Abstract
Lecithin:retinol acyltransferase (LRAT) regulates retinol (vitamin A) metabolism by esterifying retinol. LRAT expression is decreased in cultured human head and neck squamous cell carcinoma (SCCHN) relative to normal epithelial cells. We investigated whether the carcinogen 4-nitroquinoline 1-oxide (4-NQO) induced a higher incidence of oral cancer in LRAT knockout than wild type (Wt) mice. We also investigated retinol deprivation during 4-NQO treatment in LRAT−/− mice as a model for rapid retinol deficiency. We observed higher levels of secreted frizzled-related protein 2 (Sfrp2), an inhibitor of WNT signaling, in tongue tumors in LRAT−/− versus Wt. LRAT−/− embryonic stem cells also expressed higher Sfrp2 transcripts, indicating an interaction between retinol and WNT signaling. Cox-2, Cyclin D1, p21, Trop2, and RARβ2 were not differentially expressed in Wt versus LRAT−/− tongue tumors. Wt and LRAT−/− mice fed a retinol sufficient diet showed the same oral tumor incidence after 4-NQO. In contrast, tongue tumors developed in 60% of Wt and in 100% of LRAT−/− mice fed a retinol deficient diet during 4-NQO treatment (p=0.22); moreover, the BrdU labeling index was 21.0±2.4% in LRAT−/− normal tongue epithelium as compared to 9.9±0.8% in Wt (p<0.001). Thus, partial retinol deficiency during carcinogen treatment (achieved in LRAT−/−) resulted in more proliferating cells in tongue epithelia from LRAT−/− mice and ultimately a greater probability of carcinogenesis.
Keywords: stem cell, squamous cell carcinoma of the head and neck (SCCHN), retinol, cancer prevention, retinoic acid, nutrition
INTRODUCTION
Head and neck cancer is an important health threat worldwide [1, 2]. According to the National Cancer Institute, over 22,000 new oral cancer cases are diagnosed annually in the United States (http://www.cancer.gov/cancertopics/types/oral/) and they account for approximately 3 to 5 percent of all cancers. Retinoids are structurally related compounds that are derived from vitamin A (retinol). Retinoids regulate cell proliferation and differentiation [3]. All-trans retinoic acid (RA), the most biologically active metabolite of retinol, acts via binding and activating its nuclear receptors, which are transcription factors that directly regulate the transcription of certain “target” genes [4]. Two families of nuclear receptors mediate the effects of retinoids. All-trans RA binds and activates the RA receptor family (RARα, β and γ), while 9-cis RA binds to both RARs and the retinoid X receptor family (RXRα, β and γ)[3-5]. Studies have shown that alterations in retinoid signaling occur during the malignant transformation of oral epithelia [6-9].
Abnormal metabolism of retinoids and reduced expression of RARβ2 have been observed in many types of malignant cells, as well as in the transition from pre-malignant lesions to aggressive carcinomas, including oral cancers [6, 8-13]. RA treatment can up-regulate RARβ2 gene expression in normal epithelial cells, and can also partially restore the reduced RARβ2 expression in certain carcinoma cells [14, 15]. For these reasons, retinoids and drugs that modulate the functions of RARs are useful therapeutic and cancer chemopreventive agents for many types of cancers [16-19].
Lecithin:retinol acyltransferase (LRAT) catalyzes the esterification of retinol and functions as one of the enzymes which regulates the levels of tissue retinoids. Retinyl esters are stored in a variety of tissues and can be hydrolyzed to retinol by retinyl ester hydrolases [20, 21]. High LRAT activity is found in the liver [22-25], lung [26, 27] and small intestine [28, 29], but expression is also detected in eye [30-32], testis [33], skin [34, 35], mammary gland [34, 36], prostate epithelium [37] and oral cavity epithelial cells [34]. LRAT gene knockout mice do not store retinyl esters in the liver and other tissues and therefore they are more susceptible to retinol deficiency [26, 38, 39]. LRAT also plays a role in the homeostasis of retinol in both mouse embryos and adult mice as the dietary retinol level fluctuates [40, 41].
Since our previous research has shown that the expression of LRAT is much higher in normal cultured human oral epithelial cells than in oral carcinoma cell lines [34], we wanted to determine whether low or absent LRAT activity increased the incidence or severity of carcinogen induced oral carcinogenesis. We developed a mouse model of carcinogenesis in which the carcinogen 4-nitroquinoline 1-oxide (4-NQO) induces both tongue and, at a lower incidence, esophageal squamous cell carcinoma (SCC) [42]. In this model the 4-NQO is added to the drinking water for several weeks. Then, the 4-NQO is removed and over the next several weeks hyperplasias, dysplasias, papillomas, and squamous cell carcinomas develop specifically on the tongue and at a lower frequency, in the esophagus [42]. This murine oral carcinogenesis model is based on the 4-NQO rat model used previously by many researchers (eg. [43, 44]). One advantage of the murine model over the rat model is that various transgenic and knockout animals can be tested in the murine model. In this study we investigated whether the carcinogen 4-NQO induced a higher incidence of oral cancer in mice in which the LRAT gene is disrupted by homologous recombination [26].
MATERIALS AND METHODS
Animals
The LRAT gene knockout mice were produced as previously described [26] and bred into a C57BL/6 background. The genotypes were determined by polymerase chain reaction (PCR) from tail genomic DNA [26]. All studies were performed with the approval of the Research Animal Resource Center (RARC) of Weill Cornell Medical College.
4-NQO treatment
The carcinogen 4-NQO (Sigma, St. Louis, MO) was used to induce oral carcinogenesis, as described by this laboratory previously [42]. Briefly, freshly prepared 4-NQO stock solution (5 mg/ml in propylene glycol) was added to the drinking water at 60 μg/ml, and treatment was started in mice at six-weeks of age. The 4-NQO treated group contained 42 mice, including 20 wild type (Wt) mice (18 males and 2 females) and 22 LRAT−/− mice (19 males and 3 females). The control (not treated with 4-NQO) group contained 15 mice, including 8 Wt mice (6 males and 2 females) and 7 LRAT−/− mice (4 males and 3 females). No 4-NQO, and only propylene glycol was added to the drinking water for the control group. The drinking water was freely accessible at all times. After the 8 weeks of the 4-NQO treatment ended, the mice were maintained on regular water (without carcinogen) for another 16 weeks. All of these mice were maintained on standard lab chow which contains 25 IU of retinyl palmitate per gram throughout the entire experiment. The mice were then sacrificed and the lesions on the tongues were assessed by gross and histological examination. Three mice, including one Wt male, one LRAT−/− male and one LRAT−/− female, unexpectedly died during the 4-NQO treatment, and they were not included in further data analysis.
In an additional treatment group set up to test the effects of vitamin A deficiency, five Wt mice (two males and three females) and five LRAT−/− mice (three males and two females), were fed a vitamin A deficient diet (TD 88407, Harlan Teklad) for six weeks during the carcinogen 4-NQO treatment. Serum retinol levels decline by greater than 90% in the LRAT−/− mice within 2-3 weeks on this diet [26]. In contrast, there is no decline of serum retinol levels in Wt mice during the 6 weeks they are on this vitamin A deficient diet [26]. We purposely used a higher 4-NQO dose and shorter treatment period (6 weeks) so that the LRAT−/− mice would be only partially vitamin A deficient during the 4-NQO treatment. The concentration of 4-NQO in the drinking water for this group of mice was 100 μg/ml and the duration of 4-NQO treatment was six weeks. Normal drinking water and the regular vitamin A sufficient diet (25 IU of retinyl palmitate/gram) were resumed after six weeks of 4-NQO treatment. After another 16 weeks the oral tumors in this group were examined as described above.
Bromodeoxyuridine (BrdU) incorporation
At 5 hr before sacrifice, five male mice (2 Wt and 3 LRAT−/−) which had been fed the vitamin A deficient diet for six weeks during 4-NQO treatment were injected intraperitoneally with BrdU (Zymed, South San Francisco, CA; Cat# 00-0103) at 1 ml per 100 g body weight. The tongues were dissected and fixed in 4% paraformaldehyde. The tissue slides were then prepared and analyzed for BrdU incorporation using an immunohistochemical detection kit (Zymed, Cat# 93-3943) as previously described [42]. The number of BrdU positive cells was determined by counting in 4-5 high power fields (200 ×) of each sample. Data from these counts were pooled and the BrdU labeling indices were calculated as the percentage of BrdU labeled cell nuclei over a total of 300 epithelial cell nuclei in each area.
Semi-quantitative and quantitative real-time RT-PCR
The tongues were dissected and temporarily stored in RNAlater (Ambion, Austin, TX). When a tumor was present, the entire tumor or a part of the tumor, along with adjacent non-tumor tongue tissue, was harvested. Total RNA was then extracted from the tissue samples using Trizol reagent (Invitrogen, Carlsbad, CA), and one microgram of each sample of total RNA was used for reverse transcription in a 20 μl reaction using SuperScript™ II Reverse Transcriptase (Invitrogen). The cDNA produced from reverse transcription (RT) was diluted 1:5, and one microliter of the diluted cDNA was used in the PCR. All PCRs were performed three times.
The gene-specific primers for semi-quantitative RT-PCR are listed as follows: for LRAT (GenBank™ accession number AF255061), the 5′-primer 5-CTGACCAATGACAAGGAACGCACTC-3 and 3′-primer 5-CTAATCCCAAGACAGCCGAAGCAAGAC-3, and 34 cycles were used, and a 370 bp product was expected; for Sfrp2 (GenBank™ accession number NM_009144), the 5′-primer 5-CAACCTGCTGGGCCACGAGACC-3 and 3′-primer 5-GCTTGCGGATGCTGCGGGAGAT-3, and 40 cycles were used, and a 695 bp product was expected; for Sfrp4 (GenBank™ accession number NM_016687), the 5′-primer 5- TCCCTCGAACACAAGTCCCTCTCA-3 and 3′-primer 5-TGCGGCTGGCTATCTGCTTCTTGT-3, and 35 cycles were used, and a 234 bp product was expected; for Frzb (GenBank™ accession number NM_011356), the 5′-primer 5- CGACTTCCAGCACGAGCCCATCA-3 and 3′-primer 5-GCTCTGACAGGCTTACATTTG-3, and 35 cycles were used, and a 261 bp product was expected. The primers were designed around introns to avoid detection of any signal from potential genomic DNA contamination. The 36B4 (5′-primer, 5- AGAACAACCCAGCTCTGGAGAAA-3; 3′-primer, 5-ACACCCTCCAGAAAGCGAGAGT-3) was used as a control for both semi-quantitative RT-PCR (28 cycles were used and a 448 bp product was expected) and quantitative real-time PCR [45]. The semi-quantitative RT-PCR was performed using the following conditions: 94 °C for 30 sec, 58 °C for 30 sec, and 72 °C for 1 min, with a final extension at 72 °C for 10 min. Taq polymerase was purchased from Invitrogen (Cat# 18038-042). The PCR products were subjected to 1.2% agarose gel electrophoresis. The gel images, stained with ethidium bromide, were recorded and quantitated with a FluorChem 8800 system (Alpha Innotech, San Leandro, CA).
For quantitative real-time RT-PCR, the reactions were performed in 20 μl and a BioRad thermal cycler (MyiQ™ Single-Color Real-Time PCR Detection System) was used. Samples were denatured initially at 95°C for 3 min, and then 46 cycles were performed using the following conditions: 94°C for 15 sec, 58°C for 30 sec, and 72°C for 45 sec. The SYBR Green fluorescence emissions were recorded at 80°C after each cycle. The PCRs were performed in triplicate. The quantitative results were calculated using MyIQ software (BioRad) and normalized to the level of 36B4 mRNA. The primers used in the PCR reactions are listed as follows: for Cox-2 (GenBank™ accession number NM_011198), the 5′-primer 5-GCCCAGCACTTCACCCATCAG-3 and 3′-primer 5- ATCATCAGACCAGGCACCAGACC-3 were used; for Cyclin D1 (GenBank™ accession number NM_007631), the 5′-primer 5-AAGTGCGTGCAGAAGGAGATTGT-3 and 3′-primer 5-GGATAGAGTTGTCAGTGTAGATGC-3 were used; for p21 (GenBank™ accession number NM_007669), the 5′-primer 5-AGGCCCAGTACTTCCTCTGC-3 and 3′-primer 5-CAATCTGCGCTTGGAGTGATA-3 were used; for Trop2 (GenBank™ accession number NM_020047), the 5′-primer 5-CCTGCGCTGCGACGAAGTGGTG-3 and 3′-primer 5-TCTGCCGAAGCTCTATCTGAATGG-3 were used; for RARβ (GenBank™ accession number NM_011243), the 5′-primer 5-GTCATCGGGCTACCACTAT-3 and 3′-primer 5-GCTCCGCTGTCATCTCAT-3 were used; these primers detect all RARβ transcripts.
Mouse embryonic stem cells and embryoid bodies
The mouse LRAT−/− embryonic stem (ES) cell line was generated from the LRAT+/− ES cells using a method reported previously [26, 46]. The LRAT+/− ES cells were produced previously in this laboratory for making the LRAT−/− mice [26]. The Wt and LRAT−/− embryonic stem (ES) cell lines were maintained as monolayer cultures in the embryonic stem cell culture medium (ESCM) (Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 2 mM glutamine, 1× penicillin/streptomycin [Invitrogen, cat# 10378-016], 1× non-essential amino acids [Invitrogen, cat# 11140-019], 1 mM sodium pyruvate [Invitrogen, cat# 11360-070], 0.1 mM β-mercaptoethanol and leukemia inhibitory factor (LIF) (103 units/ml; ESGRO, Invitrogen). When grown in suspension without LIF, the ES cells are able to form spheroid aggregates termed embryoid bodies (EBs) [47-49]. ES cells within the developing EBs are able to differentiate into various committed cell types derived from all three primitive layers, the endoderm, mesoderm and ectoderm [50, 51]. To form the EBs, the ES cells were trypsinized and seeded at 1 × 106 cells per 100 mm ultra low culture dish (Corning, NY) in the ESCM without LIF. The EBs were maintained as a suspension culture in the ESCM without LIF and the culture medium was refreshed every 48 hrs. The EBs were harvested at the designated times for gene expression analyses.
Statistical methods
A Fisher’s exact test was employed to compare the incidence of carcinogenesis and a Chi-square test was used to compare the BrdU incorporation between Wt and LRAT−/− mice. A Difference with a p value of <0.05 was considered to be statistically significant. We consulted with Dr. Kathy Zhou of the WCMC Biostatistics Core Facility for statistical analysis of our data.
RESULTS
Effects of 4-NQO on oral carcinogenesis in Wt and LRAT−/− mice fed a diet containing a standard vitamin A level (vitamin A sufficient diet)
Our studies reported here primarily focused on the effects of the lack of LRAT activity on 4-NQO induced carcinogenesis in animals that had sufficient levels of vitamin A in their diets. These LRAT−/− mice have retinol obtained from the diet in their tissues, but they are unable to store retinol as retinyl esters in tissues. The carcinogen 4-NQO induced visible tumors on the surfaces of tongues in both Wt and LRAT−/− mice. The overall incidence of oral tumors, including both male and female mice, was 73.7% in Wt mice (14/19) and 80.0% in LRAT−/− mice (16/20). No statistically significant difference was detected between the Wt mice and LRAT−/− mice (p=0.716, Fisher’s exact test). Among the male mice, tumors were found on the tongues in 70.6% (12/17) of Wt and 77.8% (14/18) of LRAT−/− mice. No significant difference was detected between the Wt and LRAT−/− male mice in terms of percentages of mice with oral tumors (p=0.711, Fisher’s Exact Test). Tongue tumors were found in two Wt female (2/2) and two LRAT−/− female mice (2/2). These results are summarized in Table 1. No tumors were found on the tongues of the control Wt or LRAT−/− mice not treated with carcinogen (0/15).
Table 1.
Oral Carcinogenesis after 4-NQO Treatment, Vitamin A Sufficient Diet
| Incidence a | Number of Tumors |
Size of Tumors (mm)b |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | <1 | ≥1 | ≥2 | ≥3 | ||
| Wt (n=19) | 73.7% | 5 | 5 | 5 | 4 | 4 | 3 | 2 | 4 |
| LRAT−/− (n=20) | 80.8% | 4 | 8 | 4 | 4 | 3 | 4 | 4 | 5 |
No statistically different incidence in oral tumors was detected between the Wt mice and LRAT−/− mice (p=0.716, Fisher’s exact test). The incidence of tumors was determined by gross examination of the tongues. The samples with visible tumors, regardless of the number and size of the tumors, were counted as one positive case.
If a sample contained more than one tumor, the size of tumor is indicated by the largest mass on the tongue
On gross examination, the tumors appeared as single or multiple masses of various sizes, ranging from tiny lesions (diameter < 0.1 mm) to large masses (> 2.0 mm). The large masses had either a narrow or a wide base. No metastases were observed in the liver, lungs, and kidneys by gross examination. However, at these time points micro-metastases, such as the migration of the tumor progenitor cells to these organs, can’t be ruled out.
All tongue samples were subjected to further histological examination. The tongues from mice that did not develop visible tumors after 4-NQO treatment appeared normal or mildly hyperplastic. Pathological diagnoses were made in seventeen mice that developed tongue abnormalities, including nine samples from Wt mice and eight samples from LRAT−/− mice. Among the nine Wt mice, hyperplasia was seen in two cases (2/9), dysplasia in two cases (2/9), SCC in situ in three cases (3/9), and invasive SCC in two cases (2/9). The LRAT−/− mice showed a similar range of pathological changes. Among eight tongue samples from LRAT−/− mice, hyperplasia was seen in two (2/8), dysplasia in three (3/8), SCC in situ in two cases (2/8), and invasive SCC in one case (1/8). More details of the gross and histological examinations are provided (Figure 1, Table 2). Thus, we conclude that in LRAT−/− mice fed a diet with adequate vitamin A levels, the inability to store retinol as retinyl esters does not lead to a greater incidence of oral tumors after 4-NQO treatment.
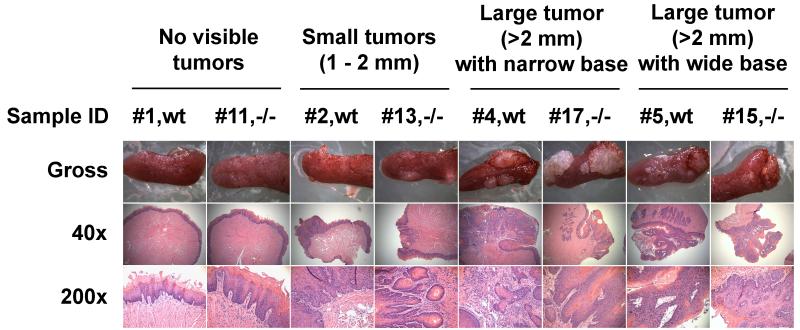
Figure 1. Gross and histological morphology of mouse tongues after 4-NQO treatment on a vitamin A sufficient diet.
Eight tongue samples from Wt and LRAT−/− male mice that were treated with 4-NQO. The genotypes and pathological diagnoses are as follows: #1, Wt, normal epithelium; #11, LRAT−/−, hyperplasia; #2, Wt, SCC in situ; #13, LRAT−/−, invasive SCC; #4, Wt, invasive SCC; #17, LRAT−/−, moderate dysplasia; #5, Wt, SCC in situ; #15, LRAT−/−, moderate dysplasia. More details are shown in Table 2. Wt, wild type; −/−, LRAT−/−.
Table 2.
Examination of the Tongues after 8 wks of 4-NQO treatment, followed by 16 wks without 4-NQOa
| Sample IDb | Gender | Genotypec | Gross Examination | Pathological Diagnosis |
|---|---|---|---|---|
| 1 | M | +/+ | No visible lesions | Normal |
| 2 | M | +/+ | 1.0×1.0 mm ×2/dorsal | In situ SCC |
| 3 | M | +/+ | 2.0×2.0 and 1.0×1.0mm/dorsal | Mild dysplasia |
| 4 | M | +/+ | 2.5×2.5 mm/dorsal; 1.0×1.0mm/ventral | Invasive SCC |
| 5 | M | +/+ | 2.5×2.5 mm and two tiny lesions/dorsal | In situ SCC |
| 6 | M | +/+ | 2.0×3.0 mm/dorsal | In situ SCC |
| 7 | M | +/+ | 2.5×3.5 and 1.0×1.0 mm/dorsal | Invasive SCC |
| 8 | M | +/+ | 3.5×3.5 mm/dorsal | Moderate dysplasia |
| 9 | F | +/+ | 1.5×1.5 mm/dorsal | Hyperplasia |
| 10 | F | +/+ | Multiple tiny lesions/dorsal & ventral | Hyperplasia |
| 11 | M | −/− | No visible lesions | Hyperplasia |
| 12 | M | −/− | 1.5×1.5 mm & multiple tiny lesions/dorsal | Mild dysplasia |
| 13 | M | −/− | 1.0×2.0 mm/lateral | Invasive SCC |
| 14 | M | −/− | 2.5×2.5 mm/dorsal | Hyperplasia |
| 15 | M | −/− | 2.5×3.5 and 1.0×1.0 mm/dorsal | Moderate dysplasia |
| 16 | M | −/− | 2.5×3.5 mm/dorsal; 1.5×1.5 mm/ventral | In situ SCC |
| 17 | M | −/− | 3.0×3.5 and 2.5×2.5 mm/dorsal | Moderate dysplasia |
| 18 | F | −/− | 1.5×1.5 mm/dorsal | Hyperplasia |
| 19 | F | −/− | 5.0×5.0 mm/dorsal | In situ SCC |
All mice were on a vitamin A sufficient diet through the experiment. The controls (Wt and LRAT−/− mice NOT treated for 8 wks with 4-NQO but on a vitamin A sufficient diet throughout the experiment) were uniformly negative for tumors. The samples without pathological diagnoses are not included in this table.
+/+ = wild type, −/− = LRAT null mice.
Expression of Cox-2, Cyclin D1, p21, RARβ and Trop2 in Tongue Tissue of 4-NQO Treated Wt and LRAT−/− Mice Fed a Vitamin A Sufficient Diet
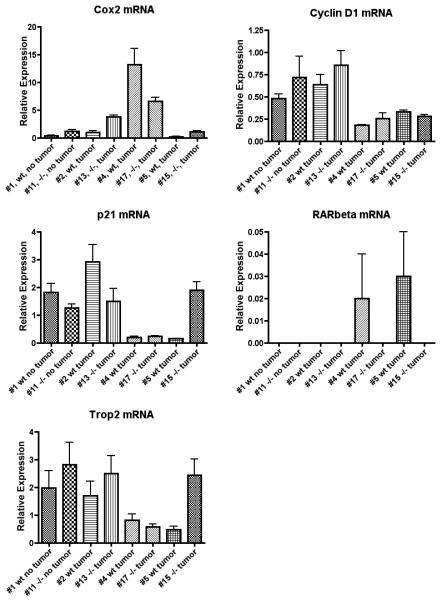
Previous reports suggested that aberrant expression of the Cox-2 [52, 53], Cyclin D1 [54-56], p21 [57], Trop2 [58] and RARβ [6, 9] genes was involved in the process of head and neck carcinogenesis. To determine the expression of these genes in the tongues of Wt and LRAT−/− mice after 4-NQO treatment on a vitamin A sufficient diet, we examined the mRNA levels of these genes in the mice that developed tumors on their tongues (seven tumor samples from 4-NQO treated Wt mice and seven tumor samples from 4-NQO treated LRAT−/− mice, all while on a vitamin A sufficient diet) using quantitative real-time RT-PCR. Transcripts of Cox-2, Cyclin D1, p21 and Trop2 were detected from all samples (7/7 in Wt and 7/7 in LRAT−/− mice). No consistently different patterns were detected between the 4-NQO treated Wt and LRAT−/− tissue samples. The level of RARβ mRNA was very low in all of the tissue samples. The fluorescent signal for RARβ mRNA passed the threshold of detection in three Wt (3/7) and three LRAT−/− (3/7) samples. The real-time RT-PCR results from eight 4-NQO treated tissue samples, including two tumor negative and six tumor positive tongue samples, are shown (Figure 2). These tongue tumor samples were selected for analysis based on their comparable gross morphology and 36B4 mRNA levels.
Figure 2. Examination of Cox-2, Cyclin D1, p21, RARβ and Trop2 mRNA levels by real-time quantitative RT-PCR.
All mice were on a vitamin A sufficient diet through the experiment. Total RNA was isolated from the samples of tongues that developed visible tumors (Wt: #2, #4 and #5; LRAT−/−: #13, #17 and #15) vs. those without visible tumors (Wt: #1; LRAT−/−: #11) after 4-NQO treatment. Each real-time PCR were performed in triplicate. The means and standard deviations were calculated and normalized to the level of 36B4 mRNA, and the graphs were plotted. The gross morphology of each sample can be found in Table 2. Wt, wild type; −/−, LRAT−/−.
Expression of Transcripts of the Sfrp family in Tongue Tumors from 4-NQO treated Wt and LRAT−/− Mice Fed a Vitamin A Sufficient Diet
Additional genes, including LRAT and members of secreted frizzled-related protein (Sfrp) gene family, were examined by semi-quantitative RT-PCR. Using the PCR conditions indicated in the Materials and Methods, LRAT mRNA was detected in all seven tongue samples of the Wt mice that developed tongue tumors (7/7). No LRAT mRNA was detected from LRAT−/− samples, as expected (Fig. 3A).
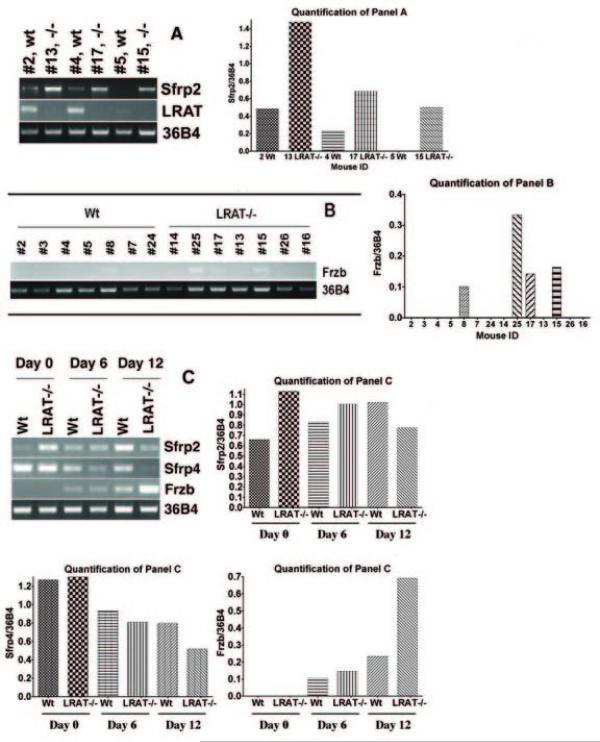
Figure 3. Examination of gene expression in the tongue tumors and embryoid bodies by semi-quantitative RT-PCR.
All mice were on a vitamin A sufficient diet through the experiment. A. The level of LRAT and Sfrp2 mRNA in six representative tongue tumor samples (Wt: #2, #4 and #5; LRAT−/−: #13, #17 and #15). Higher levels of Sfrp2 mRNA were detected in the tongue tumors of LRAT−/− mice (#13, #17 and #15) as compared to the tongue tumors of Wt mice (#2 and #4). No Sfrp2 mRNA was detected from the sample #5 (Wt). LRAT mRNA was detected in all samples of Wt mice (#2, #4 and #5) but not in LRAT−/− mice. Sample #5 showed an LRAT band with a greater number of RT-PCR cycles; a faint band can be seen in Fig. 3A. Wt, wild type; −/−, LRAT−/−. B. The level of Frzb mRNA in tongue samples. Frzb mRNA was detected in one tongue sample (#8) from Wt mice and three tongue samples (#15, #17 and #25) from LRAT−/− mice. The pathological changes of these tongue samples are as follows: #8, moderate dysplasia; #15, moderate dysplasia; #17, moderate dysplasia; #25, inflammation. C. Measurement of Sfrp2, Sfrp4 and Frzb mRNA levels in cultured, undifferentiated murine Wt and LRAT null ES cells and embryoid bodies. Wt and LRAT−/− ES cells (Day 0) and EBs (Day 6 and Day 12) were harvested. Total RNA was extracted and the mRNA levels of Sfrp2, Sfrp4 and Frzb were examined by RT-PCR. The quantitation of the RT-PCR blots in panels A, B, and C is shown to the right of each panel; the quantitation was performed using Alpha Innotech FluorChem software and data are normalized to 36B4 mRNA levels.
The Sfrp gene family encodes antagonists of the WNT pathway [59, 60]. Some members of the Sfrp gene family are silenced in human SCCHN [61, 62]. Sfrp2 mRNA was detected in three tongue tumor samples from Wt mice (3/7) and in four tongue tumor samples from LRAT−/− mice (4/7). The levels of Sfrp2 mRNA detected in the tumor samples from LRAT−/− mice were consistently higher than the levels in the tumor samples from Wt mice. The semi-quantitative RT-PCR results from representative samples are shown (Figure 3A).
We also assayed mRNA levels of Sfrp1, Sfrp4 and Frzb, which are other members of the Sfrp gene family. Frzb mRNA was detected in one tongue sample (moderate dysplasia) from 4-NQO treated Wt mice (1/7). In the 4-NQO treated LRAT−/− mice, Frzb transcripts were detected in three tongue samples (3/7) which showed lesions with inflammation (#25), or moderate dysplasia (#15 and #17) (Figure 3B). Sfrp1 and Sfrp4 transcripts were detected in tongue tumor samples from both Wt and LRAT−/− mice (3/3 in Wt and 3/3 in LRAT−/− mice) but no differences in Sfrp1 or Sfrp4 expression were detected between the Wt and LRAT−/− tissue samples (data not shown).
In order to determine how the expression of these Sfrp family members correlates with the function of the WNT pathway during oral carcinogenesis, we examined the mRNA levels of all nineteen murine WNT members in the tongue samples from Wt and LRAT−/− mice. The following WNT members were also examined by semi-quantitative PCR: WNT1, WNT2, WNT2b, WNT3, WNT3a, WNT4, WNT5a, WNT5b, WNT6, WNT7a, WNT7b, WNT8a, WNT8b, WNT9a, WNT9b, WNT10a, WNT10b, WNT11, WNT16. We did not find any differences in the mRNA levels of these Wnt genes between Wt and LRAT gene knockout mice (data not shown).
Expression of Sfrp2, Sfrp4 and Frzb Transcripts in Mouse Embryonic Stem Cells and Embryoid Bodies
To assess the physiological significance of the changes in gene expression, we assessed the levels of the Sfrp2, Sfrp4, and Frzb gene transcripts in undifferentiated mouse Wt and LRAT−/− embryonic stem (ES) cells and in the differentiated cells within the embryoid bodies (EBs) (day 6 and day 12). In undifferentiated ES cells (day 0), the level of Sfrp2 mRNA in Wt ES cells was lower than in LRAT−/− ES cells. However, upon EB differentiation (day 12) the Sfrp2 mRNA level in Wt EBs was higher than that in LRAT−/− EBs (Figure 3C). The highest level of Sfrp4 mRNA was detected in the undifferentiated ES cells (day 0). Sfrp4 mRNA levels decreased in both Wt and LRAT−/− EBs, but a greater decrease was observed in LRAT−/− as compared to Wt EBs (day 12) (Figure 3C). No Frzb mRNA was detected in undifferentiated Wt or LRAT−/− ES cells (day 0). The Frzb mRNA level increased in both Wt and LRAT−/− EBs, but a more marked increase was detected in LRAT−/− EBs (day 12) (Figure 3C). These results suggest that changes in the levels of expression of the Sfrp gene family members occur during the differentiation of both Wt and LRAT−/− ES cells, and that the patterns of these changes are different between the Wt and LRAT−/− cells.
Effects of a vitamin A deficient diet on 4-NQO induced carcinogenesis and cell proliferation
Five Wt mice (2 males and 3 females) and five LRAT−/− mice (3 males and 2 females) were fed a vitamin A deficient diet during the entire six week carcinogen 4-NQO treatment. On this diet only LRAT−/− mice, and not the Wt mice, show a large (> 90%) decrease in serum vitamin A levels [26]. Tumors were found on the tongues of three Wt mice (60%) (2/2 of the males; 1/3 of the females) and on the tongues of all five LRAT−/− mice (100%) (3/3 of the males; 2/2 of the females) (p=0.22, Fisher’s Exact Test). Although the difference between the Wt mice and LRAT−/− mice is not statistically significant because of small sample groups, there is a trend showing that partial retinol deficiency makes mice more sensitive to carcinogen induced tumorigenesis.
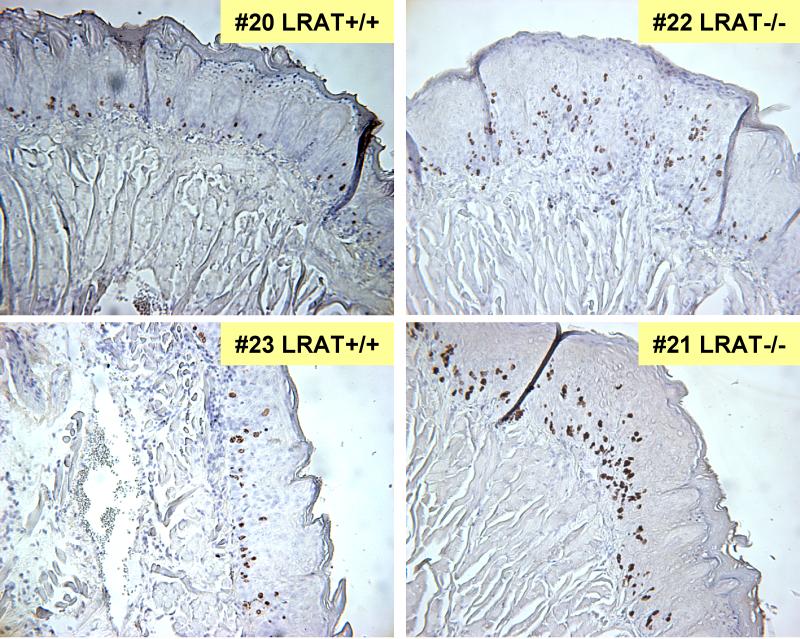
BrdU immunostaining was performed on the tongue tissue sections of Wt and LRAT−/− mice that were fed the vitamin A deficient diet during the six weeks of 4-NQO treatment and subsequently developed tumors. The regions of the tongues that appeared grossly normal were subjected to the BrdU staining. Three tongue tissue samples from control Wt mice that were fed the regular vitamin A sufficient diet and not treated with 4-NQO were stained as controls. After BrdU injection, cells are expected to incorporate BrdU in S-phase, so this assay is a measure of cell proliferation [63]. The BrdU labeling index was calculated and expressed as the percentage of BrdU-positive nuclei divided by the total nuclei counted under a microscope [42]. As compared to the BrdU labeling index (7.1% ± 0.9%, mean ± SEM) calculated from the control Wt mice (not treated with 4-NQO) on a vitamin A sufficient diet, treatment with 4-NQO on a vitamin A deficient diet resulted in a statistically significant increase in the BrdU labeling index in the tongue tissue sections of only the LRAT−/− mice. The BrdU labeling index of tongue tissue from LRAT−/− mice (21.0% ± 2.4%) was significantly higher than the BrdU index of Wt mice (9.9% ± 0.8%) after 4-NQO treatment on a vitamin A deficient diet (p<0.001, chi-square test) (Fig. 4). This result indicates that a greater proportion of proliferating cells was detected in the tongue epithelial tissues from the LRAT−/− mice than from Wt mice. As discussed above, the serum retinol in Wt mice doesn’t decrease over the six weeks on the vitamin A deficient diet, so the fact that the BrdU labeling index in these tissues is not different from the BrdU labeling index in the negative control Wt mice (7.1% ± 0.9%) is not surprising. The representative fields of BrdU immunostained tissues which correspond to the grossly normal tongue tissues adjacent to the tumors are shown (Figure 4).
Figure 4. Bromodeoxyuridine (BrdU) staining in Wild-type and LRAT−/− mouse tongue tissue sections.
The levels of BrdU in tongue epithelia were analyzed by immunohistochemical staining. Two samples of Wt mice (#20 and #23) and two samples of LRAT−/− mice (#21 and #22) are shown. The Wt and LRAT−/− mice were fed the vitamin A deficient diet during 4-NQO treatment for six weeks and then maintained on a vitamin A sufficient diet and regular drinking water for another 16 weeks. BrdU was injected intraperitoneally at 5 hr before sacrifice. The images represent the tongue tissues near the tumor. The number of BrdU positive cells was determined by counting in 4-5 high power fields (200x) of each sample. Data from these counts were pooled and the BrdU labeling indices were calculated as the percentage of BrdU labeled cell nuclei over a total of 300 epithelial cell nuclei in each area. A Chi-square test was used to compare the BrdU incorporation between Wt and LRAT−/− mice.
DISCUSSION
The process of malignant transformation of the human oral epithelium is frequently accompanied by the reduced expression of lecithin:retinol acyltransferase (LRAT) [34]. Although most retinol is converted into retinyl esters in the liver and lung, LRAT functions in many human epithelial tissues, including the oral epithelium [34]. We also showed that LRAT plays a role in retinol uptake by epithelial cells [34]. Therefore, it is important to determine whether the loss of LRAT expression, via knockout of the LRAT gene in all tissues of the mice by homologous recombination [26], contributes directly to the process of malignant transformation.
Reduced retinoid signaling has been implicated in the malignant transformation of human oral epithelia [6-9], and cancer chemopreventive effects of RA have also been observed in animal experiments and in humans [15, 64, 65]. Our results show that loss of LRAT expression does not directly affect the process of carcinogenesis when mice are given adequate retinol in their diet. However, the absence of the LRAT gene causes mice to be much more susceptible to vitamin A (retinol) deficiency [26, 38, 39]. Although inactivation of the LRAT gene itself did not result in a statistically significant increase in oral cancer incidence in LRAT−/− mice fed a vitamin A sufficient diet as compared to Wt mice fed a vitamin A sufficient diet (Tables 1 and 2), our results show a statistically significant increase in the number of proliferating tongue epithelial cells in LRAT−/− mice that were on a vitamin A deficient diet during the 4-NQO treatment as compared to Wt mice on the same diet during 4-NQO treatment. This is striking because this BrdU measurement was performed 16 weeks after the diet was changed back to a sufficient vitamin A diet (Figure 4). Thus, a secondary vitamin A deficiency resulting from the lack of the LRAT gene in the LRAT gene knockout mice may lead to reprogramming of basal epithelial cells that lasts for many weeks and results in increased proliferation and hyperplasia. This hypothesis is also supported by our data showing a trend toward a higher oral cancer incidence in LRAT−/− mice (5/5) as compared to Wt mice (3/5) fed a vitamin A deficient diet during the 4-NQO treatment.
To identify the molecular regulatory mechanisms underlying the carcinogenesis process in Wt and LRAT−/− mice, we examined the mRNA levels of many genes that have been reported to play a role in the process of head and neck carcinogenesis. The patterns of Cox-2, Cyclin D1, p21, Trop2, and RARβ2 gene expression indicate that these genes are not expressed differently in carcinogen treated Wt vs. LRAT−/− mice, consistent with the lack of any difference in tumor incidence between Wt and LRAT−/− mice when both are on a retinol sufficient diet.
In contrast, the levels of Sfrp2 mRNA were higher in LRAT−/− as compared to Wt tumor tissues from mice on the normal, vitamin A sufficient diet (Fig. 3A). Increased WNT signaling that activates oncogene pathways through β-catenin is seen in many cancer tissues, including cancer of the oral cavity [66, 67]. Silencing of the Sfrp2 gene via epigenetic inactivation by promoter hypermethylation is frequently observed in human oral squamous cell carcinomas [61, 62]. We found that Sfrp2 transcripts are higher in tongue tumors from LRAT−/− mice as compared to tongue tumors of Wt mice (Figure 3A), suggesting that expression of genes that encode some of the antagonists of the Wnt pathway is inversely correlated with LRAT gene expression. The pattern of Sfrp2 mRNA expression in tongue tumors from Wt vs. LRAT−/− mice resembles the pattern of Sfrp2 mRNA expression in cultured Wt vs. LRAT−/− ES mouse cells (Figure 3A and 3C). The Sfrp2 gene is transcriptionally activated by RA via RARγ in cultured, F9 teratocarcinoma cells; since Sfrp2 mRNA increases in response to RA in F9 Wt cells even in the presence of the protein synthesis inhibitor cycloheximide, Sfrp2 is a direct target of RA [68, 69]. These data from our laboratory provide another link between Sfrp2, Wnt signaling, and retinoid signaling.
The pattern of Frzb transcript expression in tongue samples also suggests that the WNT signaling is differentially regulated in Wt and LRAT−/− mice. Our results suggest cross-talk between vitamin A signaling, via the level of LRAT, and the WNT signaling pathway during the process of malignant transformation of the oral cavity.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Kathy Zhou for assistance in the statistical analyses of the data, Christopher Kelly for editorial assistance, and the members of the Gudas laboratory for insightful scientific input.
Funding: Agency: NIH; Grant number: RO1 DE10389 to Dr. Gudas.
Abbreviations
- BrdU
Bromodeoxyuridine
- EB
embryoid body
- ES
embryonic stem
- LRAT
lecithin:retinol acyltransferase
- 4-NQO
4-nitroquinoline 1-oxide
- RA
all-trans retinoic acid
- RARs
retinoic acid receptors
- Wt
wild type
- SCCHN
squamous cell carcinoma of the head and neck
- Sfrp2
secreted frizzled-related protein 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- [3].Gudas LJ, Sporn MB, Roberts A. Cellular biology and biochemistry of retinoids. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. Raven Press; New York: 1994. pp. 443–520. [Google Scholar]
- [4].Chambon P. A decade of molecular biology of retinoic acid receptors. Faseb J. 1996;10:940–954. [PubMed] [Google Scholar]
- [5].Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hu L, Crowe DL, Rheinwald JG, Chambon P, Gudas LJ. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991;51:3972–3981. [PubMed] [Google Scholar]
- [7].Lotan R. Retinoids and their receptors in modulation of differentiation, development, and prevention of head and neck cancers. Anticancer Res. 1996;16:2415–2419. [PubMed] [Google Scholar]
- [8].Love JM, Gudas LJ. Vitamin A, differentiation and cancer. Curr Opin Cell Biol. 1994;6:825–831. doi: 10.1016/0955-0674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- [9].Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res. 1994;54:3580–3587. [PubMed] [Google Scholar]
- [10].Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- [11].Sun SY, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41:41–55. doi: 10.1016/s1040-8428(01)00144-5. [DOI] [PubMed] [Google Scholar]
- [12].Youssef EM, Lotan D, Issa JP, Wakasa K, Fan YH, Mao L, Hassan K, Feng L, Lee JJ, Lippman SM, Hong WK, Lotan R. Hypermethylation of the retinoic acid receptor-beta(2) gene in head and neck carcinogenesis. Clin Cancer Res. 2004;10:1733–1742. doi: 10.1158/1078-0432.ccr-0989-3. [DOI] [PubMed] [Google Scholar]
- [13].Ha PK, Califano JA. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. Lancet Oncol. 2006;7:77–82. doi: 10.1016/S1470-2045(05)70540-4. [DOI] [PubMed] [Google Scholar]
- [14].Giannini F, Maestro R, Vukosavljevic T, Pomponi F, Boiocchi M. All-trans, 13-cis and 9-cis retinoic acids induce a fully reversible growth inhibition in HNSCC cell lines: implications for in vivo retinoic acid use. Int J Cancer. 1997;70:194–200. doi: 10.1002/(sici)1097-0215(19970117)70:2<194::aid-ijc10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- [15].Lotan R, Xu XC, Lippman SM, Ro JY, Lee JS, Lee JJ, Hong WK. Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- [16].Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- [17].Clarke N, Germain P, Altucci L, Gronemeyer H. Retinoids: potential in cancer prevention and therapy. Expert Rev Mol Med. 2004;6:1–23. doi: 10.1017/S1462399404008488. [DOI] [PubMed] [Google Scholar]
- [18].Lippman SM, Sudbo J, Hong WK. Oral cancer prevention and the evolution of molecular-targeted drug development. J Clin Oncol. 2005;23:346–356. doi: 10.1200/JCO.2005.09.128. [DOI] [PubMed] [Google Scholar]
- [19].Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- [20].Harrison EH. Lipases and carboxylesterases: possible roles in the hepatic utilization of vitamin A. J Nutr. 2000;130:340S–344S. doi: 10.1093/jn/130.2.340S. [DOI] [PubMed] [Google Scholar]
- [21].Harrison EH. Mechanisms of digestion and absorption of dietary vitamin A. Annu Rev Nutr. 2005;25:87–103. doi: 10.1146/annurev.nutr.25.050304.092614. [DOI] [PubMed] [Google Scholar]
- [22].Blomhoff R, Rasmussen M, Nilsson A, Norum KR, Berg T, Blaner WS, Kato M, Mertz JR, Goodman DS, Eriksson U, et al. Hepatic retinol metabolism. Distribution of retinoids, enzymes, and binding proteins in isolated rat liver cells. J Biol Chem. 1985;260:13560–13565. [PubMed] [Google Scholar]
- [23].Matsuura T, Gad MZ, Harrison EH, Ross AC. Lecithin:retinol acyltransferase and retinyl ester hydrolase activities are differentially regulated by retinoids and have distinct distributions between hepatocyte and nonparenchymal cell fractions of rat liver. J Nutr. 1997;127:218–224. doi: 10.1093/jn/127.2.218. [DOI] [PubMed] [Google Scholar]
- [24].Ong DE, MacDonald PN, Gubitosi AM. Esterification of retinol in rat liver. Possible participation by cellular retinol-binding protein and cellular retinol-binding protein II. J Biol Chem. 1988;263:5789–5796. [PubMed] [Google Scholar]
- [25].Ross AC, Zolfaghari R. Regulation of hepatic retinol metabolism: perspectives from studies on vitamin A status. J Nutr. 2004;134:269S–275S. doi: 10.1093/jn/134.1.269S. [DOI] [PubMed] [Google Scholar]
- [26].Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- [27].Zolfaghari R, Ross AC. Lecithin:retinol acyltransferase expression is regulated by dietary vitamin A and exogenous retinoic acid in the lung of adult rats. J Nutr. 2002;132:1160–1164. doi: 10.1093/jn/132.6.1160. [DOI] [PubMed] [Google Scholar]
- [28].Herr FM, Wardlaw SA, Kakkad B, Albrecht A, Quick TC, Ong DE. Intestinal vitamin A metabolism: coordinate distribution of enzymes and CRBP(II) J Lipid Res. 1993;34:1545–1554. [PubMed] [Google Scholar]
- [29].MacDonald PN, Ong DE. Evidence for a lecithin-retinol acyltransferase activity in the rat small intestine. J Biol Chem. 1988;263:12478–12482. [PubMed] [Google Scholar]
- [30].Gollapalli DR, Rando RR. All-trans-retinyl esters are the substrates for isomerization in the vertebrate visual cycle. Biochemistry. 2003;42:5809–5818. doi: 10.1021/bi0341004. [DOI] [PubMed] [Google Scholar]
- [31].Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264:8636–8640. [PubMed] [Google Scholar]
- [32].Saari JC, Bredberg DL, Farrell DF. Retinol esterification in bovine retinal pigment epithelium: reversibility of lecithin:retinol acyltransferase. Biochem J. 1993;291(Pt 3):697–700. doi: 10.1042/bj2910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shingleton JL, Skinner MK, Ong DE. Retinol esterification in Sertoli cells by lecithin-retinol acyltransferase. Biochemistry. 1989;28:9647–9653. doi: 10.1021/bi00451a016. [DOI] [PubMed] [Google Scholar]
- [34].Guo X, Ruiz A, Rando RR, Bok D, Gudas LJ. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: reduced expression of lecithin:retinol acyltransferase in carcinoma lines. Carcinogenesis. 2000;21:1925–1933. doi: 10.1093/carcin/21.11.1925. [DOI] [PubMed] [Google Scholar]
- [35].Kurlandsky SB, Duell EA, Kang S, Voorhees JJ, Fisher GJ. Auto-regulation of retinoic acid biosynthesis through regulation of retinol esterification in human keratinocytes. J Biol Chem. 1996;271:15346–15352. doi: 10.1074/jbc.271.26.15346. [DOI] [PubMed] [Google Scholar]
- [36].Randolph RK, Winkler KE, Ross AC. Fatty acyl CoA-dependent and -independent retinol esterification by rat liver and lactating mammary gland microsomes. Arch Biochem Biophys. 1991;288:500–508. doi: 10.1016/0003-9861(91)90227-a. [DOI] [PubMed] [Google Scholar]
- [37].Guo X, Knudsen BS, Peehl DM, Ruiz A, Bok D, Rando RR, Rhim JS, Nanus DM, Gudas LJ. Retinol metabolism and lecithin:retinol acyltransferase levels are reduced in cultured human prostate cancer cells and tissue specimens. Cancer Res. 2002;62:1654–1661. [PubMed] [Google Scholar]
- [38].Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kim YK, Wassef L, Hamberger L, Piantedosi R, Palczewski K, Blaner WS, Quadro L. Retinyl ester formation by lecithin:retinol acyltransferase is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem. 2008;283:5611–5621. doi: 10.1074/jbc.M708885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu L, Tang XH, Gudas LJ. Homeostasis of retinol in lecithin: retinol acyltransferase gene knockout mice fed a high retinol diet. Biochem Pharmacol. 2008;75:2316–2324. doi: 10.1016/j.bcp.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–313. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- [43].Makita H, Tanaka T, Fujitsuka H, Tatematsu N, Satoh K, Hara A, Mori H. Chemoprevention of 4-nitroquinoline 1-oxide-induced rat oral carcinogenesis by the dietary flavonoids chalcone, 2-hydroxychalcone, and quercetin. Cancer Res. 1996;56:4904–4909. [PubMed] [Google Scholar]
- [44].Tanaka T, Makita H, Ohnishi M, Mori H, Satoh K, Hara A, Sumida T, Fukutani K, Tanaka T, Ogawa H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combination. Cancer Res. 1997;57:246–252. [PubMed] [Google Scholar]
- [45].Gillespie RF, Gudas LJ. Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARbeta (2), and Cyp26A1 in F9 embryonal carcinoma cells. J Mol Biol. 2007;372:298–316. doi: 10.1016/j.jmb.2007.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mortensen RM, Conner DA, Chao S, Geisterfer-Lowrance AA, Seidman JG. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992;12:2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- [48].Kurosawa H. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–398. doi: 10.1263/jbb.103.389. [DOI] [PubMed] [Google Scholar]
- [49].Mohn D, Chen SW, Dias DC, Weinstein DC, Dyer MA, Sahr K, Ducker CE, Zahradka E, Keller G, Zaret KS, Gudas LJ, Baron MH. Mouse Mix gene is activated early during differentiation of ES and F9 stem cells and induces endoderm in frog embryos. Dev Dyn. 2003;226:446–459. doi: 10.1002/dvdy.10263. [DOI] [PubMed] [Google Scholar]
- [50].Desbaillets I, Ziegler U, Groscurth P, Gassmann M. Embryoid bodies: an in vitro model of mouse embryogenesis. Exp Physiol. 2000;85:645–651. [PubMed] [Google Scholar]
- [51].Rathjen J, Rathjen PD. Mouse ES cells: experimental exploitation of pluripotent differentiation potential. Curr Opin Genet Dev. 2001;11:587–594. doi: 10.1016/s0959-437x(00)00237-9. [DOI] [PubMed] [Google Scholar]
- [52].Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- [53].Shiotani H, Denda A, Yamamoto K, Kitayama W, Endoh T, Sasaki Y, Tsutsumi N, Sugimura M, Konishi Y. Increased expression of cyclooxygenase-2 protein in 4-nitroquinoline-1-oxide-induced rat tongue carcinomas and chemopreventive efficacy of a specific inhibitor, nimesulide. Cancer Res. 2001;61:1451–1456. [PubMed] [Google Scholar]
- [54].Gimenez-Conti IB, Collet AM, Lanfranchi H, Itoiz ME, Luna M, Xu HJ, Hu SX, Benedict WF, Conti CJ. p53, Rb, and cyclin D1 expression in human oral verrucous carcinomas. Cancer. 1996;78:17–23. doi: 10.1002/(SICI)1097-0142(19960701)78:1<17::AID-CNCR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- [55].Kotelnikov VM, Coon J.S.t., Mundle S, Kelanic S, LaFollette S, Taylor SI, Hutchinson J, Panje W, Caldarelli DD, Preisler HD. Cyclin D1 expression in squamous cell carcinomas of the head and neck and in oral mucosa in relation to proliferation and apoptosis. Clin Cancer Res. 1997;3:95–101. [PubMed] [Google Scholar]
- [56].Schoelch ML, Regezi JA, Dekker NP, Ng IO, McMillan A, Ziober BL, Le QT, Silverman S, Fu KK. Cell cycle proteins and the development of oral squamous cell carcinoma. Oral Oncol. 1999;35:333–342. doi: 10.1016/s1368-8375(98)00098-0. [DOI] [PubMed] [Google Scholar]
- [57].Todd R, Hinds PW, Munger K, Rustgi AK, Opitz OG, Suliman Y, Wong DT. Cell cycle dysregulation in oral cancer. Crit Rev Oral Biol Med. 2002;13:51–61. doi: 10.1177/154411130201300106. [DOI] [PubMed] [Google Scholar]
- [58].Fong D, Spizzo G, Gostner JM, Gastl G, Moser P, Krammel C, Gerhard S, Rasse M, Laimer K. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–191. doi: 10.1038/modpathol.3801001. [DOI] [PubMed] [Google Scholar]
- [59].Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24:811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- [60].Rattner A, Hsieh JC, Smallwood PM, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci U S A. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sogabe Y, Suzuki H, Toyota M, Ogi K, Imai T, Nojima M, Sasaki Y, Hiratsuka H, Tokino T. Epigenetic inactivation of SFRP genes in oral squamous cell carcinoma. Int J Oncol. 2008;32:1253–1261. doi: 10.3892/ijo_32_6_1253. [DOI] [PubMed] [Google Scholar]
- [62].Marsit CJ, McClean MD, Furniss CS, Kelsey KT. Epigenetic inactivation of the SFRP genes is associated with drinking, smoking and HPV in head and neck squamous cell carcinoma. Int J Cancer. 2006;119:1761–1766. doi: 10.1002/ijc.22051. [DOI] [PubMed] [Google Scholar]
- [63].Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- [64].Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, Hays GL, Goepfert H, Hong WK. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328:15–20. doi: 10.1056/NEJM199301073280103. [DOI] [PubMed] [Google Scholar]
- [65].Lotan R. Retinoids and chemoprevention of aerodigestive tract cancers. Cancer Metastasis Rev. 1997;16:349–356. doi: 10.1023/a:1005808429176. [DOI] [PubMed] [Google Scholar]
- [66].Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–6605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- [67].Uraguchi M, Morikawa M, Shirakawa M, Sanada K, Imai K. Activation of WNT family expression and signaling in squamous cell carcinomas of the oral cavity. J Dent Res. 2004;83:327–332. doi: 10.1177/154405910408300411. [DOI] [PubMed] [Google Scholar]
- [68].Su D, Gudas LJ. Retinoic acid receptor gamma activates receptor tyrosine kinase Tie1 gene transcription through transcription factor GATA4 in F9 stem cells. Exp Hematol. 2008;36:624–641. doi: 10.1016/j.exphem.2007.12.016. [DOI] [PubMed] [Google Scholar]
- [69].Su D, Gudas LJ. Gene expression profiling elucidates a specific role for RARgamma in the retinoic acid-induced differentiation of F9 teratocarcinoma stem cells. Biochem Pharmacol. 2008;75:1129–1160. doi: 10.1016/j.bcp.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]