Abstract
Human T-cell leukemia virus type 1 (HTLV-1) infection is characterized by lifelong persistence of the virus in the host. While most infected individuals remain asymptomatic, 3-5% will eventually develop adult T-cell leukemia/lymphoma (ATLL) or tropical spastic paraparesis/HTLV-associated myelopathy (TSP/HAM) after a clinical latency that can span years (TSP/HAM) to decades (ATLL). The major oncogenic determinant among HTLV-1 proteins is the Tax transactivator, which influences the expression and function of a great number of cellular proteins, drives cell proliferation, reduces cell death, and induces genetic instability.
The present review is focused on the current knowledge of p13, an HTLV-1 accessory protein targeted to the inner mitochondrial membrane and, under certain conditions, to the nucleus. In mitochondria, p13 produces an inward K+ current that results in an increased production of ROS by mitochondria. These effects are linked to the protein's effects on cell turnover which include activation of primary T cells and reduced proliferation/sensitization to cell death of tumor cells. Recent findings suggest that in the presence of Tax, p13 is subjected to ubiquitylation and partly targeted to the nucleus. Nuclear p13 binds Tax and inhibits its transcriptional activity. These findings suggest that the protein might exert distinct functions depending on its intracellular localization and influence both the turnover of infected cells and the balance between viral latency and productive infection.
Keywords: mitochondria, K+ channel, HTLV-1, apoptosis, leukemia
1. Introduction
Although HTLV-1 is recognized as the causative agent of adult T-cell leukemia/lymphoma (ATLL), neoplastic transformation is a rare event (3-5% of infected individuals) which arises after a clinical latency of 3-4 decades. A hallmark of HTLV-1 is therefore its ability to establish a life-long disease-free infection within the host (reviewed by (Lairmore and Franchini, 2007)).
HTLV-1 is characterized by a complex expression pattern that includes production of plus and minus-strand transcripts, alternative splicing and polycistronic translation. This strategy allows the virus to produce several regulatory and accessory proteins in addition to the structural proteins and virion-associated enzymes common to all retroviruses (i.e. Gag, Pro, Pol, Env). The open reading frames (ORFs) for these extra proteins, named Tax, Rex, p12, p13, p21, p30 and HBZ, are located in the 3' portion of the viral genome, with HBZ coded in minus-strand transcripts (reviewed by (Lairmore and Franchini, 2007)). Tax and Rex are essential to the viral life cycle, as they are required for production of the primary viral transcript (Tax) and for its balanced processing into unspliced versus spliced mRNAs (Rex).
The ability of HTLV-1 to expand and persist in T-cells relies mainly on division of cells already carrying the integrated viral genome (‘mitotic transmission’). It is tempting to speculate that the life-long persistence of HTLV-1-infected T-cells in the host might exploit the pathways controlling the long term persistence of normal memory T-cells (Saggioro et al., 2009). Homeostasis of the normal T-cell compartment is controlled by antigen-driven expansion and contraction of antigen-specific clones through properly timed proliferation (which precedes antigen clearance) and apoptosis (which follows antigen clearance). Only a minority of antigen-specific T-cells survive this culling phase and persist as memory cells (Sallusto et al., 2004). This process is controlled by engagement of T-cell receptor (TCR) by the antigen, which may result in either clonal expansion or clonal deletion [activation-induced cell death (AICD), reviewed by (Krammer et al., 2007)]. The switch between TCR-mediated clonal expansion and AICD is regulated by the Nuclear Factor-kappaB (NF-κB) pathway and c-FLIP, which inhibits of the “death-inducing signalling complex” (DISC), a key mediator of apoptosis triggered by engagement of death receptors (Krammer et al., 2007).
In addition to driving transcription from the viral promoter, Tax influences the expression and function of a great number of cellular proteins whose effects add up to enhance cell proliferation and reduce cell death (reviewed by (Grassmann et al., 2005)), resulting in long-term persistence of the virus in T-cells. The effects of HTLV-1 on the turnover of the host cells are likely to be cell-type specific, most of the HTLV-1 DNA is detected in the CD4+CD8-CD45RO+ memory T-cells in vivo (Richardson et al., 1990), even though the virus is capable of infecting many different cell types in vitro.
It is noteworthy that, through the activation of NF-κB, Tax increases c-FLIP expression and inhibits apoptosis in response to engagement of the CD95/Fas death receptor (Krueger et al., 2006). Microarray expression data underscore the importance of survival pathways in the development of the ATLL phenotype, with upregulation of anti-apoptotic genes and downregulation of proapoptotic genes in HTLV-1-immortalized cells and ATLL cells (Harhaj et al., 1999) (Ruckes et al., 2001) (Pise-Masison et al., 2002). Such a consistent and complex perturbation of apoptotic pathways is likely to be linked to the aneuploidy of ATLL cells which can be maintained only in the presence of mechanism overriding the activation of death pathways that are normally engaged by the “DNA damage response”. These mechanisms also play a role in the marked resistance of ATLL cells to genotoxic chemotherapeutic agents (reviewed by (Kfoury et al., 2005)).
Tax is necessary and sufficient to induce T-cell transformation, as shown in a Taxtransgenic mouse model in which Tax expression driven by the T-cell-specific Lck proximal promoter gave rise to an ATLL-like leukemia/lymphoma (Hasegawa et al., 2006). Nevertheless, the very long clinical latency (decades) and low penetrance that characterize ATLL suggest the existence of viral/host factors keeping the oncogenic potential of Tax under check. As described in other reviews in this issue of Molecular Aspects of Medicine, intense effort has been focused in understanding the roles of the viral accessory proteins p12, p30 and HBZ in the relationship of HTLV-1 with its host. The present review is focused on the role of the viral accessory protein p13 in controlling T-cell turnover and viral expression in connection to the intracellular targeting of the protein and the possible implications of these functions in the context of the HTLV-1 life cycle and pathogenic properties. Our discussion of Tax will be limited to its possible functional interactions with p13, in particular its influence on the latter's intracellular localization: as described below, our most recent studies suggests that p13 might furnish cues that counterbalance the effects of Tax both on cell turnover and viral expression.
2. p13 sequence and structural features
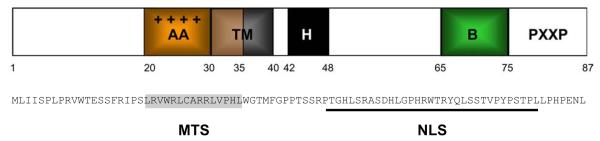
p13 is a highly basic 87-amino acid protein translated from the x-II open reading frame (ORF) of HTLV-1 in the context of a singly-spliced monocistronic mRNA. Figure 1 summarizes results of biochemical analyses (D'Agostino et al., 2002) and in silico predictions that suggest that p13 contains the following domains (i) an amphipathic alpha helix (residues 20-35) which includes the mitochondrial targeting signal (MTS) of p13 and is essential for the protein's effects on mitochondria (Ciminale et al., 1999; D'Agostino et al., 2002), (ii) a transmembrane region (residues 30-40), (iii) a region with a high flexibility score (residues 42-48), and (iv) a C-terminal region (residues 65-75) with a predicted βsheet hairpin structure that is homologous to an α-bungarotoxin-binding sequence (Schnell and Chou, 2008), suggesting that this region of p13 might represent a protein-protein interaction domain. The C-terminal portion of p13 also includes PXXP motifs that might mediate binding of p13 to proteins containing SH3 domains and also contains a cryptic nuclear localization sequence (NLS) (Ghorbel et al., 2006) which may mediate nuclear targeting of p13 in conditions in which the MTS is masked.
Figure 1.
Indicated is the domain structure of p13 suggested by biochemical and in silico analyses. AA indicates the amphipathic alpha helix (residues 20-35) which includes the mitochondrial targeting signal (MTS) of p13 and is essential for the protein's function in mitochondria. TM indicates the transmembrane region (residues 30-40); H indicates a region with a high flexibility score (residues 42-48) which is likely to form a hinge in the structure of p13. B indicates a predicted βsheet hairpin structure (residues 65-75). The C-terminal portion of p13 also includes PXXP SH3 domain-binding motifs and a cryptic nuclear localization sequence (NLS) whose exact position remains to be determined.
3. Intracellular targeting of p13
The first report describing the intracellular targeting of p13 indicated that it might accumulate in the nucleus (Koralnik et al., 1993). However, subsequent studies demonstrated that p13 is mainly localized in mitochondria where it its inserted in the inner membrane (Ciminale et al., 1999; D'Agostino et al., 2002), while nuclear localization was observed only in cells expressing higher levels of p13 (V. Ciminale, unpublished observations). The minimal mitochondrial targeting sequence (MTS) of p13, which spans amino acids 21-30, is a potent targeting signal, which is necessary and sufficient to direct mitochondrial localization of GFP; in addition its fusion to the HIV Rev protein induces a partial relocalization of the fusion protein from nucleoli to mitochondria (D'Agostino et al., 2000). The MTS of p13 is contained in the amphipathic α-helical domain, a structure that is reminiscent of canonic MTS which consist of an N-terminal positively charged, cleavable presequence and a hydrophobic sequence. Unlike canonic MTS, which are dependent on the presence of positively charged residues, and are cleaved upon import in mitochondria, substitution of the four arginines in the MTS of p13 does not affect mitochondrial targeting and the sequence is not cleaved upon import (Ciminale et al., 1999).
A recent study by Andresen et al. provides interesting clues regarding the dual localization of p13 demonstrating that, upon coexpression with Tax, p13 is partially routed to nuclear speckles (Andresen et al., submitted). These observations indicating a dual localization of p13 suggest that the protein might exert distinct mitochondrial and nuclear effects (see below).
4. Effects of p13 on isolated mitochondria
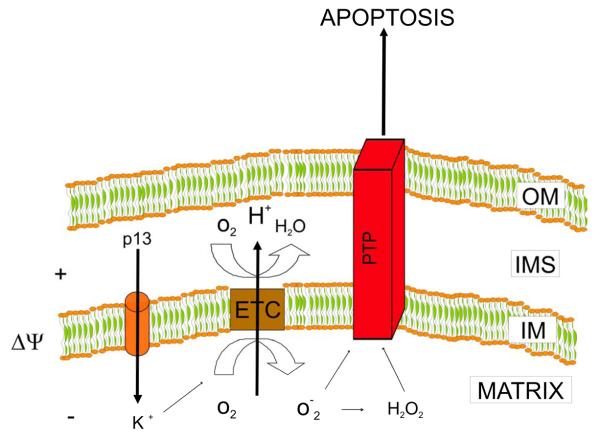
When added to isolated energized mitochondria, p13 induces an influx of K+ that is driven by the inner mitochondrial membrane potential (Δψ), as depicted in the model shown in Figure 2. Interestingly, low p13 concentrations induce low amplitude swelling that results in a crescent-like ultrastructure of mitochondria. This process is not accompanied by mitochondrial depolarization or cytochrome c release and is reverted by mitochondrial depolarization with protonophores, suggesting that it is directly linked to energy-dependent K+ influx (Silic-Benussi et al., 2009). In contrast, high p13 concentrations induce large amplitude swelling which cannot be reversed by depolarization and is accompanied by cytochrome c release (Silic-Benussi et al., 2009). This effect is reminiscent of the swelling triggered by opening of the permeability transition pore (PTP), a large conductance mitochondrial channel controlling apoptosis [reviewed by (Rasola and Bernardi, 2007)].
Figure 2.
Working model of p13 function based on the results of studies on isolated mitochondria. p13 induces inner mitochondrial membrane potential (Δψ)-driven influx of K+. The influx of K+ induced by p13 triggers an increase in the activity of the electron transport chain (ETC) that, by extruding H+ from the matrix, dampens the depolarization induced by p13. Increased ETC activity also leads to increased production of reactive oxygen species (ROS) by the ETC that lowers the opening threshold of the permeability transition pore (PTP), suggesting that p13 might trigger apoptosis. OM, outer membrane; IMS, intermembrane space; IM, inner membrane.
The influx of K+ induced by p13 triggers an increase in mitochondrial respiration, an effect that is consistent with the tight coupling between membrane potential and electron transport. The increased activity of the electron transport chain (ETC) dampens the depolarization induced by p13 by extruding H+ from the matrix. These changes lead to increased production of reactive oxygen species (ROS) by the ETC (Silic-Benussi et al., 2009) that lowers the opening threshold of the PTP in isolated mitochondria, suggesting that p13 might sensitize to apoptotic stimuli acting trough the PTP and mitochondria. At higher concentrations of p13 the magnitude of the K+ current exceeds the compensating capacity of the ETC resulting in mitochondrial depolarization and large amplitude swelling, two effects that are also likely to affect mitochondria-mediated apoptosis. All these effects are dependent on the presence of the positively charged amino acids in the α-helical MTS of the protein.
5. Mitochondrial effects of p13 in the context of cells
5.1 Effects on cell death
Early studies based on ectopic expression of p13 in HeLa cells showed that p13 induces mitochondrial fragmentation although this effect does not “per se” result in mitochondrial depolarization, release of cytochrome c from mitochondria or cell death (Ciminale et al., 1999). This p13-induced fragmentation of mitochondria is dependent on the presence of critical arginines in the α-helical domain of the protein (D'Agostino et al., 2002). The fact that these arginine residues are not essential for mitochondrial targeting, but are required for the protein's ability to induce K+ influx in mitochondria and mitochondrial fragmentation in cells, suggests that these two functions are not completely overlapping.
In a more recent study we quantitated the effects of p13 on mitochondrial inner membrane potential using the potential-dependent probe tetramethyl rhodamine methyl ester (TMRM) and GFP-tagged WT p13 vs. mutant p13. Results showed that the effects of p13 on mitochondrial membrane potential were dose-dependent, with depolarization ensuing at high levels of p13 expression, suggesting that, as observed in isolated mitochondria, the K+ influx induced by low concentrations of p13 can be compensated by an activation of the ETC. Like the effects observed in isolated mitochondria, this effect was not observed with mutants of p13 carrying substitutions of the critical arginines in the alpha helical domain of the protein (Biasiotto et al., submitted) (Figure 3).
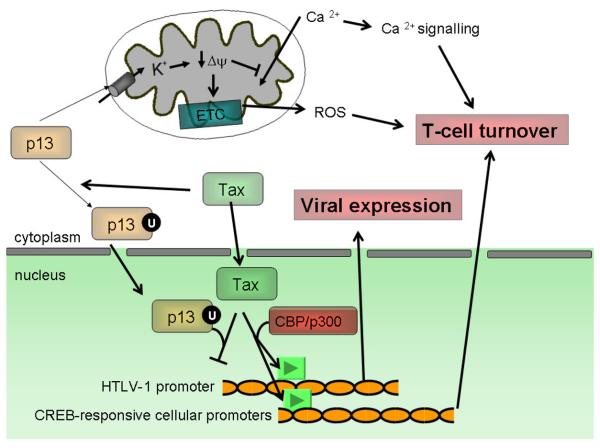
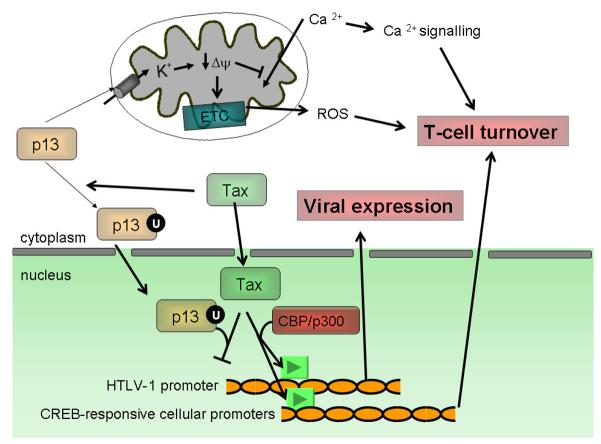
Figure 3.
Mitochondrial and nuclear functions of p13. The observations indicating a dual localization of p13 suggest that the protein might exert distinct functions depending on its intracellular localization. Effects on mitochondria, mediated through increased ROS production, control cell turnover. The effects of p13 on inner membrane potential also inhibit the mitochondrial uptake of Ca2+, which in turn may modulate Ca2+ signalling that in T cells also plays a pivotal role in controlling cell activation and death.
Nuclear localization, which follows p13 ubiquitylation (U) in the presence of Tax results in the formation of transcriptionally inactive p13-Tax dimers. This effect is likely to reduce the expression of both HTLV-1 and Tax-controlled cellular genes.
Studies of p13's effects on cell death showed that the protein does not act as a pro-apoptotic protein per se but enhances proapoptotic stimuli such as FasL and C-2 ceramide (Hiraragi et al., 2005; Silic-Benussi et al., 2004) or glucose deprivation (see below). Interestingly, the effects of p13 on FasL-mediated apoptosis are enhanced by Ras overexpression and blocked by inhibitors of Ras (Hiraragi et al., 2005; Silic-Benussi et al., 2004). Furthermore, p13 binds farnesyl pyrophosphate synthase, a key enzyme in the synthesis of substrates required for the prenylation of Ras (Lefebvre et al., 2002a; Lefebvre et al., 2002b), suggesting that p13 affects the Ras signal transduction pathway. This hypothesis goes along with the finding that p13 inhibits the growth of Ras-transformed tumors in vivo (Silic-Benussi et al., 2004).
5.2 Effects of p13 on reactive oxygen species (ROS)
Also consistent with the observations in isolated mitochondria is the finding that p13 affects ROS production in the context of cells. Interestingly, in tumor cells (e.g. transiently transfected HeLa cells or Jurkat T-cells transduced with a lentiviral vector expressing p13), p13 increases ROS production and cell death in response to glucose deprivation (Silic-Benussi et al., submitted) (Figure 3). As shown in Figure 4, the Jurkat T cells transduced with p13 lentiviral vectors also exhibit a reduced proliferation rate, suggesting that p13 might restrain the growth of transformed cells. These results suggest that, in the context of transformed cells, p13 might act as a “viral tumor suppressor” reducing proliferation and favoring cell death especially in conditions of reduced substrate availability. In contrast, expression of p13 in primary quiescent T-cells results in ROS-dependent activation, measured as an increase in CD38 expression (Silic-Benussi et al., submitted). This dual effect of p13 in normal vs. transformed cells is consistent with a current model that compares the effects of ROS on cell turnover to a rheostat (Rustin, 2002). According to this model, an increasing gradient of ROS is found from resting- to activated- to transformed- to apoptotic cells. This model is also relevant in the context of T-cell activation, as engagement of the T cell receptor (TCR) induces rapid generation of ROS which are in part produced by mitochondria. Elevated ROS act as powerful second messengers controlling redox-sensitive pathways such as the MEK, ERK, LCK, NF-κB and the permeability transition pore (PTP) (Williams and Kwon, 2004). In the context of HTLV-1 replication, p13 may therefore favor life-long viral persistence of the virus in the absence of disease by expanding the pool of normal infected T lymphocytes and eliminating cells occasionally acquiring a transformed phenotype as a result of the function of the Tax oncoprotein.
Figure 4.
Effects of p13 on T-cell proliferation. Shown is a proliferation analysis of Jurkat T cells transduced with a lentiviral vector expressing p13 or with a control empty vector. Cells were labelled with the cell membrane fluorescent probe PKH26 (sigma) on day 0 and proliferation was assessed by measuring the dilution of the fluorescent signal resulting from cell division was monitored on days 2 and 6. A) Flow cytometry analysis of PKH26 staining demonstrating a higher proportion of cells of higher generation in control compared to p13-expressing cells. Colors indicate generations of cells exhibiting progressively lower intensities of PKH26 staining (Blue, generation 1= parental; orange, generation 2; green, generation 3; purple, generation 4; light blue, generation 5); arrow indicates peak fluorescence values of parental (time o) generation for the two cell lines. B) Mean proliferation indexes over time calculated from the PKH26 analyses in control vs. p13-expressing cells.
5.3 Effects of p13 on Ca2+ homeostasis
Mitochondria are also known to play a crucial role in the control of intracellular Ca2+ homeostasis (Giacomello et al., 2007). On the other hand, uptake of Ca2+ in the mitochondrial matrix stimulates ATP production and aerobic metabolism by controlling the activity of key metabolic enzymes, while excessive calcium accumulation in mitochondria triggers PTP opening and apoptosis [reviewed by (Giacomello et al., 2007)]. In light of the mitochondrial targeting of p13 and its effects on mitochondrial morphology and function, we recently investigated whether the protein might affect calcium homeostasis. To this end we employed the Ca2+-sensitive probe aequorin targeted to mitochondria, ER or cytosol. These probes were employed to measure Ca2+ concentrations in these intracellular compartments following histamine stimulation, which mobilizes Ca2+ from intracellular stores and mediates influx of Ca2+ from the extracellular medium. Results showed that p13 specifically reduces mitochondrial Ca2+ uptake (Biasiotto et al., submitted) (Figure 3). These data shed light on previous findings showing that p13 increases phosphorylation of CREB on serine 133 in response to histamine (Silic-Benussi et al., 2004) and underscore that through its control of Ca2+ signalling p13 might control T-cell activation and death.
6. Nuclear effects of p13 and interactions with Tax
Although mainly targeted to mitochondria, in the presence of Tax p13 shows a partial redistribution to the nucleus. Tax also mediates ubiquitylation of p13, an effect that increases the stability of p13. Although the p13 sequence does not include any lysines (the canonical targets for ubiquitylation), preliminary experiments suggest that p13 might be ubiquitylated on serine and threonine. Interestingly, in the presence of Tax, ubiquitylated p13 is enriched in the nuclear fraction, suggesting that this modification might be important in controlling the intracellular sorting of p13 (Andresen et al., submitted).
In addition, Tax and p13 partly colocalize in nuclear speckles, and a fraction of p13 binds to Tax. This interaction interferes with the ability of Tax to bind the CBP/p300 co-activator, resulting in the inhibition of Tax-mediated LTR transcription and expression of Gag protein from an HTLV-1 molecular clone (Andresen et al., submitted). Taken together, these data suggest that p13 might provide a negative feedback loop in the regulation of HTLV-1 gene expression. This effect appears to be somewhat distinct from that of the p30 protein, which shares with p13 its C-terminal 87 amino acids. p30 was in fact demonstrated to selectively inhibit the viral mRNA coding for Tax and Rex by increasing its nuclear retention, while p13 inhibits HTLV-1 expression at the transcriptional level. The effects of p13 on viral gene expression and Tax activity were confirmed by ectopic expression of p13 in the context of the chronically infected cell line MT-2. In addition, also in the context of naturally infected cell lines, transfected p13 was stabilized by Tax and partially relocalized to the nucleus (Andresen et al., submitted).
Andresen et al. also showed that p13 forms dimers which are dependent on the presence of cysteine 27 and are inhibited by reducing agents such as β-mercaptoethanol. However, the functional significance of the dimerization of p13 is still unclear, as the presence of cysteine 27 did not seem to be required for either mitochondrial localization or interaction with Tax and inhibition of viral gene expression (Andresen et al., submitted).
7. FUTURE DIRECTIONS
7.1. Interactions of p13 and Tax in controlling redox homeostasis and cell turnover
The data summarized above highlight the need to study the effects of p13 (and other viral proteins) in the context of the complexity of the HTLV-1 genome. Furthermore, the fact that Tax may exert antiapoptotic functions by activating key survival pathways (e.g. NF-κB, Akt) and that p13 may trigger cells death in response to specific signals (e.g., FasL, C2-ceramide, glucose deprivation) suggest that these two viral proteins might act in concert to maintain the proliferation and survival rates of infected cells at a level compatible with long term viral persistence and survival of the host.
The effects of p13 on ROS and its recently discovered functional interactions with Tax are interesting in light of the redox alterations known to occur in HTLV-1 infection and tumour transformation. A growing body of experimental evidence highlights a prominent role of ROS as second messengers controlling proliferation, cell death and, more generally, protein function (Trachootham et al., 2009). ROS homeostasis results from the balance between ROS-generating pathways (e.g. the mitochondrial electron transport chain and NADPH oxidase) and the concerted action of scavenging systems (e.g. thioredoxin and glutathione). The finding that cancer cells exhibit characteristic alterations in their redox balance has prompted considerable interest in developing novel therapeutic approaches aimed at targeting redox alterations in cancer cells (Trachootham et al., 2009). Human thioredoxin (TRX), a powerful ROS scavenger that was first isolated from cultures of ATLL cells and described as “ATL-derived factor” (ATF). Subsequent studies showed that TRX is consistently up-regulated in cells chronically infected with HTLV-I (Yodoi and Tursz, 1991). This alteration results from the transcriptional activation of the TRX promoter by Tax (Masutani et al., 1996) and suggests that changes in the redox balance might be intimately linked to HTLV-1 infection. Further studies of TRX showed that it is overexpressed in various solid and hematological tumors and is associated with a poor prognosis [reviewed by (Kaimul et al., 2007)] and with the onset of resistance to ROS-inducing drugs (e.g. the synthetic retinoid N-4-hydroxyphenylretinamide) (Darwiche et al., 2007).
In addition to its ROS-scavenging activity, TRX also functions as a redox sensor and signalling molecule. In its reduced form TRX has strong stimulatory effects on cellular proliferation and exerts a strong anti-apoptotic activity through mechanisms involving the JNK/p38MAPK and AKT pathways. In the first mechanism, reduced TRX binds and inhibits ASK1 (apoptosis signal-regulating kinase), while oxidized TRX dissociates from ASK1, which binds TRAF-2 leading to activation of the JNK/p38MAPK pathways and cell death [reviewed by (Kaimul et al., 2007)]. Activation of the Akt survival pathway by TRX occurs either directly or indirectly through binding and inhibition of the PTEN phosphatase (Meuillet et al., 2004).
Interestingly, both an increase and a decrease in ROS production have been reported in Tax-expressing cells. Using an estradiol-inducible system in which Tax is fused at its N-terminus with the human estrogen receptor, Los et al. show increased H2O2 production in Jurkat T-cells upon stimulation with anti-CD3 antibodies and induction of Tax expression (Los et al., 1998). Upon treatment with estradiol, the ER-Tax fusion protein re-localizes to the nucleus and induces a prolonged pro-oxidative state with decreased GSH levels and increased apoptosis (Los et al., 1998). In apparent contrast with these data, Darwiche et al. demonstrated that Tax blocks the effect of chemotherapeutic agents that act by increasing ROS levels (Darwiche et al., 2007). This latter finding is consistent with the ability of Tax to activate expression of TRX. The apparent contradiction of these reports may be linked to differences in the Tax expression vectors, cell types and ROS probes employed. In addition, Tax upregulates TRX expression by interacting with the CREB, AP-1 and NF-κb trascription factors; on the other hand TXR may act as repressor of Tax thus inhibiting transcription of viral genes (Sasada et al., 2002), suggesting that redox state and viral expression might be connected and regulated through a negative feedback loop. It is worth noting that in the context of HTLV-I infected cells the downregulation of TRX-binding protein-2 (TBP-2) by promoter methylation and histone deacetylation is necessary for the infected cells to acquire independence from exogenous IL-2, a key step for the development of the transformed phenotype. TBP-2 is 46 kDa protein that binds the reduced form of TRX acting as a negative regulator of its activity. TBP-2 acts as a growth suppressor and is is downregulated in human cancer (Kaimul et al., 2007), (Ahsan et al., 2006). Interestingly, in a recent study of bovine leukemia virus (BLV), an oncogenic retrovirus related to HTLV-1, Bouzar et al. reported a correlation between a reduction in ROS levels, increased survival and increased BLV proviral loads (Bouzar et al., 2009).
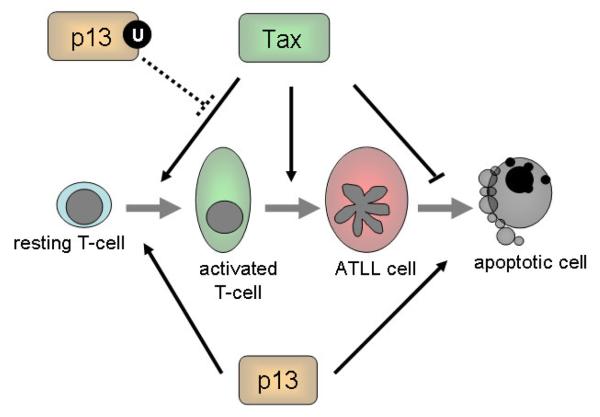
Taken together, these findings suggest that the outcome of the interactions between p13 and Tax might depend on the cellular context: in primary resting T-cells the two proteins might cooperate in driving the expansion of infected cells, while in the context of transformed cells p13 might act as a viral tumor suppressor curbing the transforming potential of Tax (Figure 5). Further studies should be aimed at investigating the combined effects of p13 and Tax on cell turnover and redox homeostasis in primary vs. tumor cells.
Figure 5.
Functional interactions of Tax and p13 in HTLV-1 persistence and T-cell transformation. Studies carried out so far suggest that Tax and p13 might act in concert to maintain the proliferation and survival rates of infected cells at a level compatible with long term viral persistence and survival of the host. Solid lines indicate demonstrated effects, while dotted lines indicate hypothetical interactions. Tax drives T-cell proliferation and reduces cell death resulting in the accumulation of infected cell pool; however due to the effects of Tax on cell cycle checkpoints and genetic stability these cells are prone to accumulate genetic lesions resulting in the emergence of neoplastic clones giving rise to ATLL. p13 activation of resting T-cells might cooperate with Tax in expanding the pool of “normal” infected T-cells; however, the proapototic effect of p13 on tumor cells might contrast with the anti-apoptotic effect of Tax, reducing the probability of ATLL transformation.
Further studies of the influence of Tax on the ubiquitylation of p13 (Andresen et al., submitted) are likely to reveal an important link between this post-translational modification and p13's subcellular localization and function. In contrast to polyubiquitylation, monoubiquitylation mediates proteasome-independent, non catabolic functions including intracellular targeting, vescicular trafficking, virus budding, chromatine remodelling, transcription and DNA repair (Mukhopadhyay and Riezman, 2007; Salmena and Pandolfi, 2007). Interestingly, recent studies demonstrated that the nuclear–cytoplasmic shuttling of the tumor suppressors p53, FOXO and PTEN is regulated through monoubiquitylation (Salmena and Pandolfi, 2007). Of particular relevance is the control of p53 export from the nucleus (where it acts as a transcription factor) to the cytoplasm, where it localizes to mitochondria and triggers apoptosis. This process is mediated through the MDM2-mediated monoubiquitylation of p53, and possibly by HAUSP, a deubiquitylating enzyme.
7.2. Interactions of p13 with p12: effects on Ca2+ homeostasis and Ca2+ signaling
The effects of p13 on Ca2+ homeostasis are also likely to intersect with the function of the p12 accessory protein of HTLV-1. p12 localizes to the endoplasmic reticulum (ER) (Ding et al., 2001; Johnson et al., 2001), which represents the major site of intracellular Ca2+ storage. p12 increases release of Ca2+ from the ER stores resulting in increased cytosolic Ca2+ concentrations and activation of the nuclear factor of activated T cells (NF-AT) (Albrecht et al., 2002; Ding et al., 2002), a key transcription factor controlling T-cell activation. Combined expression of p12 and p13 might therefore enhance the amplitude and duration of cytosolic Ca2+ transients in response to TCR stimulation, resulting in enhanced NFAT activation.
Experiments aimed at reconstructing the complexity of HTLV-1 biology/pathogenesis by testing the functional interactions among p13 and other viral proteins should shed light on the mechanisms evolved by the virus to impinge on the pathways controlling on T-cell turnover resulting in HTLV-1 propagation and persistence in the host. Such studies should not be confined to CD4+ T-cells, as recent evidence indicates that plasmacytoid dendritic cells (pDC) are key targets of HTLV-1 infection in vivo and efficiently transfer the virus to CD4+ T-cells (Jones et al., 2008). The understanding of the functional interactions of the different regulatory and accessory proteins of HTLV-1 would also benefit from a careful quantitative analysis on their relative abundance and timing of expression of the in the context of natural HTLV-1 infection, a topic that is currently under investigation in our laboratory.
ACKNOWLEDGMENTS
We thank Luigi Chieco-Bianchi, Paolo Bernardi, Fabio di Lisa and Ilaria Cavallari for valuable discussions. This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (VC), the European Union (‘The role of chronic infections in the development of cancer’; contract no. 2005-018704, to VC), the Ministero per l'Università e la Ricerca Scientifica, e Tecnologica - Progetti di Ricerca di Interesse Nazionale (VC), the Ministero della Salute (progetto RFPS-2006-2-342-010, the University of Padova (DMD, VC), and the National Institutes of Health (VA, GF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahsan MK, Masutani H, Yamaguchi Y, Kim YC, Nosaka K, Matsuoka M, Nishinaka Y, Maeda M, Yodoi J. Loss of interleukin-2-dependency in HTLV-I-infected T cells on gene silencing of thioredoxin-binding protein-2. Oncogene. 2006;25:2181–2191. doi: 10.1038/sj.onc.1209256. [DOI] [PubMed] [Google Scholar]
- Albrecht B, D'Souza CD, Ding W, Tridandapani S, Coggeshall KM, Lairmore MD. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12(I) J Virol. 2002;76:3493–3501. doi: 10.1128/JVI.76.7.3493-3501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzar AB, Boxus M, Florins A, Francois C, Reichert M, Willems L. Reduced levels of reactive oxygen species correlate with inhibition of apoptosis, rise in thioredoxin expression and increased bovine leukemia virus proviral loads. Retrovirology. 2009;6:102. doi: 10.1186/1742-4690-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciminale V, Zotti L, D'Agostino DM, Ferro T, Casareto L, Franchini G, Bernardi P, Chieco-Bianchi L. Mitochondrial targeting of the p13II protein coded by the x-II ORF of human T-cell leukemia/lymphotropic virus type I (HTLV-I) Oncogene. 1999. pp. 4505–4514. [DOI] [PubMed]
- D'Agostino DM, Ranzato L, Arrigoni G, Cavallari I, Belleudi F, Torrisi MR, Silic-Benussi M, Ferro T, Petronilli V, Marin O, et al. Mitochondrial alterations induced by the p13II protein of human T-cell leukemia virus type 1. Critical role of arginine residues. J Biol Chem. 2002;277:34424–34433. doi: 10.1074/jbc.M203023200. [DOI] [PubMed] [Google Scholar]
- D'Agostino DM, Zotti L, Ferro T, Franchini G, Chieco-Bianchi L, Ciminale V. The p13II protein of HTLV type 1: comparison with mitochondrial proteins coded by other human viruses. AIDS Res Hum Retroviruses. 2000;16:1765–1770. doi: 10.1089/08892220050193281. [DOI] [PubMed] [Google Scholar]
- Darwiche N, Abou-Lteif G, Bazarbachi A. Reactive oxygen species mediate N-(4-hydroxyphenyl)retinamide-induced cell death in malignant T cells and are inhibited by the HTLV-I oncoprotein Tax. Leukemia. 2007;21:261–269. doi: 10.1038/sj.leu.2404472. [DOI] [PubMed] [Google Scholar]
- Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim SJ, Altschuld RA, Lairmore MD. Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J Virol. 2002;76:10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Albrecht B, Luo R, Zhang W, Stanley JR, Newbound GC, Lairmore MD. Endoplasmic reticulum and cis-Golgi localization of human T-lymphotropic virus type 1 p12(I): association with calreticulin and calnexin. J Virol. 2001;75:7672–7682. doi: 10.1128/JVI.75.16.7672-7682.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbel S, Sinha-Datta U, Dundr M, Brown M, Franchini G, Nicot C. Human T-cell leukemia virus type I p30 nuclear/nucleolar retention is mediated through interactions with RNA and a constituent of the 60 S ribosomal subunit. J Biol Chem. 2006;281:37150–37158. doi: 10.1074/jbc.M603981200. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Pizzo P, Pozzan T. Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ. 2007;14:1267–1274. doi: 10.1038/sj.cdd.4402147. [DOI] [PubMed] [Google Scholar]
- Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24:5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- Harhaj EW, Good L, Xiao G, Sun SC. Gene expression profiles in HTLV-I-immortalized T cells: deregulated expression of genes involved in apoptosis regulation. Oncogene. 1999;18:1341–1349. doi: 10.1038/sj.onc.1202405. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Sawa H, Lewis MJ, Orba Y, Sheehy N, Yamamoto Y, Ichinohe T, Tsunetsugu-Yokota Y, Katano H, Takahashi H, et al. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat Med. 2006;12:466–472. doi: 10.1038/nm1389. [DOI] [PubMed] [Google Scholar]
- Hiraragi H, Michael B, Nair A, Silic-Benussi M, Ciminale V, Lairmore M. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13II sensitizes Jurkat T cells to Ras-mediated apoptosis. J Virol. 2005;79:9449–9457. doi: 10.1128/JVI.79.15.9449-9457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Nicot C, Fullen J, Ciminale V, Casareto L, Mulloy JC, Jacobson S, Franchini G. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12(I) protein. J Virol. 2001;75:6086–6094. doi: 10.1128/JVI.75.13.6086-6094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14:429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- Kaimul AM, Nakamura H, Masutani H, Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free radical biology & medicine. 2007;43:861–868. doi: 10.1016/j.freeradbiomed.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Kfoury Y, Nasr R, Hermine O, de The H, Bazarbachi A. Proapoptotic regimes for HTLV-I-transformed cells: targeting Tax and the NF-kappaB pathway. Cell Death Differ. 2005;12 Suppl 1:871–877. doi: 10.1038/sj.cdd.4401624. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Fullen J, Franchini G. The p12I, p13II, and p30II proteins encoded by human T-cell leukemia/lymphotropic virus type I open reading frames I and II are localized in three different cellular compartments. J Virol. 1993;67:2360–2366. doi: 10.1128/jvi.67.4.2360-2366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. doi: 10.1038/nri2115. [DOI] [PubMed] [Google Scholar]
- Krueger A, Fas SC, Giaisi M, Bleumink M, Merling A, Stumpf C, Baumann S, Holtkotte D, Bosch V, Krammer PH, et al. HTLV-1 Tax protects against CD95-mediated apoptosis by induction of the cellular FLICE-inhibitory protein (c-FLIP) Blood. 2006;107:3933–3939. doi: 10.1182/blood-2005-06-2567. [DOI] [PubMed] [Google Scholar]
- Lairmore M, Franchini G. Human T-cell leukemia virus types 1 and 2. In: DM K, PM H, editors. Fields Virology. Fifth Edition Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 2071–2106. [Google Scholar]
- Lefebvre L, Ciminale V, Vanderplasschen A, D'Agostino D, Burny A, Willems L, Kettmann R. Subcellular localization of the bovine leukemia virus R3 and G4 accessory proteins. J Virol. 2002a;76:7843–7854. doi: 10.1128/JVI.76.15.7843-7854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre L, Vanderplasschen A, Ciminale V, Heremans H, Dangoisse O, Jauniaux JC, Toussaint JF, Zelnik V, Burny A, Kettmann R, et al. Oncoviral bovine leukemia virus G4 and human T-cell leukemia virus type 1 p13(II) accessory proteins interact with farnesyl pyrophosphate synthetase. J Virol. 2002b;76:1400–1414. doi: 10.1128/JVI.76.3.1400-1414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los M, Khazaie K, Schulze-Osthoff K, Baeuerle PA, Schirrmacher V, Chlichlia K. Human T cell leukemia virus-I (HTLV-I) Tax-mediated apoptosis in activated T cells requires an enhanced intracellular prooxidant state. J Immunol. 1998;161:3050–3055. [PubMed] [Google Scholar]
- Masutani H, Hirota K, Sasada T, Ueda-Taniguchi Y, Taniguchi Y, Sono H, Yodoi J. Transactivation of an inducible anti-oxidative stress protein, human thioredoxin by HTLV-I Tax. Immunology letters. 1996;54:67–71. doi: 10.1016/s0165-2478(96)02651-x. [DOI] [PubMed] [Google Scholar]
- Meuillet EJ, Mahadevan D, Berggren M, Coon A, Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN's lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN's tumor suppressor activity. Arch Biochem Biophys. 2004;429:123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–205. doi: 10.1126/science.1127085. [DOI] [PubMed] [Google Scholar]
- Pise-Masison CA, Radonovich M, Mahieux R, Chatterjee P, Whiteford C, Duvall J, Guillerm C, Gessain A, Brady JN. Transcription profile of cells infected with human T-cell leukemia virus type I compared with activated lymphocytes. Cancer Res. 2002;62:3562–3571. [PubMed] [Google Scholar]
- Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- Richardson JH, Edwards AJ, Cruickshank JK, Rudge P, Dalgleish AG. In vivo cellular tropism of human T-cell leukemia virus type 1. J Virol. 1990;64:5682–5687. doi: 10.1128/jvi.64.11.5682-5687.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckes T, Saul D, Van Snick J, Hermine O, Grassmann R. Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood. 2001;98:1150–1159. doi: 10.1182/blood.v98.4.1150. [DOI] [PubMed] [Google Scholar]
- Rustin P. Mitochondria, from cell death to proliferation. Nat Genet. 2002;30:352–353. doi: 10.1038/ng0402-352. [DOI] [PubMed] [Google Scholar]
- Saggioro D, Silic-Benussi M, Biasiotto R, D'Agostino DM, Ciminale V. Control of cell death pathways by HTLV-1 proteins. Front Biosci. 2009;14:3338–3351. doi: 10.2741/3456. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Salmena L, Pandolfi PP. Changing venues for tumour suppression: balancing destruction and localization by monoubiquitylation. Nat Rev Cancer. 2007;7:409–413. doi: 10.1038/nrc2145. [DOI] [PubMed] [Google Scholar]
- Sasada T, Nakamura H, Masutani H, Ueda S, Sono H, Takabayashi A, Yodoi J. Thioredoxin-mediated redox control of human T cell lymphotropic virus type I (HTLV-I) gene expression. Molecular immunology. 2002;38:723–732. doi: 10.1016/s0161-5890(01)00109-2. [DOI] [PubMed] [Google Scholar]
- Schnell JR, Chou JJ. Structure and mechanism of the M2 proton channel of influenza A virus. Nature. 2008;451:591–595. doi: 10.1038/nature06531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silic-Benussi M, Cannizzaro E, Venerando A, Cavallari I, Petronilli V, La Rocca N, Marin O, Chieco-Bianchi L, Di Lisa F, D'Agostino DM, et al. Modulation of mitochondrial K(+) permeability and reactive oxygen species production by the p13 protein of human T-cell leukemia virus type 1. Biochim Biophys Acta. 2009;1787:947–954. doi: 10.1016/j.bbabio.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Silic-Benussi M, Cavallari I, Zorzan T, Rossi E, Hiraragi H, Rosato A, Horie K, Saggioro D, Lairmore MD, Willems L, et al. Suppression of tumor growth and cell proliferation by p13II, a mitochondrial protein of human T cell leukemia virus type 1. Proc Natl Acad Sci U S A. 2004;101:6629–6634. doi: 10.1073/pnas.0305502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Williams MS, Kwon J. T cell receptor stimulation, reactive oxygen species, and cell signaling. Free radical biology & medicine. 2004;37:1144–1151. doi: 10.1016/j.freeradbiomed.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Yodoi J, Tursz T. ADF, a growth-promoting factor derived from adult T cell leukemia and homologous to thioredoxin: involvement in lymphocyte immortalization by HTLV-I and EBV. Adv Cancer Res. 1991;57:381–411. doi: 10.1016/s0065-230x(08)61004-0. [DOI] [PubMed] [Google Scholar]