In our study directly comparing in vivo human imaging of the right coronary artery at 7 T and 3 T in young healthy volunteers, quantitative parameters related to image quality attained at 7 T equal or surpass those at 3 T.
Abstract
Purpose:
To objectively compare quantitative parameters related to image quality attained at coronary magnetic resonance (MR) angiography of the right coronary artery (RCA) performed at 7 T and 3 T.
Materials and Methods:
Institutional review board approval was obtained, and volunteers provided signed informed consent. Ten healthy adult volunteers (mean age ± standard deviation, 25 years ± 4; seven men, three women) underwent navigator-gated three-dimensional MR angiography of the RCA at 7 T and 3 T. For 7 T, a custom-built quadrature radiofrequency transmit-receive surface coil was used. At 3 T, a commercial body radiofrequency transmit coil and a cardiac coil array for signal reception were used. Segmented k-space gradient-echo imaging with spectrally selective adiabatic fat suppression was performed, and imaging parameters were similar at both field strengths. Contrast-to-noise ratio between blood and epicardial fat; signal-to-noise ratio of the blood pool; RCA vessel sharpness, diameter, and length; and navigator efficiency were quantified at both field strengths and compared by using a Mann-Whitney U test.
Results:
The contrast-to-noise ratio between blood and epicardial fat was significantly improved at 7 T when compared with that at 3 T (87 ± 34 versus 52 ± 13; P = .01). Signal-to-noise ratio of the blood pool was increased at 7 T (109 ± 47 versus 67 ± 19; P = .02). Vessel sharpness obtained at 7 T was also higher (58% ± 9 versus 50% ± 5; P = .04). At the same time, RCA vessel diameter and length and navigator efficiency showed no significant field strength–dependent difference.
Conclusion:
In our quantitative and qualitative study comparing in vivo human imaging of the RCA at 7 T and 3 T in young healthy volunteers, parameters related to image quality attained at 7 T equal or surpass those from 3 T.
© RSNA, 2010
Introduction
Currently, relatively few (approximately 40) 7-T magnetic resonance (MR) imaging systems are available for human use, and most of them are situated in research centers. High-field-strength cardiac MR imaging initially was thought to be problematic due to magnetic field inhomogeneity and specific absorption rate constraints. Furthermore, contemporary commercial 7-T units are not routinely equipped with body radiofrequency (RF) transmit coils or surface RF receive coils. Despite these major challenges, a number of research groups already have demonstrated the feasibility of cardiac imaging at 7 T and beyond (1–5). Initial attempts focusing on coronary artery imaging at 7 T showed that these barriers can be removed successfully, and initial in vivo human images were promising (6). Although these early 7-T studies were conducted by using single-channel RF transmit-receive coil architecture, recent advances in surface coil technology seem particularly promising. However, although an improvement in image quality may be expected at higher magnetic field strength (7), there have been no reports, to our knowledge, on cardiac MR imaging studies in which investigators directly and objectively compare parameters related to image quality at 7 T with those obtained at lower field strength. Therefore, the purpose of our study was to objectively compare quantitative parameters related to image quality attained at coronary MR angiography of the right coronary artery (RCA) performed at 7 T and 3 T.
Materials and Methods
Our study was approved by our institutional review board, and all volunteers provided signed informed consent. Three-dimensional (3D) MR angiography of the right coronary system was performed in 10 healthy young adult volunteers (mean age ± standard deviation, 25 years ± 4; seven men, three women) who underwent imaging at 7 T and 3 T (Achieva; Philips Healthcare, Best, the Netherlands) in a prospective study design. For practical reasons, 7-T imaging always occurred before 3-T imaging. Coronary MR angiography was performed with prospective navigator technology and vector electrocardiographic (ECG) triggering (8). All volunteers underwent imaging in a head-first, supine position. None of the volunteers received nitroglycerin before MR imaging. The mean interval between the two examinations was 8 weeks ± 5.
Imaging at 7 T
A quadrature transmit-receive surface coil consisting of two overlapping loops (13-cm diameter each) was constructed in-house (Fig 1). The coil size is larger than described previously (4,6) to improve volumetric coverage. First, non–ECG-triggered scout images in coronal, transverse, and sagittal orientations were acquired to plan subsequent images and to localize the two-dimensional selective navigator. At 7 T, the navigator was placed at the lung-heart interface because of the limited sensitive volume of the surface coil. Second, ECG-triggered, breath-hold multisection transverse cine scout imaging was performed for both the determination of the period of minimal coronary motion (trigger delay) and the volume targeting of the 3D stack in parallel with the middiastolic RCA. Finally, volume-targeted coronary MR angiography was performed by using a 3D segmented k-space gradient-echo imaging technique (parameters are in Table 1) combined with a spectrally selective adiabatic inversion-recovery pulse (inversion time, 200 msec) for fat saturation. First-order local volume shimming at the anatomic level of the RCA was performed in all cases.
Figure 1:

Custom-built quadrature RF transmit-receive surface coil consisting of two 13-cm elements used for our study at 7 T.
Table 1.
Imaging Parameters at 7-T and 3-T Coronary MR Angiography
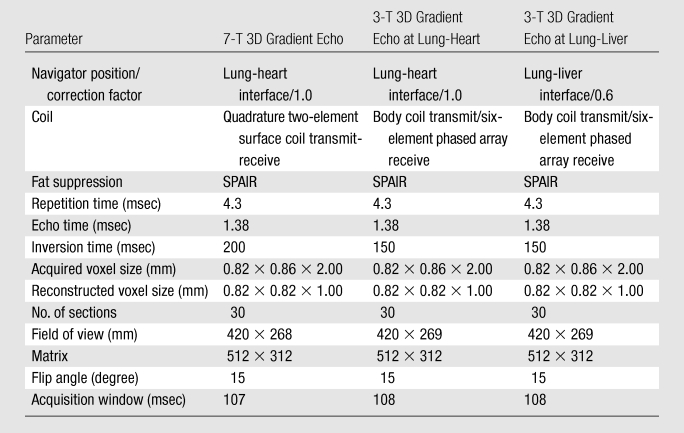
Note.—SPAIR = spectrally selective adiabatic inversion recovery.
Imaging at 3 T
On the 3-T system, the body coil was used for RF transmission, with a commercial six-element cardiac coil array for signal reception. Scout imaging included free-breathing, retrospectively ECG-gated, two-dimensional cine balanced fast gradient-echo imaging in a horizontal long-axis view (four-chamber) to determine the trigger delay. Furthermore, ECG-triggered free-breathing navigator-gated and corrected 3D gradient-echo whole-heart imaging was performed for the anatomic localization of the RCA. After scout imaging, two coronary imaging sequences were performed at 3 T with different navigator localization in random order: (a) with navigator localization at the lung-heart interface (navigator at heart) and (b) with navigator localization at the lung-liver interface (navigator at liver). Both 3-T coronary imaging sequences consisted of a 3D segmented k-space gradient-echo technique with spectrally selective adiabatic inversion recovery (inversion time, 150 msec) for fat saturation. The coronary MR angiography imaging parameters were similar (Table 1) at both field strengths to support a fair quantitative comparison.
Data Analysis
Images were processed and analyzed by using the Soap-Bubble tool (9). Both a visual qualitative description and a direct quantitative comparison between 7-T and 3-T images were performed. All data analyses were performed by one physician (S.G.C.v.E., with 4 years of experience in cardiac MR imaging) with the supervision of a senior researcher (M.S., with 18 years of experience in cardiac MR imaging).
The following parameters were measured: contrast-to-noise ratio (CNR) between the blood pool and the epicardial fat, signal-to-noise ratio (SNR) of the blood pool, RCA vessel sharpness and diameter of the first 4 cm, and visible vessel length. The CNR was defined as the difference in signal intensity between a manually placed region of interest (ROI) in the aortic root (mean ROI area, 1.80 cm2 ± 0.60) near the offshoot of the RCA, and that of an ROI placed in the epicardial fat adjacent to the proximal RCA (mean ROI area, 0.90 cm2 ± 0.61), divided by the standard deviation of the background signal from an ROI positioned anterior to the chest wall (noise; mean ROI area, 9.95 cm2 ± 3.98). The SNR was calculated for the blood signal intensity in the described ROI localized in the aortic root. The average signal intensity from this ROI was divided by the noise. Vessel sharpness was measured by using signal intensity gradients perpendicular to the 3D course of the RCA and was calculated for the proximal 4 cm of the RCA (9). The RCA vessel length was measured manually, and both vessel sharpness and diameter for the proximal 4 cm of the RCA were calculated automatically by the software.
Statistical Analysis
Data are presented as mean ± standard deviation. Comparisons were made between the results obtained at 7 T and 3 T and between the results obtained with the different navigator positions at 3 T. For comparisons, a nonparametric Mann-Whitney U test was used. P < .05 was considered to indicate a statistically significant difference.
Results
Coronary MR angiography was performed successfully in all volunteers at both field strengths. At 7 T, one study volunteer complained about vertigo while the table was moving. All quantitative findings are listed in Table 2. Total time in the magnet was on average 30 minutes at 7 T and 20 minutes at 3 T.
Table 2.
Quantitative Results Obtained from 10 Healthy Volunteers
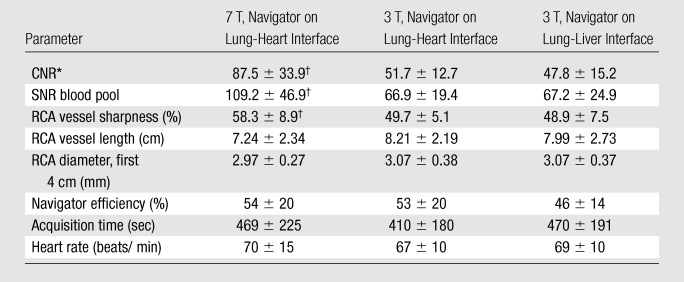
Note.—Data are mean ± standard deviation.
CNR between the blood pool and epicardial fat surrounding the proximal RCA.
Significantly different from 3 T (P < .05).
Figures 2–4 illustrate example coronary MR angiography reformations obtained at 7 T and at 3 T. All images show high signal intensity of the coronary artery lumen, while that of the surrounding epicardial fat is suppressed. At 7 T, suppression of the epicardial fat appears visually improved when compared with that at 3 T (Fig 2). Consistent with these findings, quantitative CNR between the blood pool and epicardial fat was significantly improved at 7 T (7 T versus 3 T navigator at heart, P = .013; 7 T versus 3 T navigator at liver, P = .009). Visually, the contrast between the myocardium and the blood pool in the left ventricle is rather shallow at 7 T as shown in Figures 3 and 4. When compared with that at 3 T, the SNR of the blood pool measured on the 7 T images was 60% higher (7 T versus 3 T navigator at heart, P = .023; 7 T versus 3 T navigator at liver, P = .027). Improved delineation of the RCA at 7 T is visible in Figure 3, with good depiction of RCA branches and distal segments. Consistent with these findings, objective vessel sharpness analysis demonstrated improved quantitative vessel conspicuity at 7 T (versus 3 T navigator at heart, P = .038; versus 3 T navigator at liver; P = .031). Even though constraints related to the B1 field and coil sensitivity adversely affected the signal more distant to the surface transmit-receive coil at 7 T (Figs 2–4), there was no significant difference in vessel length (7 T versus 3 T navigator at heart, P = .233; 7 T versus 3 T navigator at liver, P = .414) or vessel diameter (7 T versus 3 T navigator at heart, P = .653; 7 T versus 3 T navigator at liver, P = .567) measurements among the images from the different field strengths. Navigator efficiency (7 T versus 3 T navigator at heart, P = .970; 7 T versus 3 T navigator at liver, P = .272) and total data acquisition time (7 T versus 3 T navigator at heart, P = .520; 7 T versus 3 T navigator at liver, P = .821) also were not field strength dependent. No significant difference in CNR between the blood pool and epicardial fat, SNR of the blood pool, RCA vessel sharpness, vessel length, vessel diameter, navigator efficiency, or acquisition time was found between the 3-T images acquired with the different navigator localizations.
Figure 2a:
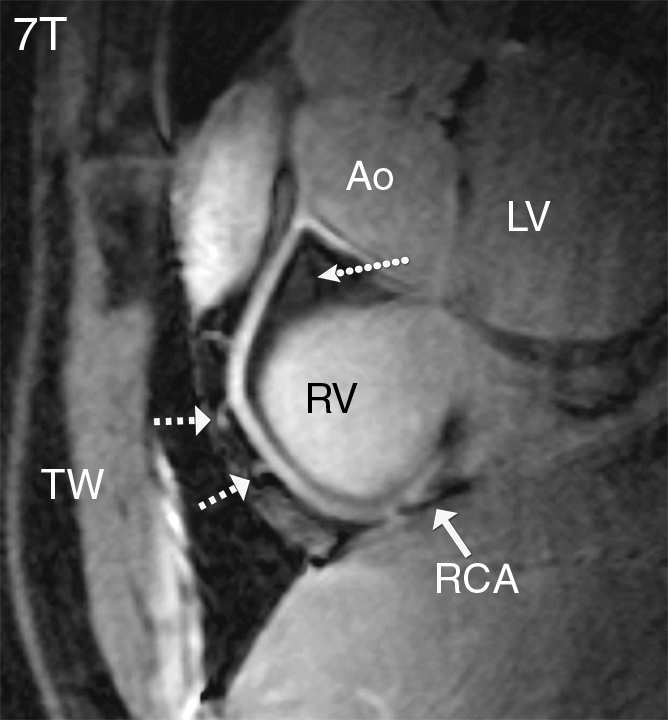
Coronary MR angiograms of the RCA obtained at (a) 7 T and (b) 3 T in the same healthy 18-year-old man (double oblique volume targeted plane parallel to the RCA). Improved suppression of the epicardial fat (long dotted arrow) with high contrast between the blood and epicardial fat is visible at 7 T. At both field strengths, a number of small branching vessels are depicted (short dashed arrows). Also at 7 T, a long portion of the RCA is visible (solid arrow = distal part of RCA). Ao = aortic root, LV = left ventricle, RV = right ventricle, TW = thoracic wall.
Figure 4a:
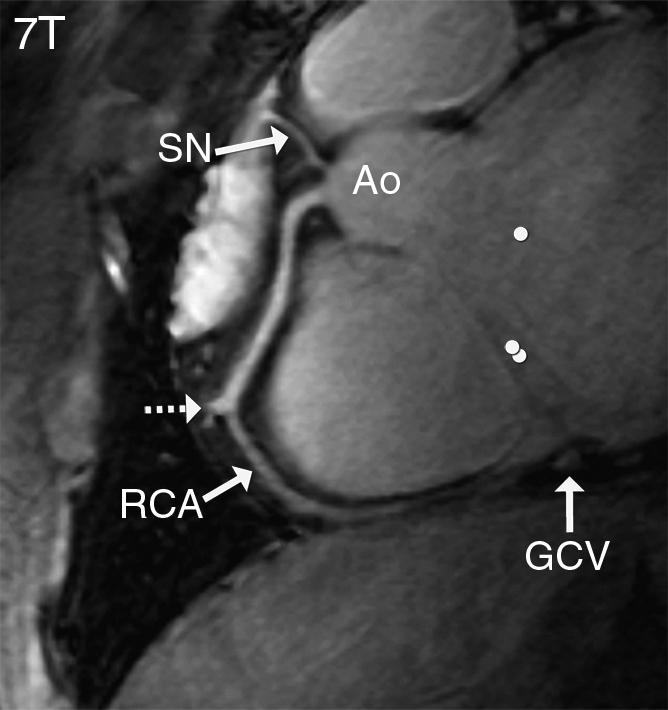
Sinoatrial nodal artery (SN) and a proximal branch of the RCA (image obtained in a double oblique volume targeted plane parallel to the RCA) in a healthy 28-year-old man. (a) At 7 T, there is not much difference in signal intensity between the blood pool (●) and the myocardium (●●). (b) At 3 T, this contrast is slightly improved. On b, the great cardiac vein (GCV) can be identified easily, but this structure is less visible on a, likely because of shortened T2* at 7 T, as well as limited surface coil RF penetration. Ao = aortic root, dashed arrow = RCA side branch.
Figure 3a:
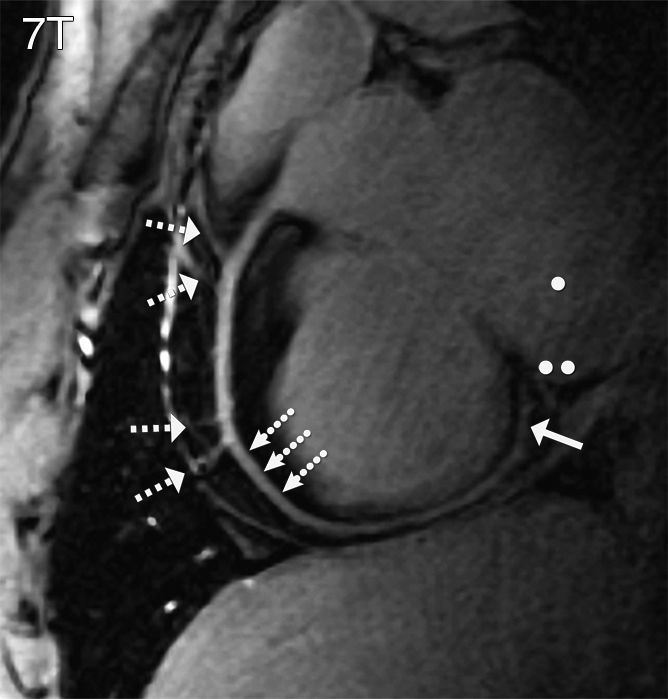
RCA images (double oblique volume targeted plane parallel to the RCA) in a healthy 26-year-old man display a high visual vessel definition (dotted arrows) in (a) the 7 T image compared with that at (b) 3 T. At 7 T, contrast is limited between the myocardium (●●) and the blood pool (●). Multiple RCA side branches (dashed arrows) and distal parts of the RCA (solid arrow) are depicted clearly at both field strengths.
Figure 2b:
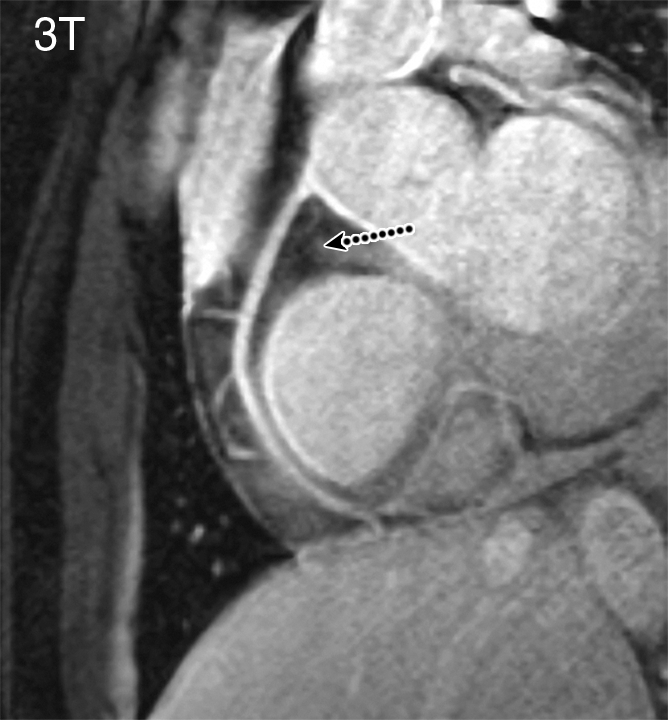
Coronary MR angiograms of the RCA obtained at (a) 7 T and (b) 3 T in the same healthy 18-year-old man (double oblique volume targeted plane parallel to the RCA). Improved suppression of the epicardial fat (long dotted arrow) with high contrast between the blood and epicardial fat is visible at 7 T. At both field strengths, a number of small branching vessels are depicted (short dashed arrows). Also at 7 T, a long portion of the RCA is visible (solid arrow = distal part of RCA). Ao = aortic root, LV = left ventricle, RV = right ventricle, TW = thoracic wall.
Figure 3b:
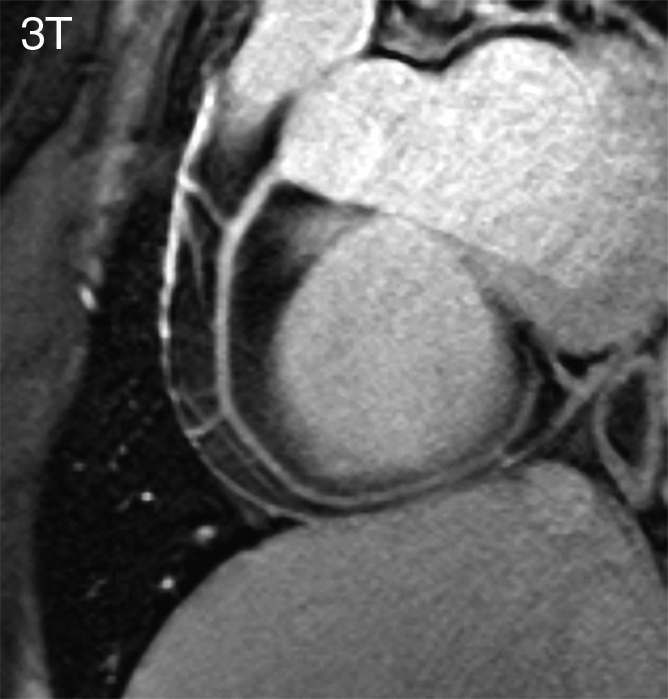
RCA images (double oblique volume targeted plane parallel to the RCA) in a healthy 26-year-old man display a high visual vessel definition (dotted arrows) in (a) the 7 T image compared with that at (b) 3 T. At 7 T, contrast is limited between the myocardium (●●) and the blood pool (●). Multiple RCA side branches (dashed arrows) and distal parts of the RCA (solid arrow) are depicted clearly at both field strengths.
Figure 4b:
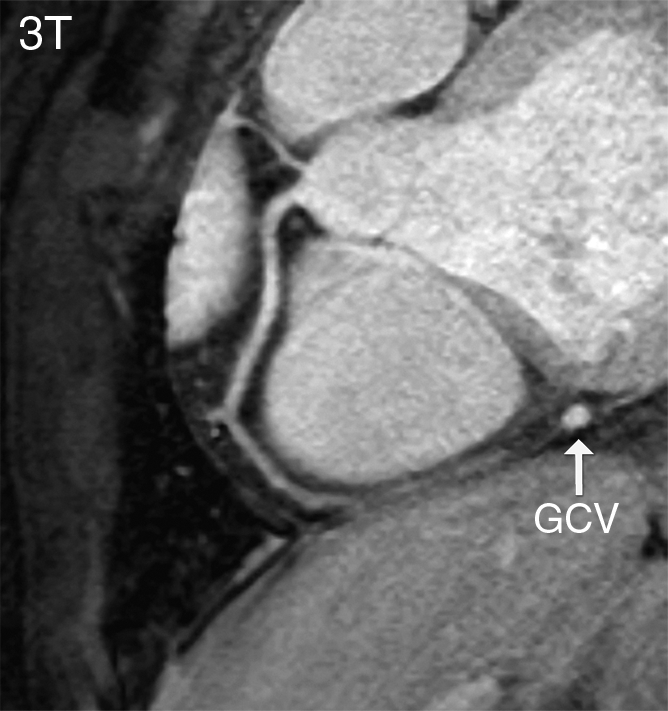
Sinoatrial nodal artery (SN) and a proximal branch of the RCA (image obtained in a double oblique volume targeted plane parallel to the RCA) in a healthy 28-year-old man. (a) At 7 T, there is not much difference in signal intensity between the blood pool (●) and the myocardium (●●). (b) At 3 T, this contrast is slightly improved. On b, the great cardiac vein (GCV) can be identified easily, but this structure is less visible on a, likely because of shortened T2* at 7 T, as well as limited surface coil RF penetration. Ao = aortic root, dashed arrow = RCA side branch.
Discussion
In our study comparing 7-T and 3-T RCA coronary MR angiography in young healthy volunteers, we found improved CNR between the blood pool and the epicardial fat, enhanced SNR of the blood pool, and increased vessel sharpness at 7 T. These findings may have implications, since vessel conspicuity and well-defined borders of the coronary arteries support improved identification of significant coronary artery stenoses.
The results of a multicenter study indicate that multidetector computed tomography (CT) is highly promising and superior to MR angiography for the noninvasive evaluation of significant proximal coronary stenoses (10). However, the most recent data from a coronary MR angiography multicenter trial (11) demonstrate substantial progress, and further improvement in SNR diagnostic quality approaching that of CT angiography may be expected soon; therefore, the development of new MR imaging methodology at higher magnetic field strength is of particular interest.
Approximately a twofold increase in SNR is predicted at 7 T when compared with 3 T (7,12), whereas a 60% SNR improvement was found in our study, which may be explained with the prolonged T1 at 7 T and the depth penetration of the RF transmit-receive coil, which was clearly inferior to that at 3 T. However, a significant 60% increase in SNR with a simple coil design is encouraging and emphasizes the need for further developments in coil technology. Recent advances in RF transmit arrays (2,3) are a step in this direction. Findings from previous studies in which investigators compared 1.5-T and 3-T coronary MR angiography (13–15) clearly demonstrated that the expected 100% improvement in SNR could not be obtained. At 7 T, the lack of commercially available RF coils and the only recent availability of 7 T for whole-body human use constitute additional 7-T–specific limitations. Despite these limitations, the reported 60% improvement in SNR, when going from 3 T to 7 T, is consistent with that reported for direct 1.5 T versus 3 T.
Our findings of increased vessel sharpness at 7 T suggest that motion suppression works effectively at 7 T, since vessel sharpness depends on the performance of ECG triggering and the respiratory navigator. Two similar imaging sequences with different navigator localizations were performed at 3 T to exclude the influence of navigator localization on the quantitative parameters related to image quality. Consistent with earlier findings (16), no navigator-dependent quantitative differences were observed.
The results from the RCA vessel length and diameter measurements were similar for both field strengths. This finding suggests that coverage and RF penetration of the surface coil at 7 T may not be limiting factors for visualization of the RCA.
The situation for the left coronary system is different. On the one hand, the penetration depth of the current transmit-receive coil is limited, so parts of the left coronary system may not be visualized easily. On the other hand, an enhancement of the contrast between myocardium and the blood pool is mandatory for visualizing the left coronary system. At 3 T, such contrast enhancement has been obtained with adiabatic T2 preparation (17) or with the combination of extracellular contrast agents and inversion recovery (18). However, at 7 T, specific absorption rate constraints preclude the use of the T2 preparation, and alternative 7-T–specific solutions with or without contrast agents remain to be explored. Finally, although a 60% increase in SNR was obtained at 7 T, we have not used this gain for increased spatial resolution. However, the objective of our work was a direct, quantitative, objective comparison with 3 T.
In conclusion, although substantial challenges are associated with 7-T cardiac MR imaging, a number of them have been addressed successfully. In our study directly comparing in vivo human imaging of the RCA at 7 T and 3 T in young healthy volunteers, quantitative parameters related to image quality attained at 7 T equal or surpass those from 3 T. Our results clearly warrant further evaluation in patients with coronary artery disease to assess the potential of our 7-T approach for the visualization of luminal RCA disease. Future work will concentrate on refinements in coil technology and contrast generation to support concomitant imaging of the left coronary system.
Advance in Knowledge.
At 7 T, the right coronary artery (RCA) is visualized with better-defined vessel borders and higher signal-to-noise ratio (SNR) when compared with 3-T acquisitions with identical acquisition duration.
Implications for Patient Care.
Improved contrast between blood and epicardial fat, better coronary vessel sharpness, and increased blood signal intensity of the RCA are obtained at 7 T than at 3 T.
Better-defined vessel borders and high SNR are potentially promising for the more accurate noninvasive identification of clinically important luminal coronary artery disease without ionizing radiation.
Received March 23, 2010; revision requested May 21; final revision received June 15; accepted June 18; final version accepted June 23.
Supported by the Dutch Organization for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek [NWO]).
Funding: This research was supported by the National Institutes of Health (grant RO1 HL084186).
Authors stated no financial relationship to disclose.
Abbreviations:
- CNR
- contrast-to-noise ratio
- ECG
- electrocardiography
- RCA
- right coronary artery
- RF
- radiofrequency
- ROI
- region of interest
- SNR
- signal-to-noise ratio
- 3D
- three-dimensional
References
- 1.Maderwald S, Orzada S, Schafer LC, et al. 7T human in vivo cardiac imaging with an 8-channel transmit/receive array [abstr]. In: Proceedings of the Seventeenth Meeting of the International Society for Magnetic Resonance in Medicine. Berkeley, Calif: International Society for Magnetic Resonance in Medicine, 2009; 821 [Google Scholar]
- 2.Snyder CJ, DelaBarre L, Metzger GJ, et al. Initial results of cardiac imaging at 7 Tesla. Magn Reson Med 2009;61(3):517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaughan JT, Snyder CJ, DelaBarre LJ, et al. Whole-body imaging at 7T: preliminary results. Magn Reson Med 2009;61(1):244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Versluis MJ, Tsekos N, Smith NB, Webb AG. Simple RF design for human functional and morphological cardiac imaging at 7 tesla. J Magn Reson 2009;200(1):161–166 [DOI] [PubMed] [Google Scholar]
- 5.Frauenrath T, Hezel F, Heinrichs U, et al. Feasibility of cardiac gating free of interference with electro-magnetic fields at 1.5 Tesla, 3.0 Tesla and 7.0 Tesla using an MR-stethoscope. Invest Radiol 2009;44(9):539–547 [DOI] [PubMed] [Google Scholar]
- 6.van Elderen SG, Versluis MJ, Webb AG, et al. Initial results on in vivo human coronary MR angiography at 7 T. Magn Reson Med 2009;62(6):1379–1384 [DOI] [PubMed] [Google Scholar]
- 7.Wen H, Denison TJ, Singerman RW, Balaban RS. The intrinsic signal-to-noise ratio in human cardiac imaging at 1.5, 3, and 4 T. J Magn Reson 1997;125(1):65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer SE, Wickline SA, Lorenz CH. Novel real-time R-wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance acquisitions. Magn Reson Med 1999;42(2):361–370 [DOI] [PubMed] [Google Scholar]
- 9.Etienne A, Botnar RM, Van Muiswinkel AM, Boesiger P, Manning WJ, Stuber M. “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med 2002;48(4):658–666 [DOI] [PubMed] [Google Scholar]
- 10.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359(22):2324–2336 [DOI] [PubMed] [Google Scholar]
- 11.Kato S, Kitagawa K, Ishida N, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial. J Am Coll Cardiol (in press). [DOI] [PubMed] [Google Scholar]
- 12.Vaughan JT, Garwood M, Collins CM, et al. 7T vs. 4T: RF power, homogeneity, and signal-to-noise comparison in head images. Magn Reson Med 2001;46(1):24–30 [DOI] [PubMed] [Google Scholar]
- 13.Bi X, Deshpande V, Simonetti O, Laub G, Li D. Three-dimensional breathhold SSFP coronary MRA: a comparison between 1.5T and 3.0T. J Magn Reson Imaging 2005;22(2):206–212 [DOI] [PubMed] [Google Scholar]
- 14.Sommer T, Hackenbroch M, Hofer U, et al. Coronary MR angiography at 3.0 T versus that at 1.5 T: initial results in patients suspected of having coronary artery disease. Radiology 2005;234(3):718–725 [DOI] [PubMed] [Google Scholar]
- 15.Yang PC, Nguyen P, Shimakawa A, et al. Spiral magnetic resonance coronary angiography: direct comparison of 1.5 Tesla vs. 3 Tesla. J Cardiovasc Magn Reson 2004;6(4):877–884 [DOI] [PubMed] [Google Scholar]
- 16.Stuber M, Botnar RM, Danias PG, Kissinger KV, Manning WJ. Submillimeter three-dimensional coronary MR angiography with real-time navigator correction: comparison of navigator locations. Radiology 1999;212(2):579–587 [DOI] [PubMed] [Google Scholar]
- 17.Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation 1999;99(24):3139–3148 [DOI] [PubMed] [Google Scholar]
- 18.Bi X, Li D. Coronary arteries at 3.0 T: contrast-enhanced magnetization-prepared three-dimensional breathhold MR angiography. J Magn Reson Imaging 2005;21(2):133–139 [DOI] [PubMed] [Google Scholar]


