Abstract
Background
Negative margins in breast conservation therapy (BCT) decrease local recurrence risk. Excision may be performed via two techniques: either as a single lumpectomy specimen or as a central segment with simultaneously resected peripheral segments (PSs). There is little data directly comparing these methods for their effect on margin status.
Methods
A retrospective review of all patients undergoing BCT for invasive breast cancer was conducted to evaluate and compare the two techniques. Presentation, pathologic characteristics, surgical technique, specimen volume, and final margin status were recorded.
Results
Among 259 cancers in 257 women, 33 had positive margins. A single segment was removed in 69 patients, while 190 patients had 1-6 PSs simultaneously removed. By univariate analysis, smaller tumor size (p=0.017) and greater numbers of segments removed (p=0.01) lowered the risk of positive margins. In a multivariate model, smaller tumor size (p=0.0024), lack of EIC (p=0.049), and greater numbers of segments removed (p=0.0061) lowered the risk of margin positivity. Despite this last predictor, the total resected specimen volume did not increase with the number of PSs removed (p=0.4). There was no residual tumor in 49.2% of PSs despite a compromised primary segment margin.
Conclusions
Smaller tumor size, lack of EIC and greater numbers of simultaneous PSs excised decrease the likelihood of positive margins, despite a lack of correlation between segment numbers and excised volume. These findings suggest that excision of simultaneous PSs may assist in achieving negative margins, in part, due to avoidance of pathologic artifact.
INTRODUCTION
Successful breast conservation surgery (BCS) maximizes local control when obtaining negative margins prior to irradiation. Although it is well established that compromised margins increase the risk of local recurrence,1-4 no universally accepted definition of a close or negative margin exists5 because trials have varied widely in their margin definitions, inclusion criteria, and local recurrence rates.6, 7
Irrespective of the definition used, negative margins remain the standard of care for BCS, and although the number of re-excisions doesn’t affect local recurrence8 initial efforts to achieve margin negativity may help prevent reoperation. Repeated excisions not only add time, expense, and potential morbidity to treatment, but may result in poorer cosmesis9 and delay adjuvant therapy.6 Wider resection at the first therapeutic excision may increase the likelihood of negative margins, but may also worsen the cosmetic outcome,6, 9, 10 demonstrating the importance of balance between the two goals.
Factors thought to contribute to compromised margins include multifocality,11-13 tumor size,11, 13-16 extensive intraductal component (EIC),7, 8, 17, 18 lobular histology,8, 16, 19 younger age,6, 13, 19 mammographically dense breasts,12 and palpability. The method of surgical resection of simultaneous peripheral segments (PSs) at the edges of the primary segment resected, however, has received little attention for its contribution to obtaining the desired margin status in BCS.15, 20, 21 The primary goal of this study was to determine whether the resection of a primary segment with PSs reduces the incidence of compromised margins when compared with resection of a primary segment alone. The secondary aim was to determine whether a benefit of removing additional segments was derived from an increase in tissue volume excised or other factors.
METHODS
After institutional review board approval, 257 patients undergoing treatment between January 2006 and August 2008 were retrospectively reviewed. Patients were included if they had a diagnosis of invasive breast cancer. Patients having a total mastectomy, neoadjuvant chemotherapy, or solely ductal carcinoma in situ (DCIS) were excluded. Because excisional biopsy has been suggested to increase the likelihood of positive margins at first therapeutic excision,19, 22 patients having an excisional biopsy prior to therapeutic excision were excluded. Charts were reviewed for palpability of the primary tumor, mammographic appearance, final pathologic tumor size and grade, tumor histology, EIC, primary segment and PS volume, number, and margin status, and the presence of tumor in those additional segments.
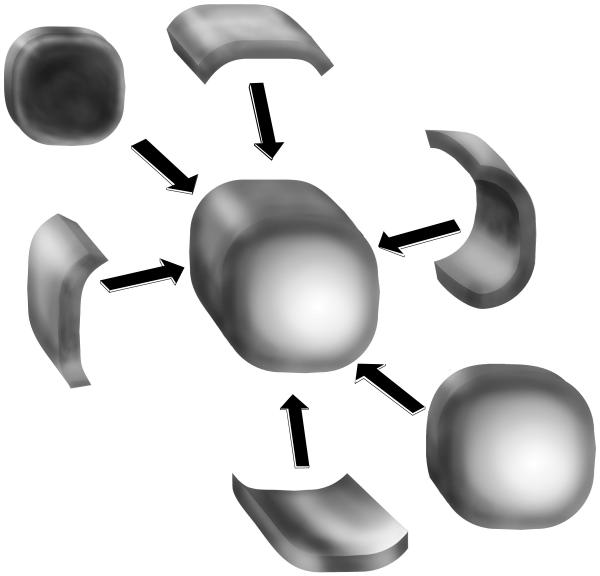
A tumor was considered palpable when a thickening or mass was documented in the patient’s physical examination. For this study, a segment refers to a volume of tissue resected while a margin refers to the distance from tumor to the inked edge of that tissue. A primary or central segment was defined as the central or main tumor resection specimen, while PSs were defined as those tissue volumes removed that surrounded a primary segment. PSs were universally oriented as to which margin they abutted off the primary segment (Figure 1). Margins were defined as positive when tumor was present at the inked surface on microscopic evaluation; close when the tumor-free distance to ink was < 2 mm; and negative when the margin was ≥ 2 mm. The margin status for a procedure was defined by the least favorable margin for the entire procedure (e.g. a procedure having two close margins and one positive margin was defined as positive). For analysis, close and negative margins were grouped together as there is no consensus as to what constitutes a close margin.5 EIC was defined as DCIS spanning ≥25% of the overall volume.
Figure 1.
Method of excision. One or more PSs surrounding the primary (central) segment are removed at the discretion of the surgeon, after assessment either by palpation or after intraoperative specimen imaging.
Use of needle localization was based on the judgement of the surgeon as to whether the tumor was sufficiently discrete on examination to use palpation guidance alone at operation. Specimens undergoing needle localization were radiographically imaged intraoperatively to confirm removal of the core biopsy marker, the entire needle localization wire, calcifications, and/or mass. Proximity of the tumor to the primary segment edge was assessed intraoperatively by palpation, intraoperative review of the specimen radiographs, or both. The decision to resect 1 or more PSs (Figure 1) was made at the discretion of the surgeon. All specimens were oriented for pathology and sent for permanent section analysis. Intraoperative pathologic margin assessment was not performed as per our institutional standard procedure. Compromised margins on skin or chest wall did not undergo re-resection of skin or muscle. Primary and peripheral segment volumes were calculated by multiplying specimen length × width × height (cm3),17 which are uniformly reported in our pathology reports, understanding that this is a best estimation and that each specimen is not cubical.
The means procedure was used to evaluate whether volume increased with the number of segments removed. The Wilcoxon test was used to correlate tumor size to the number of margins excised, and the number of margins excised to volume. The Pearson correlation test was used to determine whether there was a linear relationship between tumor size and volume of tissue excised. To evaluate margin positivity as the number of segments excised increased, the Junckheere-Terpstra test was utilized. Logistic regression was used to evaluate the risk of positive margins relative to tumor size, tumor grade, age, EIC, volume of the primary segment, total volume, removal of PSs, palpability, and the use of needle localization. A multiple logistic model was used to further evaluate margin status, removal of PSs, tumor size and grade, the use of needle-localization, EIC, volume resected, and palpability with respect to margin status. A value of ≤0.05 was designated as significant.
RESULTS
There were 259 cancers diagnosed in 257 women, whose average and median patient ages were 60 and 61 years, respectively (range 30 to 92). Tumors were not palpable in 144 cases (56%, Table 1) and were palpable in 115(44%) of cases. Radiographic characteristics are summarized in Table 1. Palpation guidance was used to resect 106 of 115 palpable lesions (41% of the total cases) while needle localization was used in the remaining 153 (59%) cases. For 69 patients (27%), a single primary segment was removed. In 190 (73%) patients ≥1 PS was removed (range 1-6). The mean and median total specimen volumes were 159.6 and 127.9 cm3, respectively (range = 16.5 to 1102 cm3). Two specimens were greater than 1000 cm3 with primary segments measuring 9 × 8 × 4.5 cm (volume of 6 additional segments=746.1 cm3) and 14 × 14 × 5.5 cm (volume of 1 additional segment = 25.0 cm3) each. The average total specimen volumes were smallest when no PSs were excised and largest with the removal of 4 PSs. Distribution of volumes resected by number of segments is demonstrated in Figure 2 and Table 2.
Table 1. Cohort characteristics.
Numbers in parentheses are percentages, unless otherwise indicated
| n Peripheral Segments: | 0 (n=69) | 1-6 (n=190) |
|---|---|---|
| Age: Mean, Median (Range) | 60, 59 (37-91) | 60, 61 (30-90) |
|
| ||
| Method of Detection | ||
| Nonpalpable (n=144) | 40 (58.0) | 104 (54.7) |
| Palpable (n=115) | 29 (42.0) | 86 (45.3) |
|
| ||
| Imaging Characteristics | ||
| Calcifications (n=37) | 12 (17.0) | 25 (13.0) |
| Mass (n=173) | 50 (72.0) | 123 (65.0) |
| Asymmetry (n=34) | 4 (6.0) | 30 (15.0) |
| Negative (n=10) | 1 (1.0) | 9 (5.0) |
| Unknown (n=5) | 2 (4.0) | 3 (2.0) |
|
| ||
| Primary Tumor Histologic Type | ||
| Invasive ductal carcinoma (n=215) | 59 (86.0) | 156 (82.0) |
| Invasive lobular carcinoma (n=28) | 7 (10.0) | 21 (11.0) |
| Invasive mammary carcinoma (n=16) | 3 (4.0) | 13 (7.0) |
Figure 2.
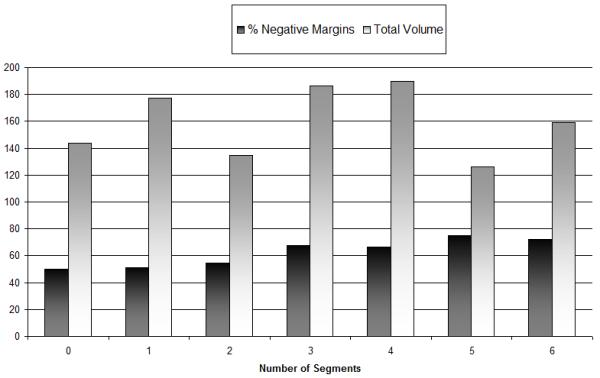
Margin positivity rates. Number of segments compared to volume and percentage of negative margins. As the number of segments removed increase, there is a decrease in positive margins.
Table 2. Tumor size versus resection volumes by number of segments resected.
Numbers in each column represent means.
| Peripheral Segments (n) |
Primary tumor size (diameter, cm) |
Primary segment volume (cm3) |
Total peripheral segment volume (cm3) |
Total resected volume (cm3) |
|---|---|---|---|---|
| 0 (69) | 1.4 | 143.6 | N/A | 143.6 |
| 1 (56) | 1.7 | 158.0 | 20.5 | 177.3 |
| 2 (43) | 1.7 | 111.0 | 19.0 | 135.0 |
| 3 (28) | 1.7 | 142.7 | 43.3 | 186.3 |
| 4 (12) | 2.0 | 125.9 | 63.6 | 189.5 |
| 5 (4) | 2.2 | 66.0 | 60.0 | 126.0 |
| 6 (47) | 2.0 | 92.2 | 67.0 | 159.2 |
Volumes of the primary segments and PSs, in relation to tumor size, are listed in Table 2. By the Wilcoxon test, there was no difference in the average total specimen volume as the number of segments removed increased (p= 0.10), because as greater numbers of segments were resected, primary segment volumes became smaller (p=0.01). With an increase in tumor size, the number of segments removed did not increase (p=0.08). Final margin positivity did not correlate to primary segment volume (p=1.0). By the Pearson Correlation test, the total volume excised increased as tumor size increased (p<0.0001), although increasing volume did not assist in achieving negative margins by univariate analysis (p=0.79, Table 4). However, as the number of PSs increased, margin positivity decreased (p=.01) via the Jonckheere-Terpstra test.
Table 4. Modeling of predictors of margin status.
| Factor | Univariate p value |
Multivariate p value |
OR (95% CI) |
|---|---|---|---|
| Tumor size (cm) | 0.017 | 0.0024 | 1.8 (1.2-2.5) |
| Histology | 0.5 (ilc v. imc v. idc) | 0.29 (ILC v. IDC) | 2.2 (0.5-9.8) |
| 0.25 (ILC v. IMC) | 2.4 (0.53-11.1) | ||
| EIC | 0.06 | .049 | 2.7 (1.0-7.4) |
| Grade | 0.75 (1 v. 2 v. 3) | 0.68 (1 v. 3) | 1.4 (0.32-5.8) |
| 0.17 (2 v. 3) | 2.0 (0.7-5.2) | ||
| PSs (0 v1-6) | 0.01 | 0.006 | 3.4 (1.4-8.0) |
| Total Volume (cm3) | 0.79 | 0.35 | 1.0 (1.0-1.002) |
| Primary Segment Volume | 1.0 | N/A | N/A |
| Palpability | 0.54 | 0.94 | 1.1 (0.2-5.5) |
| Use of needle localization | 0.33 | 0.38 | 2.1 (0.4-11.0) |
| Age | 0.17 | 0.24 | 1.0 (0.95-1.0) |
Overall mean and median tumor diameters were 1.7 cm and 1.5 cm, respectively (range 0.1 cm – 8.0 cm). Among the 190 patients who had ≥1 PSs resected, 119 (63%) had negative final margins, 53 (28%) had close margins, and 18 (9%) had positive margins. In 69 cases, only a single primary segment was resected. Among these, margins were negative in 34 (49%), close in 20 (29%), and positive in 15 (22.0 %).
There were 215 ductal (83%), 28 lobular (11%), and 16 mammary (6%) carcinomas (mixed ductal and lobular features). Among the 33 cases with positive margins, 25 were IDC (76%), 5 (15%) were ILC, and 3(9%) were IMC. There was no significant difference in the likelihood of positive margins for lobular cancers versus all others by univariate (p=0.5, Table 4) or multivariate analyses (p=0.29, ILC vs. IDC and p=0.25, ILC vs. IMC). Final peripheral margins are listed by tumor type in Table 3.
Table 3.
Tumor type and procedural margin status, representing the margin of the primary segment when resected alone, or of the PSs when removed with a primary segment. Numbers represent n (row %)
| Histology | Negative | Close | Positive |
|---|---|---|---|
| Invasive ductal carcinoma (n=215) | 125 (58.0) | 65 (30.0) | 25 (12.0) |
| Invasive lobular carcinoma (n=28) | 19 (68.0) | 4 (14.0) | 5 (18.0) |
| Invasive mammary carcinoma (n=16) | 9 (56.0) | 4 (25.0) | 3 (19.0) |
Age (p=0.17), tumor palpability (p=0.54), and use of needle localization (p=0.33) also did not assist in achieving negative margins (Table 4) by univariate analysis. The presence of EIC demonstrated a trend towards margin positivity (p=0.06), while higher tumor grade showed no association (p=0.75, Table 4).
Because an increase in volume did not explain the margin negativity, pathology reports were reviewed to correlate residual disease found in the PSs with the primary segment’s margin status and orientation to help assess whether artifact may contribute to margin status. Among 190 cases, 576 additional PSs were resected at the time of initial lumpectomy, 62 (10.8 %) of which contained additional DCIS or invasive cancer. For 119 patients who had 1-6 simultaneous PSs removed and negative margins, 64 (54%) had negative margins in the primary segment and no disease in the PSs, while 41 (34%) had a positive or close primary segment margin, but no residual disease in the corresponding PSs removed. Thirteen (11%) patients had a compromised primary segment margin and cancer in the corresponding PS, and 1(1%) patient who had negative primary segment margins and a small focus of DCIS in a PS. Three PSs (0.5%) that were adjacent to negative primary segment margins contained additional disease. Among 122 compromised primary segment margins, 62 (50.8%) had additional disease in the corresponding PS. Re-excision was performed in 29 of 73 patients with close margins, demonstrating 7/29 (24%) with residual disease. In the 33 patients having initially positive margins, 17 patients underwent re-excision with residual tumor found in 8/17 (47%) patients.
A multivariate model analyzed margin status, segment numbers, tumor size, use of needle-localization, volumes resected, EIC, age, histology, grade, and palpability with respect to margin status. Smaller tumor size (p=0.0024), EIC (p=0.049) and 1 or more PSs removed (p=0.006), significantly lowered the risk of margin positivity (Table 4).
DISCUSSION
Successful BCS depends upon the ability to obtain negative margins to minimize local recurrence. Efforts that improve the negative margin rate may have a significant clinical impact. There are few studies evaluating simultaneous excision of PSs. In a study by Jacobson and colleagues21 all specimens had additional segments resected, and most had all 6 PSs removed. The authors found that the number of patients requiring re-excision would have dropped from 66% (the number having a positive central segment margin) to 49% (the number of peripheral segments having negative margins), demonstrating a potential benefit of 17%. This is in contrast to 50% of their patients whose peripheral resection did not affect margin status, either because central segments already had negative margins, or because the PSs also had positive margins. Although the overall specimen volumes were measured, there was no analysis to determine if resection volume affected margin status overall, and the reader must make a judgment as to whether unnecessary resection of additional breast volume in 50% of patients is worth saving 17% of patients from reoperation. Because none of the patients underwent resection of one large central segment alone, it is difficult to make any conclusions about how this compares to removing a single lumpectomy specimen.
In a study by Huston et al,23 the patients were divided into groups of 0, 1-3 and 4-6 PSs, unlike our analysis. The authors noted that 56% and 36% of these latter respective groups had positive central segment margins, and negative PS margins, suggesting the value of simultaneous PS removal. This study contrasted with our own in that they found that the total specimen volume increased with PS resection numbers. This increased volume could account for the improvement in margin status, consistent with other data,18 invalidating the need for simultaneous PS resection over simply removing larger volumes. In contrast, we found an increasing benefit with PS numbers even though the volumes did not increase, suggesting that the benefit is not volume dependent.
Certain characteristics are predictive of margin status, but are innate to the patient or biology of the tumor. Larger tumor size here, as elsewhere,7, 14-16, 18 increased the likelihood of positive margins. EIC7, 8, 17, 18 and invasive lobular carcinoma8, 16, 19 have also been implicated as factors contributing to the likelihood of margin compromise. Although lobular carcinoma was not correlated with positive margins here, possibly due to the paucity of such tumors, there was a trend towards margin positivity when EIC was present. Multifocality may also contribute to margin positivity,11-13 but that data was unavailable. Younger age has been associated with positive margins,6, 13, 19 but we found no such association, consistent with other studies.15, 16 Finally, grade was also not a predictor for margin status, in accordance with other series.11, 13, 19
Prior studies have evaluated factors other than resection technique that are associated with negative margins.6, 11, 12, 16, 19 Neither needle localization nor palpability were of assistance, despite the theoretical possibility that these may help to target a tumor more accurately. This is consistent with data demonstrating that bracketed localization of a lesion may not assist in improving margin status.24 Although this may seem counterintuitive, it is likely due to the lack of perfect correlation between imaging and histologic tumor extent.25 The most extreme example of this would be breast MRI, which claims the highest sensitivity, but no demonstrable utility to date in decreasing the likelihood of compromised margins.26, 27
Intraoperative assessment, if accurate, might spare the patient a second procedure. Gross evaluation by the surgeon can be difficult, irrespective of the histology of the primary tumor, possibly because DCIS may surround a lesion, and may not be palpable.28, 29 Visual inspection of the specimen and cavity,14 frozen section analysis30, 31 intraoperative imprint cytology,32-34 and, radiofrequency spectroscopy35 have all been proposed, but only variably successful. Intraoperative frozen section evaluation has limitations because it is time consuming, increases cost,31 and the fat content of the breast makes such evaluation inaccurate due to artifact from the freezing process.36 Intraoperative imprint cytology has been evaluated for initial surgery32, 33 and for re-excisions,34 but unfortunately, results also vary widely by institution.
Further difficulties may arise in the accuracy of orientation, resulting in inaccurate margin assessment. In a study evaluating 122 lumpectomy specimens, Molina and colleagues37 noted that the surgeon’s orientation sutures were interpreted incorrectly by the pathologist in over 30% of cases, suggesting that part of the disparity between margin status and residual tumor in margin-directed re-excisions may be due to incorrect assessment of which margin to re-excise. Specimen compression has also been suggested to increase the risk of a false-positive margin,38 and while it has been suggested that this might be avoided if the surgeon supervises specimen compression for imaging,39 resection itself causes a loss of dimension without compression.40 The outcome impact of such artifact on local recurrence is likely to be small and would be difficult to demonstrate, as local recurrence rates overall for breast conservation are low, even for node-positive disease.41 The clinical implication here, however, is not whether PS resection will change long term outcomes, but whether it allows fewer return trips to the operating room.
In light of these difficulties, removing additional tissue would seem to be the simplest option, but cosmesis must be considered in BCS technique. We found no correlation between volume resected and PS numbers. This method of PS resection, which increases the negative margin rate, but doesn’t increase in the volume resected, should not substantially compromise cosmesis. This advantage of PS resection appears to result from the ability to minimize artifact by having additional pathologic specimens to evaluate.
Sabel and colleagues previously evaluated 130 patients with close margins, where 67% of re-excisions demonstrating the biopsy cavity or scar, but showed no evidence of tumor in the re-excised margin(s).13 In theory, some of this may be due to destruction of residual tissue by cautery at that margin or an inflammatory effect from the procedure. 36, 42 Inking artifact should also be considered,36 where ink may bleed into the crevices of a specimen, artificially making the margin appear closer to ink than the true in vivo distance. Similar to the findings of Sabel et al,13 we noted that for 52% of patients with positive margins and 76% of patients with close margins evaluated with re-excision, no residual disease was found at re-operation. Similarly, in those patients with PSs removed having negative margins, 34% had positive or close margins on the primary segment and no residual disease in the corresponding PSs, while 10% had residual disease in the PS corresponding to the positive margin on the primary segment. Of the 54 patients with compromised primary segments, 76% avoided a return trip to the operating room since they had PSs removed at the time of initial lumpectomy that were negative for additional disease, confirming the benefit of the technique.
This study is limited by its retrospective nature, and the possibility of a selection bias. This bias, however, is largely a function of the clinical judgement of the surgeon, and may demonstrate the advantage of that technique when used judiciously. Unfortunately, data regarding breast size and multifocality, which may affect margin positivity and the volume of resection, were not available for analysis. Finally, not all patients with close or positive margins underwent re-excision. Data on residual disease in the lumpectomy cavity was not available for these patients, although the overwhelming majority of these compromised margins were on skin or chest wall, precluding such a re-excision.
In short, this study demonstrated that increasing the number of PSs helps decrease margin positivity, without necessarily increasing volume. Further analysis demonstrated that the benefit may be due to a decrease in artifact. Thus, while excision of additional PSs does not ensure negative margins, this technique may decrease additional surgeries secondary to artifact without increasing the total tissue volume excised or having a negative impact on cosmesis.
Acknowledgments
This work was supported, in part, by US Public Health Services grant 5P30 CA06927 and by an appropriation from the Commonwealth of Pennsylvania
Footnotes
Presented, in part, at the Annual Meeting of the American Society of Breast Surgeons, San Diego, CA, April, 24, 2009
REFERENCES
- 1.Fisher ER, Dignam J, Tan-Chiu E, Costantino J, Fisher B, Paik S, Wolmark N. Pathologic findings from the National Surgical Adjuvant Breast Project (NSABP) eight-year update of Protocol B-17: intraductal carcinoma. Cancer. 1999;86:429–438. doi: 10.1002/(sici)1097-0142(19990801)86:3<429::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–1150. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 3.Besana-Ciani I, Greenall MJ. The importance of margins status after breast conservative surgery and radiotherapy in node positive patients: a follow-up of 10-15 years. Int Semin Surg Oncol. 2008;5:13. doi: 10.1186/1477-7800-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunne C, Burke JP, Morrow M, Kell MR. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27:1615–1620. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 5.Taghian A, Mohiuddin M, Jagsi R, Goldberg S, Ceilley E, Powell S. Current perceptions regarding surgical margin status after breast-conserving therapy: results of a survey. Ann Surg. 2005;241:629–639. doi: 10.1097/01.sla.0000157272.04803.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menes TS, Tartter PI, Bleiweiss I, Godbold JH, Estabrook A, Smith SR. The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients. Ann Surg Oncol. 2005;12:881–885. doi: 10.1245/ASO.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Freedman G, Fowble B, Hanlon A, et al. Patients with early stage invasive cancer with close or positive margins treated with conservative surgery and radiation have an increased risk of breast recurrence that is delayed by adjuvant systemic therapy. Int J Radiat Oncol Biol Phys. 1999;44:1005–1015. doi: 10.1016/s0360-3016(99)00112-1. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan MJ, Li T, Freedman G, Morrow M. The effect of multiple reexcisions on the risk of local recurrence after breast conserving surgery. Ann Surg Oncol. 2007;14:3133–3140. doi: 10.1245/s10434-007-9523-4. [DOI] [PubMed] [Google Scholar]
- 9.Wazer DE, DiPetrillo T, Schmidt-Ullrich R, Weld L, Smith TJ, Marchant DJ, Robert NJ. Factors influencing cosmetic outcome and complication risk after conservative surgery and radiotherapy for early-stage breast carcinoma. J Clin Oncol. 1992;10:356–363. doi: 10.1200/JCO.1992.10.3.356. [DOI] [PubMed] [Google Scholar]
- 10.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184:383–393. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 11.Ramanah R, Pivot X, Sautiere JL, Maillet R, Riethmuller D. Predictors of re-excision for positive or close margins in breast-conservation therapy for pT1 tumors. Am J Surg. 2008;195:770–774. doi: 10.1016/j.amjsurg.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Bani MR, Lux MP, Heusinger K, et al. Factors correlating with re-excision after breast-conserving therapy. Eur J Surg Oncol. 2009;35:32–37. doi: 10.1016/j.ejso.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Sabel MS, Rogers K, Griffith K, et al. Residual disease after re-excision lumpectomy for close margins. J Surg Oncol. 2009;99:99–103. doi: 10.1002/jso.21215. [DOI] [PubMed] [Google Scholar]
- 14.Cabioglu N, Hunt KK, Sahin AA, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007;14:1458–1471. doi: 10.1245/s10434-006-9236-0. [DOI] [PubMed] [Google Scholar]
- 15.Keskek M, Kothari M, Ardehali B, Betambeau N, Nasiri N, Gui GP. Factors predisposing to cavity margin positivity following conservation surgery for breast cancer. Eur J Surg Oncol. 2004;30:1058–1064. doi: 10.1016/j.ejso.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Chagpar AB, Martin RC, 2nd, Hagendoorn LJ, Chao C, McMasters KM. Lumpectomy margins are affected by tumor size and histologic subtype but not by biopsy technique. Am J Surg. 2004;188:399–402. doi: 10.1016/j.amjsurg.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 17.Gage I, Schnitt SJ, Nixon AJ, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer. 1996;78:1921–1928. doi: 10.1002/(sici)1097-0142(19961101)78:9<1921::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Park CC, Mitsumori M, Nixon A, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol. 2000;18:1668–1675. doi: 10.1200/JCO.2000.18.8.1668. [DOI] [PubMed] [Google Scholar]
- 19.Waljee JF, Hu ES, Newman LA, Alderman AK. Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol. 2008;15:1297–1303. doi: 10.1245/s10434-007-9777-x. [DOI] [PubMed] [Google Scholar]
- 20.Cao D, Lin C, Woo SH, Vang R, Tsangaris TN, Argani P. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for re-excisions. Am J Surg Pathol. 2005;29:1625–1632. doi: 10.1097/01.pas.0000180448.08203.70. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson AF, Asad J, Boolbol SK, Osborne MP, Boachie-Adjei K, Feldman SM. Do additional shaved margins at the time of lumpectomy eliminate the need for re-excision? Am J Surg. 2008;196:556–558. doi: 10.1016/j.amjsurg.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 22.White RR, Halperin TJ, Olson JA, Jr., Soo MS, Bentley RC, Seigler HF. Impact of core-needle breast biopsy on the surgical management of mammographic abnormalities. Ann Surg. 2001;233:769–777. doi: 10.1097/00000658-200106000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huston TL, Pigalarga R, Osborne MP, Tousimis E. The influence of additional surgical margins on the total specimen volume excised and the reoperative rate after breast-conserving surgery. Am J Surg. 2006;192:509–512. doi: 10.1016/j.amjsurg.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Liberman L, Kaplan J, Van Zee KJ, Morris EA, LaTrenta LR, Abramson AF, Dershaw DD. Bracketing wires for preoperative breast needle localization. AJR Am J Roentgenol. 2001;177:565–572. doi: 10.2214/ajr.177.3.1770565. [DOI] [PubMed] [Google Scholar]
- 25.Mumtaz H, Hall-Craggs MA, Davidson T, Walmsley K, Thurell W, Kissin MW, Taylor I. Staging of symptomatic primary breast cancer with MR imaging. AJR Am J Roentgenol. 1997;169:417–424. doi: 10.2214/ajr.169.2.9242745. [DOI] [PubMed] [Google Scholar]
- 26.Bleicher RJ, Ciocca RM, Egleston BL, Sesa L, Evers K, Sigurdson ER, Morrow M. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009;209:180–187. doi: 10.1016/j.jamcollsurg.2009.04.010. quiz 294-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang N, Schiller DE, Crystal P, Maki E, McCready DR. Magnetic Resonance Imaging in the Planning of Initial Lumpectomy for Invasive Breast Carcinoma: Its Effect on Ipsilateral Breast Tumor Recurrence After Breast-Conservation Therapy. Ann Surg Oncol. 2009 doi: 10.1245/s10434-009-0607-1. [DOI] [PubMed] [Google Scholar]
- 28.Gould EW, Robinson PG. The pathologist’s examination of the “lumpectomy”-- the pathologists’ view of surgical margins. Semin Surg Oncol. 1992;8:129–135. [PubMed] [Google Scholar]
- 29.Holland R, Connolly JL, Gelman R, et al. The presence of an extensive intraductal component following a limited excision correlates with prominent residual disease in the remainder of the breast. J Clin Oncol. 1990;8:113–118. doi: 10.1200/JCO.1990.8.1.113. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi M, Minami M, Earashi M, et al. Pathologic Assessment of Surgical Margins on Frozen and Permanent Sections in Breast Conserving Surgery. Breast Cancer. 1995;2:27–33. doi: 10.1007/BF02966893. [DOI] [PubMed] [Google Scholar]
- 31.Olson TP, Harter J, Munoz A, Mahvi DM, Breslin T. Frozen section analysis for intraoperative margin assessment during breast-conserving surgery results in low rates of re-excision and local recurrence. Ann Surg Oncol. 2007;14:2953–2960. doi: 10.1245/s10434-007-9437-1. [DOI] [PubMed] [Google Scholar]
- 32.Klimberg VS, Westbrook KC, Korourian S. Use of touch preps for diagnosis and evaluation of surgical margins in breast cancer. Ann Surg Oncol. 1998;5:220–226. doi: 10.1007/BF02303776. [DOI] [PubMed] [Google Scholar]
- 33.Cox CE, Ku NN, Reintgen DS, Greenberg HM, Nicosia SV, Wangensteen S. Touch preparation cytology of breast lumpectomy margins with histologic correlation. Arch Surg. 1991;126:490–493. doi: 10.1001/archsurg.1991.01410280094014. [DOI] [PubMed] [Google Scholar]
- 34.Valdes EK, Boolbol SK, Ali I, Feldman SM, Cohen JM. Intraoperative touch preparation cytology for margin assessment in breast-conservation surgery: does it work for lobular carcinoma? Ann Surg Oncol. 2007;14:2940–2945. doi: 10.1245/s10434-007-9364-1. [DOI] [PubMed] [Google Scholar]
- 35.Karni T, Pappo I, Sandbank J, et al. A device for real-time, intraoperative margin assessment in breast-conservation surgery. Am J Surg. 2007;194:467–473. doi: 10.1016/j.amjsurg.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Schnitt SJ, Connolly JL. Processing and evaluation of breast excision specimens. A clinically oriented approach. Am J Clin Pathol. 1992;98:125–137. doi: 10.1093/ajcp/98.1.125. [DOI] [PubMed] [Google Scholar]
- 37.Molina MA, Snell S, Franceschi D, et al. Breast specimen orientation. Ann Surg Oncol. 2009;16:285–288. doi: 10.1245/s10434-008-0245-z. [DOI] [PubMed] [Google Scholar]
- 38.Dooley WC, Parker J. Understanding the mechanisms creating false positive lumpectomy margins. Am J Surg. 2005;190:606–608. doi: 10.1016/j.amjsurg.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Clingan R, Griffin M, Phillips J, Coberly W, Jennings W. Potential margin distortion in breast tissue by specimen mammography. Arch Surg. 2003;138:1371–1374. doi: 10.1001/archsurg.138.12.1371. [DOI] [PubMed] [Google Scholar]
- 40.Graham RA, Homer MJ, Katz J, Rothschild J, Safaii H, Supran S. The pancake phenomenon contributes to the inaccuracy of margin assessment in patients with breast cancer. Am J Surg. 2002;184:89–93. doi: 10.1016/s0002-9610(02)00902-9. [DOI] [PubMed] [Google Scholar]
- 41.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five National Surgical Adjuvant Breast and Bowel Project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 42.Schnitt SJ, Abner A, Gelman R, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer. 1994;74:1746–1751. doi: 10.1002/1097-0142(19940915)74:6<1746::aid-cncr2820740617>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]