Abstract
Aims
Matrix metalloproteinase (MMP) activity is central to the development of left ventricular (LV) remodelling and dysfunction after acute myocardial infarction (AMI). We assessed the relationships with LV structure and function and outcome, of tissue inhibitors of metalloproteinase-1 (TIMP-1) and MMP-9, and compared with N-terminal pro-B-type natriuretic peptide (NTproBNP).
Methods and results
We studied 404 patients with AMI. Primary outcome measures were the associations of TIMP-1, MMP-9, and NTproBNP with death or heart failure, and with LV dimensions, function and remodelling (ΔLVEDV, change in LV end-diastolic volume between discharge and follow-up). Cut-off concentrations for prediction of death or heart failure were identified from receiver operator characteristic (ROC) curves. In multivariable analysis, TIMP-1 and NTproBNP had predictive value for LV ejection fraction pre-discharge (TIMP-1 P = 0.023; N-BNP P = 0.007) and at follow-up (TIMP-1 P = 0.001; N-BNP P = 0.003). MMP-9, TIMP-1, and NTproBNP correlated directly with LV volumes. MMP-9 (P = 0.005) and TIMP-1 (P = 0.036), but not NTproBNP, correlated with ΔLVEDV. For the combined endpoint of death or heart failure the area under the ROC curve was 0.640 for MMP-9, 0.799 for NTproBNP and 0.811 for TIMP-1. Patients with TIMP-1 > 135 ng/mL (P < 0.001) or NTproBNP >1472 fmol/mL (P < 0.001) had increased risk of endpoint. Consideration of both NTproBNP and TIMP-1 further improved risk stratification.
Conclusion
TIMP-1 and MMP-9 correlate with echocardiographic parameters of LV dysfunction and remodelling after AMI and may identify patients at risk of subsequent LV remodelling and adverse prognosis.
Keywords: Matrix metalloproteinase, Ventricular remodelling, Myocardial infarction, Prognosis
Introduction
The development of left ventricular (LV) remodelling is a powerful indicator of adverse prognosis after acute myocardial infarction (AMI). Remodelling involves maladaptive changes to cellular and extracellular elements of the myocardium, in both infarcted and non-infarcted areas of the ventricle.1 The extent of the resultant progressive ventricular enlargement and impairment of contractile function associate closely with increased risk of adverse outcome.2
In the healthy state, structural integrity of the myocardial extracellular matrix (ECM) is maintained by activity of a family of endogenous zinc-dependent endopeptidases, the matrix metalloproteinases (MMPs). MMP enzyme activity, and consequently ECM matrix degradation, is regulated by endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). In particular, excessive degradation of ECM components, the result of enhanced MMP proteolytic activity, is critical to the development of LV dilatation and heart failure. Studies of MMP inhibition support a likely role for enhanced MMP activity in LV remodelling after AMI. In experimental models of AMI, MMP inhibition is associated with attenuated LV dilatation3,4 and reduced wall stress.4 Altered activity of the gelatinase MMP-9 has been suggested to be of particular importance in LV remodelling after experimental AMI.5,6 Clinical studies are in keeping with these observations; plasma MMP-9 correlates with both LV diameter and mass7 and increased plasma MMP-9, resulting from a MMP-9 gene polymorphism, has been associated with elevated cardiovascular risk.8
In addition to the physiological regulation of MMP activity, altered TIMP activity may play a direct role in remodelling. In particular TIMP-1, which binds a number of MMPs with high affinity, appears integral to the regulation of myocardial structure and function. Mice deficient in TIMP-1 show increased LV remodelling after experimental AMI.9 In small clinical studies, patients with hypertension,10 and in a community-based cohort,11 higher plasma TIMP-1 concentration was associated with greater LV mass and dimensions. Elevated plasma TIMP-1 concentrations are seen after AMI in man12 and have been linked with increased cardiovascular risk in patients with cardiovascular disease13 or undergoing diagnostic coronary arteriography.14 In a recent nested Case–Control study within the LIPID trial, TIMP-1 was shown to be an independent predictor of adverse events in subjects with previous myocardial infarction (MI).15 Our group16 and others17,18 have described a specific temporal profile of plasma MMP-9 after AMI. We have also demonstrated direct correlation of plasma MMP-9 concentration with the severity of LV dysfunction and increased LV volumes in this setting.19 No previous study has described the relationship of TIMP-1 with myocardial structure and function after AMI.
In recent years, a variety of plasma markers of LV dysfunction and adverse prognosis after AMI have been identified. Of these, elevated plasma concentrations of both B-type natriuretic peptide (BNP)20,21 and the N-terminal fragment of its prohormone, N-terminal pro-BNP (NTproBNP)22,23 show powerful association with the development of LV dysfunction and heart failure, and with adverse clinical outcome. We hypothesized that alterations in plasma concentrations of metalloproteinase activity will show association with measures of LV dysfunction and remodelling, and with clinical outcome in the post-AMI period. In the current study we examined the relationship of plasma MMP-9 and TIMP-1 with LV structure and function, and with prognosis, after AMI. We assessed the predictive value of plasma concentrations of MMP-9 and TIMP-1, and N-BNP, a ‘gold standard’ marker of LV dysfunction after AMI.
Methods
Study population and design
We conducted a prospective, cohort study, enrolling 404 patients admitted with AMI to the Coronary Care Unit of our hospital between 1 September 2004 and 28 February 2006. The pre-specified primary endpoint was the combination of death or heart failure occurring by 6 months of the index event. Power calculations were based on previous data in our population indicating an event rate of ∼ 19% per annum. Thus, in a study sample of 400 patients recruited >18 months with a follow-up of 6 months we expected to see ∼ 75 events, of which ∼25 would be deaths and 50 would be individuals with heart failure episodes. We have shown that this sample size is sufficient to allow robust determination of relationships between N-BNP and clinical outcomes,24 between plasma MMP and NTproBNP16 and between plasma MMP and echocardiographic parameters of remodelling.16
The diagnosis of AMI was based on appropriate symptoms of myocardial ischaemia in conjunction with appropriate, dynamic ECG changes (ST segment elevation, STEMI, n = 329) or ST segment/T-wave changes (NSTEMI, n = 75) and elevation in plasma markers of myocardial necrosis [creatine kinase (CK) or troponin I]. Predefined primary outcome was the composite of all-cause death or heart failure episode during follow-up. The latter was defined as an unplanned hospital admission, the primary reason for which was clinical heart failure requiring high dose diuretic, intravenous nitrate or inotropic support. Secondary outcome measures were death or heart failure hospitalization considered individually, and re-infarction. In addition we assessed the relationships between plasma concentrations of MMP-9, TIMP-1 and NTproBNP, and LV volume and function, measured both during the index admission and at follow-up.
Endpoints were identified through the hospital patient tracking system, with review of medical records for each endpoint. Checks were made by telephone contact with all surviving patients at the end of the study. Echocardiographic examination was carried out immediately prior to discharge and at follow-up. During the index admission, venous blood was drawn from all patients for the assay of plasma levels of MMP-9, NTproBNP, and TIMP-1. The local research ethics committee approved the study and patients gave written consent to participation. The conduct of the study was in keeping with the declaration of Helsinki.
Laboratory methods
We previously reported stronger correlation with LV structure and function for MMP-9 measured within 24 h of admission, compared with later times,19 and for NTproBNP measured pre-discharge compared with earlier times after AMI.25 Thus MMP-9 was measured within 24 h of admission, and NTproBNP immediately pre-discharge, with plasma TIMP-1 being assayed on the latter sample. Samples were centrifuged within 30 min and plasma stored at −70°C until assayed.
Our non-competitive assays for plasma NTproBNP23 and MMP-919 have been described previously. The lower limit of detection for NTproBNP was 0.4 ng/mL and for MMP-9 was 0.4 ng/mL. Inter-assay coefficient of variation was <8%. Plasma TIMP-1 was measured using a commercially available solid phase ELISA kit (Quantikine-Cat. no. DTM100). Samples and standards were added to plates pre-coated with TIMP-1-specific monoclonal antibody. After washing, an enzyme linked polyclonal antibody specific for TIMP-1 was added. Following a further wash to remove unbound antibody-enzyme reagent, a substrate solution was added to the wells, allowing colour to develop in proportion to the amount of TIMP-1. Colour intensity was measured using Dynex Revelation 4.24 ELISA reader. The lower limit of detection was 0.08 ng/mL. Intra- and inter-assay coefficient of variation were <10%. Presented plasma concentrations of NTproBNP, MMP-9, and TIMP-1 represent the mean of duplicate measurements.
Echocardiographic assessment
Echocardiography was carried out immediately prior to discharge and at follow-up by a single operator (DK) using a Sonos 5500 or IE33 scanner. LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV) and LV ejection fraction (LVEF) were estimated using the bi-planar modified Simpson's rule from apical two- and four-chamber views. LV wall motion index score (WMIS) was measured using a standard 16-segment model from parasternal long and short axes, and apical two- and four-chamber views. Each LV segment is scored as 0 – hyperkinetic, 1 – normal, 2 – hypokinetic, 3 – akinetic, and 4 – dyskinetic. The total divided by the number of segments analysed gives an overall score, higher values indicating more impaired LV function.
The degree of ventricular remodelling after AMI was assessed from the change in LVEDV (ΔEDV) and LVESV (ΔESV) between pre-discharge and follow-up examinations, expressed as a percentage of the pre-discharge measurement. When assessing remodelling, we considered only data pertaining to patients with adequate pre-discharge and follow-up echocardiographic examinations. Intra-observer variation, assessed in a subset (N = 45; Mean ±+ SD) was 0.36% ± 1.75 for WMIS, 5.2% ± 3.9 for EDV, 6.0% ± 6.6 for ESV, and 6.7% ± 7.6 for LVEF. Pearson's correlation between analyses were all >0.9, P < 0.001.
Statistical analysis
For all variables with non-Gaussian distribution (N-BNP, MMP-9, TIMP-1, CK, TnI, WMIS), log-transformed values were used in analyses. Associations of N-BNP, MMP-9 or TIMP-1 levels with categorical variables were assessed using Student's t-test, or Mann–Whitney U test for non-normally distributed variables, and with continuous variables using Pearson's correlation coefficient. Factors having biologically plausible effects on LV function and with univariable association (P < 0.1) were entered into a multivariable linear regression model using forced entry for the prediction of LV function (LVEF and WMIS). Differences between groups experiencing or not experiencing each clinical endpoint were assessed using χ2 analysis for categorical variables and Mann–Whitney U-test for continuous variables. Factors with univariate association with each endpoint at significance level of P < 0.1 were entered into multivariable Cox proportional hazards model. We constructed individual Cox models, entering candidate variables together with plasma NTproBNP, MMP-9 or TIMP-1 entered individually.
The strength of association with endpoints is expressed as hazards ratio (HR) per log-transformed unit increase in plasma concentration of TIMP-1, MMP-9, and N-BNP. When considering the primary endpoint of death or heart failure we assessed time to first event, patients being removed from further analysis thereafter. Event-free survival was assessed over 6 months follow-up.
We estimated the strength of association with outcome using individual plasma concentrations of NTproBNP and TIMP-1 identified from receiver operator characteristic (ROC) curves of these biomarkers. These cut-off points were identified as the point on the curve showing the highest combination of sensitivity and specificity and used to assess the strength of association with adverse outcome using Kaplan–Meier assessment. The original data set was subjected to bootstrapped sampling with replacement 50 times, and the area under the curve for the ROC and optimal TIMP or NTproBNP cut-off concentration computed for each sample.
For all analyses, P < 0.05 was regarded as significant and two-sided tests were used where appropriate. Statistical analyses were carried out using SPSS version 14. The authors had full access to the data, accept responsibility for their validity, and have read and agreed to the manuscript as submitted.
Results
The admission demographic features of the population are in Table 1. Approximately 75% of the population were male, the admission ECG showed ST-elevation in >80%, and median CK was >1300 I.U. Of the 329 patients presenting with STEMI, 203 (62%) received thrombolytic therapy. No patient received primary percutaneous revascularization. Few patients (n < 10) considered for inclusion in this study were deemed unsuitable or refused consent. Echocardiography was undertaken in all patients prior to discharge and in 343 survivors attending for follow-up assessment. Prior to discharge, LVEF and WMSI were measurable in 351 (86.9%) and 356 (88.1%) patients, respectively. Corresponding figures at follow-up were 310 (90.3%) and 305 (88.9%). Follow-up ranged from 1 to 619 days with a median of 314 days. Vital status at study end was available for all patients.
Table 1. Population demographics at admission.
| Median | Range | |
|---|---|---|
| Age (years) | 62.1 | 24–91 |
| CK (I.U., NR 0–200) | 1391 | 41–7384 |
| Troponin I (NR < 0.06) | 27.1 | 0.06–150 |
| Number (%) | ||
| Male/female | 305/99 (75.5/24.5) | |
| STEMI | 329 (81.4) | |
| Anterior/inferior site | 165/221 (40.8/54.7) | |
| Thrombolysis | 203 (50.2) | |
| Current smoker | 147 (36.4) | |
| Diabetes | 69 (17.1) | |
| Hypertension | 169 (41.8) | |
| Previous MI | 38 (9.4) | |
| Previous revascularization | 10 (2.5) | |
| Medications | Admission | Discharge |
| Aspirin | 82 (20.2) | 331 (90.6) |
| Clopidogrel | 14 (3.5) | 86 (23.6) |
| Beta-blocker | 82 (20.3) | 336 (92.1) |
| ACE-I/ARB | 98 (24.3) | 363 (89.9) |
| Statin | 86 (21.3) | 354 (97) |
| Furosemide | 44 (10.9) | 56 (13.9) |
CK, creatine kinase; NR, normal range; ACE-I, angiotensin converting enzyme inhibitor.
Extent of infarction
Plasma NTproBNP, TIMP-1, and MMP-9 correlated with infarction size as assessed via peak levels of CK (r = 0.192, P < 0.001; r = 0.167, P = 0.001; and r = 0.151, P = 0.004) and troponin (r = 0.198, P < 0.001; r = 0.151, P = 0.006; and r = 0.075, P = 0.174, respectively). There were direct correlations between our markers: MMP-9/NTproBNP, r = 0.151, P = 0.003; MMP-9/TIMP-1, r = 0.137, P = 0.006; TIMP-1/NTproBNP r = 0.404, P < 0.001.
Left ventricular structure and function
Plasma MMP-9, TIMP-1, and NTproBNP each correlated with greater impairment of LV function measured pre-discharge, as indicated by direct correlation with WMIS and inverse correlation with LVEF (Table 2). For NTproBNP and TIMP-1, similar relationships were evident at follow-up. Plasma MMP-9, TIMP-1, and NTproBNP correlated directly with LV volumes (Table 2).
Table 2. Univariable correlations between tissue inhibitors of metalloproteinase-1 (TIMP-1), matrix metalloproteinase-9 (MMP-9), and N-terminal B-type natriuretic peptide (N-BNP) with markers of left ventricular (LV) function.
| WMIS | LVEF | LVEDD | LVESD | LVEDV | LVESV | ΔLVEDV | ΔLVESV | |
|---|---|---|---|---|---|---|---|---|
| Pre-discharge | ||||||||
| TIMP-1 | ||||||||
| r | 0.290 | −0.244 | 0.053 | 0.148 | 0.092 | 0.191 | ||
| P-value | <0.001 | <0.001 | 0.314 | 0.005 | 0.088 | <0.001 | ||
| MMP-9 | ||||||||
| r | 0.146 | −0.176 | 0.036 | 0.125 | 0.187 | 0.199 | ||
| P-value | 0.009 | 0.002 | 0.519 | 0.026 | 0.001 | 0.001 | ||
| N-BNP | ||||||||
| r | 0.389 | −0.311 | 0.054 | 0.197 | 0.076 | 0.204 | ||
| P-value | <0.001 | <0.001 | 0.302 | 0.006 | 0.156 | <0.001 | ||
| Follow-up | ||||||||
| TIMP-1 | ||||||||
| r | 0.224 | −0.318 | 0.053 | 0.135 | 0.148 | 0.237 | 0.124 | 0.116 |
| P-value | <0.001 | <0.001 | 0.355 | 0.019 | 0.009 | <0.001 | 0.036 | 0.05 |
| MMP-9 | ||||||||
| r | 0.093 | −0.077 | 0.101 | 0.089 | 0.172 | 0.128 | 0.174 | 0.040 |
| P-value | 0.126 | 0.205 | 0.095 | 0.148 | 0.004 | 0.033 | 0.005 | 0.520 |
| N-BNP | ||||||||
| r | 0.340 | −0.328 | 0.041 | 0.128 | 0.033 | 0.158 | 0.019 | −0.008 |
| P-value | <0.001 | <0.001 | 0.479 | 0.026 | 0.562 | 0.005 | 0.753 | 0.895 |
WMIS, wall motion index score; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; ΔLVEDV/ΔLVESV, change in left ventricular end-diastolic/systolic volume.
Left ventricular remodelling
Both TIMP-1 and MMP-9 correlated with the degree of LV remodelling as assessed by ΔLVEDV (Table 2). For those patients in whom LVEDV increased (N = 142), MMP-9 was higher (median 82.5 ng/mL, range 17.1–652.1) than in those for whom LVEDV fell (N = 150; median 55.0 ng/mL, range 9.5–489.6, P = 0.006). No differences in TIMP-1 or NTproBNP were noted between these groups (EDV: TIMP-1, 116.2 vs. 108.5 ng/mL, P = 0.099; NTproBNP, 680.5 vs. 673.7 fmol/mL, P = 0.519; ESV: TIMP-1, 115.0 vs. 114.4 ng/mL, P = 0.375; NTproBNP 680.5 vs. 659.8 fmol/mL, P = 0.578).
Multivariable model
We entered into multivariable analysis those factors showing univariable association with LVEF and WMIS (age, previous angina or MI, territory of infarct, peak CK, log TIMP-1, log MMP-9, and log NTproBNP). When entered individually, TIMP-1, MMP-9, and NTproBNP each had independent predictive value for LVEF pre-discharge (TIMP-1, P = 0.001; MMP-9, P = 0.028; N-BNP, P < 0.001); TIMP-1 and NTproBNP had independent predictive values for WMIS (TIMP-1, P = 0.001; NTproBNP, P < 0.001) pre-discharge. Both NTproBNP (P = 0.003) and TIMP-1 (P < 0.001) had predictive value for LVEF at follow-up and NTproBNP had predictive value for WMIS (P = 0.001). When TIMP-1, MMP-9, and NTproBNP were entered into the model simultaneously, both TIMP-1 and NTproBNP, but not MMP-9, retained independent predictive value for LVEF (TIMP-1, P = 0.023; N-BNP, P = 0.007) and WMIS (TIMP-1, P = 0.005; NTproBNP, P = 0.001) pre-discharge. TIMP-1 and NTproBNP retained association with LVEF (TIMP-1, P = 0.001; NTproBNP, P = 0.003) and NTproBNP with WMIS (P < 0.001) at follow-up.
Clinical endpoints
During follow-up 35 (8.7%) patients died and 46 (11.4%) experienced a heart failure episode. The combined endpoint or death or heart failure was reached by 63 individual patients (15.6%). Plasma NTproBNP, MMP-9, and TIMP-1 were higher in these 63 patients and in those reaching each individual component of the primary endpoint (Table 3). There was no association of NTproBNP, MMP-9 or TIMP-1 with the occurrence of re-infarction.
Table 3. Tissue inhibitors of metalloproteinase-1 (TIMP-1), matrix metalloproteinase-9 (MMP-9), and N-terminal B-type natriuretic peptide (N-BNP) in relation to the occurrence of clinical endpoints.
| Endpoint | TIMP-1 (ng/mL) median [range] |
MMP-9 (ng/mL) median [range] |
N-BNP (fmol/mL) median [range] |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Event | No event | P-value | Event | No event | P-value | Event | No event | P-value | |
| Death/heart failure (N = 63) | 166.7 [46.5–465.9] | 108.4 [7.7–408.6] | <0.001 | 114.9 [21.2–585.1] | 67.1 [9.5–652.1] | 0.001 | 2344.7 [9.0–9856.7] | 595.8 [0.3–9635.2] | <0.001 |
| Death (N = 35) | 173.9 [84.0–465.9] | 110.8 [7.7–408.6] | <0.001 | 87.0 [21.1–585.1] | 70.0 [9.5–652.1] | 0.042 | 2266.0 [9.0–9856.7] | 672.3 [0.3–9635.2] | <0.001 |
| Heart failure (N = 46) | 163.4 [46.5–381.7] | 110.6 [36.8–465.9] | <0.001 | 103.4 [30.0–450.7] | 67.8 [9.5–652.1] | 0.011 | 2162.9 [507.3–9598.4] | 633.0 [0.3–9856.7] | <0.001 |
| Re-infarction (N = 44) | 122.1 [37.5–299.2] | 114.8 [36.8–465.9] | 0.351 | 72.8 [22.3–307.7] | 71 [9.52–652.1] | 0.78 | 586.3 [12.6–9635.2] | 776.3 [0.3–9856.7] | 0.746 |
Prediction of outcome
We constructed Cox Proportional Hazards models for the multivariable prediction of 6-month event-free survival. In any one Cox model we entered NTproBNP, MMP-9 or TIMP-1 individually together with the other factors showing univariable association with the primary endpoint (Table 4). In these analyses, NTproBNP (HR 1.51, 95% CI 1.23–1.86, P < 0.001), MMP-9 (HR 1.44, 95% CI 1.11–1.88, P = 0 .007), and TIMP-1 (HR 2.75, 95% CI 1.64–4.61, P = 0.001), each had independent association with the primary endpoint.
Table 4. Prediction of death or heart failure episode, univariate associations.
| Factor | Univariable HR | P-value | Multivariable HR | P-value |
|---|---|---|---|---|
| Anterior territory | 1.93 [1.14–3.26] | 0.015 | 1.40 [0.80–2.47] | 0.242 |
| History of hypertension | 1.84 [1.12–3.03] | 0.016 | 1.32 [0.74–2.38] | 0.350 |
| Age | 1.08 [1.05–1.10] | 0.001 | 1.04 [1.01–1.07] | 0.024 |
| Creatinine | 1.01 [1.01–1.02] | 0.001 | 1.01 [1.00–1.01] | 0.017 |
| Glucose | 1.07 [1.03–1.11] | 0.001 | 1.04 [1.00–1.09] | 0.07 |
| Log NTproBNP | 1.69 [1.44–1.99] | 0.001 | 1.51 [1.23–1.86] | 0.001 |
| Log MMP-9 | 1.49 [1.17–1.90] | 0.001 | 1.44 [1.11–1.88] | 0.007 |
| Log TIMP-1 | 4.72 [3.26–6.82] | 0.001 | 2.75 [1.64–4.61] | 0.001 |
Hazards ratio (HR) for continuous variables [age (years), creatinine (μmol/L), glucose (mmol/L), log N-BNP (N-terminal B-type natriuretic peptide), log MMP-9 (matrix metalloproteinase-9), and log TIMP-1 (tissue inhibitors of metalloproteinase-1)] are per unit increase. HR for N-BNP, MMP-9, and TIMP-1 refer to each of these factors entered singly into multivariable analysis.
Factors with independent association with death (NTproBNP, MMP-9, and TIMP-1 considered individually) were NTproBNP (HR 1.49, 95% CI 1.16–1.92, P = 0.002), TIMP-1 (HR 4.10, 95% CI 2.27–7.44, P < 0.001), MMP-9 (HR1.62, 95% CI 1.15–2.27, P = 0.005), anterior territory of infarct (HR 2.03, 95% CI 1.00–4.17, P = 0.05) and age (HR = 1.06, 95% CI 1.02–1.10, per year increase, P = 0.004).
Factors with independent association with heart failure were NTproBNP (HR 1.47, 95% CI 1.15–1.90, P = 0.003), TIMP-1 (HR 4.26, 95% CI 2.29–7.93, P < 0.001), and age (HR 1.05, 95% CI 1.01–1.09, P = 0.011). The addition of WMIS to multivariable analysis did not materially affect the association with death or heart failure of either NTproBNP or TIMP-1. Addition of LVEF did not affect NTproBNP but attenuated the strength of association for TIMP-1.
The area under the ROC curve for prediction of the primary outcome was 0.640 (95% CI 0.563–0.717; P = 0.001) for MMP-9, 0.799 (95% CI 0.743–0.855; P < 0.001) for NTproBNP, and 0.811 (95% CI 0.751–0.870; P < 0.001) for TIMP-1.
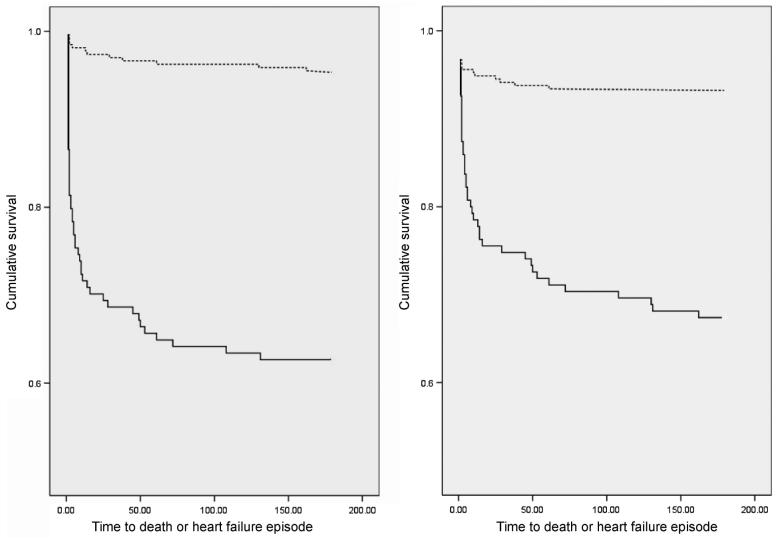
From ROC curves we identified plasma concentrations of NTproBNP (1472 fmol/mL, 95% CI 1420–1524 fmol/mL) or TIMP-1 (135 ng/mL, 95% CI 131–139 ng/mL) having best combination of sensitivity and specificity for prediction of the primary endpoint. Patients for whom TIMP-1 was >135 ng/mL (HR 12.39, 95% CI 6.43–23.90, log rank P < 0.001) or for whom NTproBNP was >1472 fmol/mL (HR = 6.46, 95% CI 3.59–11.65, log rank P < 0.001) had markedly increased risk of death or heart failure (Figure 1). The positive and negative predictive values of these cut-off points for endpoint were 39 and 97%, respectively for TIMP-1 and 34 and 93% for NTproBNP.
Figure 1.
Kaplan–Meier survival curves for patients stratified by – left panel: NTproBNP (N-terminal pro-B-type natriuretic peptide) <1472 fmol/mL (broken line) or ≥1472 fmol/mL (continuous line) and right panel: TIMP-1 (tissue inhibitors of metalloproteinase-1) <135 ng/mL (broken line) or ≥135 ng/mL (continuous line).
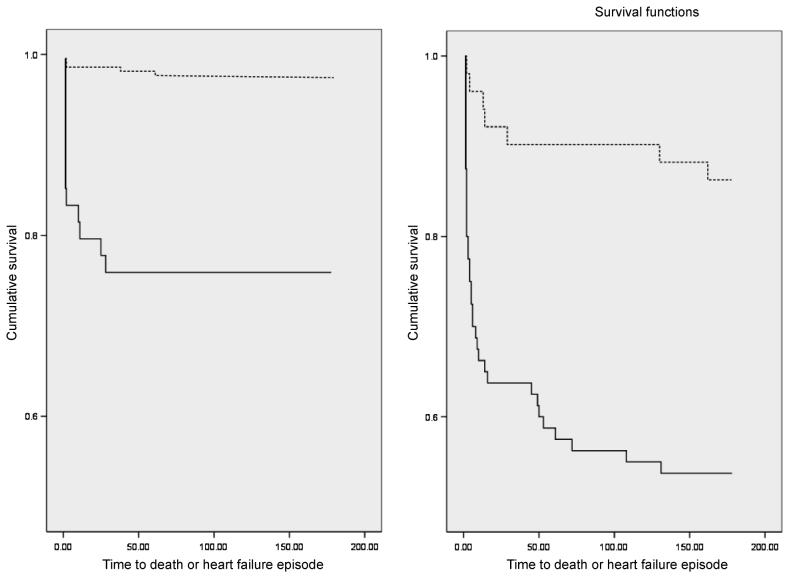
Consideration of both NTproBNP and TIMP-1 further improved risk stratification. For patients with NTproBNP <1472 fmol/mL, the risk of the combined endpoint was higher for those patients in whom TIMP-1 was ≥135 ng/mL (HR 12.25, 95% CI 4.44–33.78; log rank P < 0.001). In addition, in patients with NTproBNP ≥1472 fmol/mL, those subjects with TIMP-1 above optimum had higher risk than those below (HR 5.69, 95% CI 2.29–145.13; log rank P = 0.001) (Figure 2).
Figure 2.
Kaplan–Meier survival curves for patients stratified first by NTproBNP (N-terminal pro-B-type natriuretic peptide), then by TIMP-1 (tissue inhibitors of metalloproteinase-1). Left panel: low-risk BNP (B-type natriuretic peptide) group (NTproBNP <1472 fmol/mL) and TIMP-1 <135 ng/mL (broken line) vs. ≥135 ng/mL (solid line). Right panel: high-risk BNP group (NTproBNP >1472 fmol/mL) and TIMP-1 <135 ng/mL (broken line) vs. ≥135 ng/mL (solid line).
We used the same concentrations of NTproBNP and TIMP-1 for the individual endpoints of death or heart failure. For death, compared with patients with both markers below the respective cut-off, risk was elevated to the greatest extent in patients for whom NTproBNP and TIMP-1 were both above these values (HR 23.35, 95% CI 7.78–70.08; log rank P < 0.001). Risk was elevated to a lesser degree (HR 5.46, 95% CI 1.67–17.84; log rank P = 0.003) for patients with either NTproBNP or TIMP-1 above the cut-off. Similarly, compared with patients with both below the cut-off, risk of heart failure was highest in patients for whom NTproBNP and TIMP-1 were both elevated (HR 13.76, 95% CI 5.65–33.50; log rank P < 0.001) and intermediate in patients with either NTproBNP N-BNP or TIMP-1 above the cut-off (HR 5.94, 95% CI 2.40–14.73, log rank P = 0.001).
Discussion
This study is the most extensive examination of the relationship of elements of the MMP system with LV remodelling and prognosis after AMI in man. Our study confirms previous findings of association of plasma MMP-9 with greater LV volumes and dysfunction after AMI,16,17,19 and extends many of these observations to TIMP-1, for which association with LV remodelling, and with risk of mortality and morbidity were even more powerful. In this context, plasma TIMP-1 has prognostic value similar to that of plasma NTproBNP, the gold-standard peptide marker of prognosis after AMI.
Our study adds considerably to what is known about the MMP system in remodelling after MI. Altered MMP activity is crucial to LV remodelling after experimental AMI,3-8,26,27 and enhanced MMP-9 activity has been implicated in clinical studies of post-AMI remodelling.16-19,28 These reports have been limited by small number of patients and for the most part considered MMP-9 as a dichotomized variable.17,18,28 A true relationship between a biomarker and a measurable pathophysiological response is likely to be linear. Our unique observations of linear relationships for MMP-9 with parameters of LV volume and dysfunction, and with the extent of LV remodelling after AMI, support a true pathophysiological relationship between circulating MMP-9 and LV remodelling after AMI.
The TIMPs are low molecular weight proteins, the main action of which is to inhibit, by binding to the active domain, MMP proteolytic activity. However, TIMPs have additional biological actions, to an extent separate from their actions in inhibiting MMP activity.29 These actions include promotion of growth, and inhibition of both programmed cell death and of angiogenesis, and there is mounting evidence for a role for TIMP-1 in particular in the maintenance of LV structure and function. Spontaneous LV dilatation in TIMP-1 knockout mice suggests an intrinsic role for TIMP-1 in the maintenance of normal cardiac structure. Moreover, in this species, TIMP-1 deficiency is associated with a hypertrophic response to, and exaggerated remodelling after, experimental AMI.9 In man, higher plasma TIMP-1 concentrations have been reported as a marker of myocardial fibrosis in hypertension,30 and are associated with greater LV mass and dimensions in hypertensive10 and community-based11 populations. In addition, anti-hypertensive treatment with ACE (angiotensin converting enzyme) inhibition is associated with reduction in plasma TIMP-1 concentrations.31
The present study establishes for the first time a clear link between circulating TIMP-1 and the extent of LV dysfunction after AMI. We observed consistent associations of TIMP-1 with echocardiographic markers of LV dimension and dysfunction. Once again these associations were linear, were evident in the first few days after AMI, and persisted some weeks later. Further to this, the current study is the first demonstration of associations between plasma TIMP-1 with prognosis after AMI. Together with our observations regarding MMP-9, our study provides powerful evidence of the importance of altered activity of elements of the MMP system in remodelling following myocardial injury in man. The recent STARS-BNP study has demonstrated the use of BNP to tailor therapy in heart failure subjects.32 In a similar manner it may be suggested that both TIMP-1 and MMP-9 may be used to risk stratify patients post-AMI, potentially identifying those requiring enhanced therapy.
The nature of the association between TIMP-1 and LV remodelling cannot be assessed from our data. Previous studies noted association of plasma TIMP-1 with LV hypertrophy and myocardial collagen turnover.10,30 We noted higher TIMP-1 in male patients, smokers and those with a history of hypertension (data not shown), suggesting plasma TIMP-1 may be a marker of adverse remodelling predating the index AMI. Although this may be true in part, a likelier explanation for our observations is that plasma TIMP-1 is a true measure of the post-AMI disruption of the normal equilibrium between ECM synthesis and breakdown, in favour of proteolysis. In this context, higher plasma TIMP-1 concentrations may represent a physiological response to enhanced MMP activity, and as such reflect an attempt to regulate excessive proteolytic activity. The observation of increased TIMP-1 activity in the myocardium of patients with dilated cardiomyopathy33-35 may support this concept.
We have identified TIMP-1 as a strong predictor of prognosis after AMI, with similar predictive value for death or heart failure to that of NTproBNP, a peptide with powerful association with outcome after AMI.20-23 It may be argued that plasma TIMP-1 provides very similar information to that obtained from plasma NTproBNP, and indeed both showed similar pattern and strength of associations with echocardiographic parameters of LV volume and function. However, we have demonstrated the additive prognostic value of considering both TIMP-1 and NTproBNP. We may postulate mechanistic reasons for these observations. Plasma concentrations of the BNPs correlate inversely with LV function20,22,25 and are powerful markers of adverse outcome after AMI. Natriuretic peptides are synthesized and released in response to increased myocardial wall stress. In the setting of AMI, the extent of release is proportional to the degree of LV damage, dysfunction and remodelling. The current study has established a clinically relevant association between plasma TIMP-1, with its important role in the maintenance of LV structure, and the extent of LV remodelling. In this context, the observed association between TIMP-1 and N-BNP is biologically plausible, and indeed, logical.
Our study has some limitations. Although our findings are from a single centre, we included a large cohort with a wide spectrum of cardiac damage and LV dysfunction. We analysed the predictive value of the biomarkers using individual cut-off concentrations derived from ROC curves. Thus our results can be considered to apply only to our cohort and require validation in other large populations. However, this limitation applies to any biomarker dichotomized at any single value derived from a given population, and is one of the major limitations of the use of biomarkers in clinical practice. Moreover, our findings regarding the predictive value of MMP-9 and TIMP-1 are in keeping with our observations of linear associations between these entities and markers of LV dysfunction and remodelling. The relatively low positive predictive value for detection of endpoint is a reflection of the low event rate in our population, whereas negative predictive value remains high.
Differences in drug therapy may have influenced our observations. However, we observed no effect on MMP levels of thrombolysis, and drug treatment after admission was relatively uniform, the vast majority of patients receiving anti-platelet therapy, beta-blocker, renin–angiotensin system inhibition and lipid-lowering therapy.
In summary, plasma concentrations of MMP-9, and in particular TIMP-1, are associated with the extent of LV remodelling after AMI. Higher plasma TIMP-1 is associated powerfully with greater risk of adverse prognosis. In this context, TIMP-1 has comparable prognostic power with that of N-BNP; consideration of both TIMP-1 and NTproBNP provides additive prognostic information. Plasma TIMP-1 represents a novel marker of adverse prognosis after AMI in man.
Acknowledgments
Funding
This research was supported by the British Heart Foundation.
Footnotes
Conflict of interest: none declared.
References
- 1.Bolognese L, Cerisano G. Early predictors of left ventricular remodeling after acute myocardial infarction. Am Heart J. 1999;138:S79–S83. doi: 10.1016/s0002-8703(99)70325-x. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 4.Spinale FG, Coker ML, Krombach SR, Mukherjee R, Hallak H, Houck WV, Clair MJ, Kribbs SB, Johnson LL, Peterson JT, Zile MR. Matrix metalloproteinase inhibition during the development of congestive heart failure: effects on left ventricular dimensions and function. Circ Res. 1999;85:364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 5.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nube O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med. 1999;5:1135–1142. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 7.Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Sutherland P, Wilson PW, Vasan RS. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–2856. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 8.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 9.Creemers EE, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2003;284:H364–H371. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 10.Timms PM, Wright A, Maxwell P, Campbell S, Dawnay AB, Srikanthan V. Plasma tissue inhibitor of metalloproteinase-1 levels are elevated in essential hypertension and related to left ventricular hypertrophy. Am J Hypertens. 2002;15:269–272. doi: 10.1016/s0895-7061(01)02316-0. [DOI] [PubMed] [Google Scholar]
- 11.Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Wilson PW, Vasan RS. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham Heart Study. Eur Heart J. 2004;25:1509–1516. doi: 10.1016/j.ehj.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Hirohata S, Kusachi S, Murakami M, Murakami T, Sano I, Watanabe T, Komatsubara I, Kondo J, Tsuji T. Time-dependent alterations of serum matrix metalloproteinase-1 and metalloproteinase-1 tissue inhibitor after successful reperfusion of acute myocardial infarction. Heart. 1997;78:278–284. doi: 10.1136/hrt.78.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubos E, Schnabel R, Rupprecht HJ, Bickel C, Messow CM, Prigge S, Cambien F, Tiret L, Munzel T, Blankenberg S. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: results from the AtheroGene Study. Eur Heart J. 2006;27:150–156. doi: 10.1093/eurheartj/ehi582. [DOI] [PubMed] [Google Scholar]
- 14.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J. 2006;151:1101. doi: 10.1016/j.ahj.2006.02.029. e1–e8. [DOI] [PubMed] [Google Scholar]
- 15.West MJ, Nestel PJ, Kirby AC, Schnabel R, Sullivan D, Simes RJ, Pollicino C, Lubos E, Munzel TF, White HD, Tonkin AM, Bickel C, Tiret L, Blankenberg S. The value of N-terminal fragment of brain natriuretic peptide and tissue inhibitor of metalloproteinase-1 levels as predictors of cardiovascular outcome in the LIPID study. Eur Heart J. 2008;29:923–931. doi: 10.1093/eurheartj/ehn007. [DOI] [PubMed] [Google Scholar]
- 16.Squire IB, Evans J, Ng LL, Loftus IM, Thompson MM. Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail. 2004;10:328–333. doi: 10.1016/j.cardfail.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–1027. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 18.Wagner DR, Delagardelle C, Ernens I, Rouy D, Vaillant M, Beissel J. Matrix metalloproteinase-9 is a marker of heart failure after acute myocardial infarction. J Card Fail. 2006;12:66–72. doi: 10.1016/j.cardfail.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, Squire IB. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J. 2007;28:711–718. doi: 10.1093/eurheartj/ehm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omland T, Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, Sundsfjord JA, Dickstein K. Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996;93:1963–1969. doi: 10.1161/01.cir.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 21.Arakawa N, Nakamura M, Aoki H, Hiramori K. Plasma brain natriuretic peptide concentrations predict survival after acute myocardial infarction. J Am Coll Cardiol. 1996;27:1656–1661. doi: 10.1016/0735-1097(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 22.Richards AM, Nicholls MG, Yandle TG, Frampton C, Espiner EA, Turner JG, Buttimore RC, Lainchbury JG, Elliott JM, Ikram H, Crozier IG, Smyth DW. Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998;97:1921–1929. doi: 10.1161/01.cir.97.19.1921. [DOI] [PubMed] [Google Scholar]
- 23.Omland T, Persson A, Ng L, O'Brien R, Karlsson T, Herlitz J, Hartford M, Caidahl K. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 24.Squire IB, O'Brien RJ, Demme B, Davies JE, Ng LL. N-terminal pro-atrial natriuretic peptide (N-ANP) and N-terminal pro-B-type natriuretic peptide (N-BNP) in the prediction of death and heart failure in unselected patients following acute myocardial infarction. Clin Sci (Lond) 2004;107:309–316. doi: 10.1042/CS20040087. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien RJ, Squire IB, Demme B, Davies JE, Ng LL. Pre-discharge, but not admission, levels of NT-proBNP predict adverse prognosis following acute LVF. Eur J Heart Fail. 2003;5:499–506. doi: 10.1016/s1388-9842(03)00098-9. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JT, Hallak H, Johnson L, Li H, O'Brien PM, Sliskovic DR, Bocan TM, Coker ML, Etoh T, Spinale FG. Matrix metalloproteinase inhibition attenuates left ventricular remodeling and dysfunction in a rat model of progressive heart failure. Circulation. 2001;103:2303–2309. doi: 10.1161/01.cir.103.18.2303. [DOI] [PubMed] [Google Scholar]
- 27.Lindsey ML, Gannon J, Aikawa M, Schoen FJ, Rabkin E, Lopresti-Morrow L, Crawford J, Black S, Libby P, Mitchell PG, Lee RT. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation. 2002;105:753–758. doi: 10.1161/hc0602.103674. [DOI] [PubMed] [Google Scholar]
- 28.Matsunaga T, Abe N, Kameda K, Hagii J, Fujita N, Onodera H, Kamata T, Ishizaka H, Hanada H, Osanai T, Okumura K. Circulating level of gelatinase activity predicts ventricular remodeling in patients with acute myocardial infarction. Int J Cardiol. 2005;105:203–208. doi: 10.1016/j.ijcard.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 30.Lindsay MM, Maxwell P, Dunn FG. TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension. 2002;40:136–141. doi: 10.1161/01.hyp.0000024573.17293.23. [DOI] [PubMed] [Google Scholar]
- 31.Laviades C, Varo N, Fernandez J, Mayor G, Gil MJ, Monreal I, Diez J. Abnormalities of the extracellular degradation of collagen type I in essential hypertension. Circulation. 1998;98:535–540. doi: 10.1161/01.cir.98.6.535. [DOI] [PubMed] [Google Scholar]
- 32.Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, Aupetit JF, Aumont MC, Galinier M, Eicher JC, Cohen-Solal A, Juilliere Y. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733–1739. doi: 10.1016/j.jacc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 33.Schwartzkopff B, Fassbach M, Pelzer B, Brehm M, Strauer BE. Elevated serum markers of collagen degradation in patients with mild to moderate dilated cardiomyopathy. Eur J Heart Fail. 2002;4:439–434. doi: 10.1016/s1388-9842(02)00092-2. [DOI] [PubMed] [Google Scholar]
- 34.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 35.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, III, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]