multiple AEDs. Generic drugs were not permitted (Nuwer et al., 1990). A pharmacotherapy committee monitored the AED literature during the course of the study and updated the pharmacotherapy protocol as new information about AEDs became available. Adherence to an unbiased strategy of pharmacotherapy for both treatment groups (medical and surgical) was monitored by a second pharmacotherapy review committee with expertise in clinical pharmacology of AEDs, not directly involved in the surgical treatment of epilepsy. This committee blindly reviewed, within one month of receipt of information, the treatment of all cases from both arms of the trial at 12 and 24 months to detect and correct any deviation from the pharmacotherapy protocol.
Because seizure recurrence was the primary outcome variable in this trial, the minimal effective dose in patients who were seizure free was not determined by reducing medication until a seizure occurred, but instead by reducing medication only to eliminate unacceptable side effects if they occurred. The maximal tolerated dosage was defined as the highest dosage of drug that did not produce unacceptable side effects. Consistency of treatment among centers was facilitated by guidelines for maximal starting dosages, maximal incremental rates during titration, and maximal tolerated doses.
Blood levels to monitor compliance were obtained quarterly. In addition, blood levels were required when a participant reached the optimal dosage and was at steady state, and as soon as possible following breakthrough seizures. All AEDs used were recorded on case report forms. Reasons for changes in drug regimen were documented. Gender issues were taken into account; for example, all women of childbearing age were treated with folic acid. Women who became pregnant during the follow-up period continued participation in the study if willing.
Outcome variables
Primary outcome variable
Although all seizure types were recorded for two years, the primary outcome variable was freedom from disabling seizures. Because the prognostic significance of seizures occurring early after surgery remains unclear (Engel et al., 1993; Jehi, 2008), seizure freedom was determined for the second year of follow up. Epileptic seizure types reported by each participant during a 30-day baseline period were fully described and classified by a central seizure adjudication committee that was blinded to treatment assignment. The frequency of each seizure type during the year immediately preceding randomization was ascertained by participant and family report. During the two-year follow-up period, participants entered each seizure on a seizure log and described each seizure on a seizure report form. These data were reviewed with the site investigator at each quarterly follow-up outpatient visit.
The seizure adjudication committee also reviewed new seizure types occurring during the follow-up period to determine whether they should be considered epileptic and, if so, how they should be classified. Discrepancies between the site reports and the Seizure Adjudication Committee were resolved by the Steering Committee without knowledge of the treatment group assignment. Seizure outcomes of secondary interest included seizure frequency, overall and by seizure type.
Secondary outcome variables
HRQOL was the most important secondary outcome domain. This was assessed at baseline and every six months with the self-administered questionnaires Quality of Life in Epilepsy 89 (QOLIE-89) (Devinsky et al., 1995) for adults and Quality of Life in Epilepsy 48 (QOLIE AD-48) (Cramer et al., 1999) and Child Health Questionnaire – Parent Form (CHQ98-PF) (Landgraf and Abetz, 1996) for adolescents (ages 12–16). A variety of self-or parent-completed validated questionnaires were also administered prior to randomization and periodically throughout the trial to assess psychopathology, behavior, socialization, locus of control, neuroticism, family function, and stressful life events (Byles et al., 1988; Coddington, 1972; Derogatis, 1994; Peters and Derry, 2001; Rotter, 1966; Sarasan et al., 1978; Shields et al., 1992; Vickrey et al., 1992) (Table S1).
Other ancillary outcome measures included changes in cognitive function, medical, employment, educational, housing, and driving status, and use of community support services. Adverse events (including surgical complications) and subject disposition (including withdrawal and treatment cross-over) were carefully monitored. Lobectomy specimens were subjected to a standard pathological tissue protocol (Levesque et al., 1991) and hippocampal cell counts and Timm's stain were also performed if required to substantiate a diagnosis of hippocampal sclerosis.
The schedule of follow-up studies is shown in Table S2.
Statistical Analysis, Sample Size Considerations and Interim Monitoring
Details of the statistical analysis, justification for sample size (200), and interim monitoring of accumulating trial data are addressed in the accompanying supporting information.
DISCUSSION
ERSET was designed to compare medical and surgical therapy early in the course of pharmacoresistance, at which time the superiority of surgery is not proven and equipoise exists. The equipoise required was established by choosing a definition of “early” for which the demonstrated beneficial effects of surgical treatment are balanced by: 1) the possibility that seizures will be controlled by pharmacotherapy within one or two years and 2) the risks of adverse effects of surgery, including disturbances in memory and learning in individuals whose seizures have not persisted long enough to produce such cognitive deficits. The definition of “early” for this study was based on duration of pharmacoresistance; consequently pharmacoresistance first had to be defined.
Although an influential study reported that only 11% of patients became seizure free following failure of the first AED due to inefficacy and not intolerance, and only 3% became seizure free after failing two AEDs (Kwan and Brodie, 2000),subsequent studies have suggested that there are populations of patients who have a much higher likelihood of becoming seizure free after failing two or three AED trials (Bauer et al., 2008; Callaghan et al., 2007; Llimatainen et al., 2008; Luciano and Shorvon, 2007). ERSET defined pharmacoresistance as failure of two adequate AED trials due to inefficacy and not intolerance, and the International League against Epilepsy (ILAE) recently issued a report defining pharmacoresistance similarly (Kwan et al., 2009).
ERSET investigators arbitrarily defined “early” as within two years of a diagnosis of pharmacoresistance; however, this proved to be difficult to operationally apply, given that patients with MTLE commonly experience a stuttering course with long periods of remission (Berg et al., 2003). Consequently, this criterion was qualified by inserting the word “consecutive,” which was defined as no inter-seizure intervals of six months or greater during the two year period. This allowed inclusion of patients who met criteria for pharmacoresistance, but then entered a long period of remission within two years. Such patients would be eligible for ERSET if seizures later recurred at the required rate of one seizure day or more per two month period.
It was the intention of ERSET investigators to include as pure a population of MTLE patients as possible. MTLE, however, is not a single, homogeneous condition (Engel et al., 2008; Wieser et al., 2004). Although it was anticipated that most participants would have unilateral hippocampal sclerosis, patients with MRI evidence of mesial temporal lesions other than hippocampal sclerosis were also included. The important criterion was that all participants included in ERSET would be considered candidates for anteromesial temporal resection at most epilepsy surgery centers prior to randomization. Candidacy had to be established by noninvasive presurgical evaluation because it would be unethical to perform intracranial monitoring in patients randomized to pharmacotherapy. The standardized presurgical evaluation protocol included some studies that are not routinely performed at many epilepsy surgery centers. FDG-PET studies were required in all patients, in part because PET is capable of identifying mesial temporal abnormalities in patients with normal MRIs (Hogan et al., 2008), but it is also important to know whether FDG-PET might contribute additional information of value even when MRIs are positive. Also, bilateral IAPs were performed in all patients, not only to ensure that the contralateral hemisphere could support memory, but to determine whether this procedure might be useful in predicting postoperative memory deficits in patients early in the course of MTLE who might not be experiencing memory disturbances.
ERSET excluded patients under the age of 12 years. Although MTLE typically begins in late childhood and takes several years to become pharmacoresistant, it is conceivable that early surgical intervention in some patients ought to occur in children younger than 12 years old. However, this lower age limit was necessitated by the fact that HRQOL was the most important secondary outcome domain, and none of the available quantitative instruments are valid below the age of 12.
An important feature of the ERSET experimental design was the standardized progressive pharmacotherapy protocol overseen by an independent pharmacotherapy committee made up of specialists in clinical pharmacology of AEDs who are not involved in epilepsy surgery. This was intended to ensure that although study sites were epilepsy surgery centers, investigators were committed to making every effort to eliminate disabling seizures as quickly as possible in participants randomized to the medical arm.
Standardization of surgical therapy required considerable effort to enlist the cooperation of surgeons, who had to agree, on occasion, to change their surgical approaches. To clarify situations in which surgical results can be generally applied, it was also necessary to establish specific minimal experience criteria not only for epilepsy surgery centers, but for the surgeons participating in the study. It is anticipated that results similar to those to be reported for the ERSET study might not be obtained at centers, and by surgeons, who do not meet these experience criteria.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants R21 NS037897 and U01 NS042372 from the National Institutes of Neurological Disorders and Stroke.
Footnotes
DISCLOSURE We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
None of the authors has any conflict of interest to disclose.
REFERENCES
- Achenbach T, Edelbrock C. Manual for the Child Behavior Checklist and 4–18 and 1991 Profile. Department of Psychiatry, University of Vermont; 1991. [Google Scholar]
- Bauer J, Buchmüller L, Reuber M, Burr W. Which patients become seizure free with antiepileptic drugs? An observational study in 821 patients with epilepsy. Acta Neurologica Scandinavica. 2008;117:55–59. doi: 10.1111/j.1600-0404.2007.00940.x. [DOI] [PubMed] [Google Scholar]
- Benedict RHB. Brief Visuo-spatial Memory Test-Revised. Psychological Assessment Resources, Inc.; Odessa, FL: 1997. [Google Scholar]
- Berg AT, Langfitt J, Shinnar S, Vickrey BG, Sperling MR, Walczak T, Bazil C, Pacia SV, Spencer SS. How long does it take for partial epilepsy to become intractable? Neurology. 2003;60:186–190. doi: 10.1212/01.wnl.0000031792.89992.ec. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM. Newly-diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40:445–452. doi: 10.1111/j.1528-1157.1999.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Byles J, Byrne C, Boyle MH, Offord DR. Ontario Child Health Study: reliability and validity of the general functioning subscale of the McMaster Family Assessment Device. Fam.Process. 1988;27:97–104. doi: 10.1111/j.1545-5300.1988.00097.x. [DOI] [PubMed] [Google Scholar]
- Callaghan BC, Anand K, Hesdorrffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62:382–389. doi: 10.1002/ana.21166. [DOI] [PubMed] [Google Scholar]
- Coddington RD. The signifance of life events as etiologic factors in the diseases of children. I. A survey of professional workers. J Psychosom Res. 1972;16:7–18. doi: 10.1016/0022-3999(72)90018-9. [DOI] [PubMed] [Google Scholar]
- Cramer J, Westbrook L, Devinsky O, Perrine K, Glassman MB, Camfield C. Development of the Quality of Life in Epilepsy Inventory for Adolescents: the QOLIE-AD-48. Epilepsia. 1999;40:1114–1121. doi: 10.1111/j.1528-1157.1999.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory (BSI) Administration, Scoring, and Procedures Manual. 3rd Ed. National Computer System; Minneapolis: 1994. [Google Scholar]
- Devinsky O, Vickrey BG, Cramer J, Perrine K, Hermann B, Meador K, et al. Development of the quality of life in epilepsy inventory. Epilepsia. 1995;36:1089–1104. doi: 10.1111/j.1528-1157.1995.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr. Surgery for seizures. N Engl J Med. 1996;334:647–652. doi: 10.1056/NEJM199603073341008. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr. Finally, a randomized controlled trial of epilepsy surgery. N Engl J Med. 2001;345:365–367. doi: 10.1056/NEJM200108023450510. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr. The goals of epilepsy therapy: No seizures, no side effects, as soon as possible. CNS Spectrums. 2004;9:95–96. doi: 10.1017/s1092852900008452. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Van Ness P, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical Treatment of the Epilepsies. 2nd Edition Raven Press; New York: 1993. pp. 609–621. [Google Scholar]
- Engel J, Jr, Wiebe S, French J, Gumnit R, Spencer D, Sperling M, Williamson P, Zahn C, Westbrook E, Enos B. Practice parameter: Temporal lobe and localized neocortical resections for epilepsy. Neurology. 2003;60:538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy with hippocampal sclerosis. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Second Edition Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 2479–2486. [Google Scholar]
- Hogan RE, Carne RP, Kilpatrick CJ, Cook MJ, Patel A, King L, O'Brien TJ. Hippocampal deformation mapping in MRI negative PET positive temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2008;79:636–640. doi: 10.1136/jnnp.2007.123406. [DOI] [PubMed] [Google Scholar]
- Jallon P, Loiseau P, Loiseau J. Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Epilepsia. 2001;42:464–475. doi: 10.1046/j.1528-1157.2001.31400.x. [DOI] [PubMed] [Google Scholar]
- Jehi L. Mesial temporal lobectomy: post surgical seizure frequency. In: Lüders HO, editor. Textbook of Epilepsy Surgery. Informa Health Care; London: 2008. pp. 1223–1235. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed Lippincott, Williams and Wilkins; Philadelphia: 2001. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, et al. Schedule for affective disorders and schizophrenia for school-age children – present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2009;50:3–11. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- Landgraf JM, Abetz LN. Measuring Health Outcomes in Pediatric Populations: Issues in Psychometrics and Application. In: Spilker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 793–802. [Google Scholar]
- Levesque MF, Nakasato N, Vinters HV, Babb TL. Surgical treatment of limbic epilepsy associated with extrahippocampal lesions – the problem of dual pathology. J Neurosurg. 1991;75:364–370. doi: 10.3171/jns.1991.75.3.0364. [DOI] [PubMed] [Google Scholar]
- Liimatainen SP, Raitanen JA, Ylinen AM, Peltola MA, Peltola JT. The benefit of active drug trials is dependent on the aetiology in refractory focal epilepsy. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:808–812. doi: 10.1136/jnnp.2007.132811. [DOI] [PubMed] [Google Scholar]
- Loring DW, Hermann BP, Meador KJ, Lee GP, Gallagher BB, King DW, Murro AM, Smith JR, Wyler AR. Amnesia after unilateral temporal lobectomy: a case report. Epilepsia. 1994;35:757–763. doi: 10.1111/j.1528-1157.1994.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, et al. Wada memory asymmetries predict verbal memory decline after anterior temporal lobectomy. Neurology. 1995;45:1329–1333. doi: 10.1212/wnl.45.7.1329. [DOI] [PubMed] [Google Scholar]
- Loring DW, Murro AM, Meador KJ, Lee GP, Gratton CA, Nichols ME, Gallagher BB, King DW, Smith JR. Wada memory testing and hippocampal volume measurements in the evaluation for temporal lobectomy. Neurology. 1993;43:1789–1793. doi: 10.1212/wnl.43.9.1789. [DOI] [PubMed] [Google Scholar]
- Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol. 2007;62:375–381. doi: 10.1002/ana.21064. [DOI] [PubMed] [Google Scholar]
- National Association of Epilepsy Centers Guidelines for essential services, personnel, and facilities in specialized epilepsy centers in the United States. Epilepsia. 2001;42:804–814. doi: 10.1046/j.1528-1157.2001.08701.x. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Panel Consensus Conference on Surgery for Epilepsy. JAMA. 1990;264:729–33. [PubMed] [Google Scholar]
- Nuwer M, Browne TR, Dodson EW, Dreifuss FE, Engel J, Jr, Leppik IE, Mattson RH, Penry JK, Treiman DM, Wilder BJ. Generic substitutions for antiepileptic drugs. Neurology. 1990;40:1647–1651. doi: 10.1212/wnl.40.11.1647. [DOI] [PubMed] [Google Scholar]
- Peters AL, Derry PA. The role of neuroticism in subjective and objective memory difficulties in patients with epilepsy. University of Western Ontario: 2001. pp. 1–33. (Thesis/Dissertation) [Google Scholar]
- Risinger MW, Engel J, Jr, Van Ness PC, Henry TR, Crandall PH. Ictal localization of temporal lobe seizures with scalp/sphenoidal recordings. Neurology. 1989;39:1288–1293. doi: 10.1212/wnl.39.10.1288. [DOI] [PubMed] [Google Scholar]
- Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80:1–28. [PubMed] [Google Scholar]
- Sarasan IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experience Survey. J Consult Clin. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Rey Auditory Verbal Learning Test. Western Psycholgical Services; Los Angeles: 1996. [Google Scholar]
- Semah F, Picot M-C, Adam C, Broglin D, Arzimanoglou A, Bazin B, Cavalcanti D, Baulac M. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Janavs J, Knapp E, Weiller E, Bonora LI, Amorim P, Lepine JP, Sheehan MF, Baker RR, Sheehan KH. Mini International Neuropsychiatric Interview (M.I.N.I.) University of South Florida Institute for Research in Psychiatry; INSERM-Hôpital de la Salpêtrière; Tampa, Florida: Paris, France: 1994. [Google Scholar]
- Shields CG, Franks P, Harp JJ, McDaniel SH. Development of the Family Emotional Involvement and Criticism Scale (FEICS): A self-report scale to measure expressed emotion. Journal of Marital and Family Therapy. 1992;18:395–407. [Google Scholar]
- Spencer DD, Spencer SS, Mattson RH, Williamson PD, Novelly RA. Access to the posterior medial temporal lobe structure in surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15:667–671. doi: 10.1227/00006123-198411000-00005. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests; Administration, Norms and Commentary. 3rd ed Oxford; New York: 2006. [Google Scholar]
- Swarztrauber K, Dewar S, Engel J., Jr. Patient attitudes about treatments for intractable epilepsy. Epilepsy and Behavior. 2003;4:19–25. doi: 10.1016/s1525-5050(02)00687-x. [DOI] [PubMed] [Google Scholar]
- Vickrey BG, Hays RD, Graber J, Rausch R, Engel J, Jr, Brook RH. A health-related quality of life instrument for patients evaluated for epilepsy surgery. Med Care. 1992:30299–319. doi: 10.1097/00005650-199204000-00002. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scales -III. The Psychological Corporation; 1997. [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal lobe epilepsy. N Engl J Med. 2001;345:311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wieser H-G, Özkara Ç , Engel J, Jr, Hauser AW, Moshé SL, Avanzini G, Helmstaedter C, Henry TR, Sperling MR. Mesial temporal lobe epilepsy with hippocampal sclerosis: Report of the ILAE Commission on Neurosurgery of Epilepsy. Epilepsia. 2004;45:695–714. doi: 10.1111/j.0013-9580.2004.09004.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.