The expression of serotonin may be associated with stromal fibrosis, and fibrosis, in turn, can cause stenosis of the pancreatic duct.
Abstract
Purpose:
To determine if serotonin production by pancreatic endocrine neoplasms is associated with the pancreatic duct stenosis seen in patients with stenosis that is out of proportion to the size of the tumors seen on computed tomographic images.
Materials and Methods:
Institutional approval was obtained for this HIPAA-compliant study. Informed consent was waived. Clinical and radiologic findings in six patients were reviewed. Gross and histologic findings in the resected pancreata were also assessed. Formalin-fixed paraffin-embedded tumor sections were immunolabeled with antibodies to serotonin. Tissue microarrays constructed from 47 pancreatic endocrine neoplasms from the institutional tissue bank served as controls. Histologic and serotonin immunoreactivity findings were compared between the two groups. The Fisher exact test was used to compare serotonin immunoreactivity.
Results:
Only one of the six study patients had a large dominant tumor (4 cm in the pancreatic head). All others were 2.5 cm or smaller. Four of the six pancreatic endocrine neoplasms with associated pancreatic duct stricture had prominent stromal fibrosis. Serotonin immunoreactivity was present in five (83%) patients, and this labeling was strong and diffuse in the four patients with prominent fibrosis. By contrast, stromal fibrosis was minimal in the nonimmunoreactive case. Only three (6%) of the 47 control pancreatic endocrine neoplasms were immunoreactive for serotonin (P < .01, Fisher exact test).
Conclusion:
These data suggest that serotonin produced by pancreatic endocrine neoplasms may be associated with local fibrosis and stenosis of the pancreatic duct. Clinicians should be aware that small pancreatic endocrine neoplasms can produce pancreatic duct stenosis resulting in ductal dilatation and/or upstream pancreatic atrophy out of proportion to the size of the tumor.
© RSNA, 2010
Introduction
Pancreatic endocrine neoplasms are relatively rare, with an incidence rate of approximating five cases per one million person-years (1). Some pancreatic endocrine neoplasms release hormones into the blood stream that cause clinical syndromes, whereas others are nonsyndromic and present as a mass lesion (2,3). Computed tomography (CT) is emerging as the reference standard for evaluating pancreatic masses, and pancreatic endocrine neoplasms typically produce hyperenhanced well-demarcated lesions that are best seen on arterial phase images (4). Necrosis and cystic change can be seen in larger lesions, but the main pancreatic duct is usually not dilated. Cystic change can be seen in small endocrine neoplasms of the pancreas as well. We recently observed a series of small pancreatic endocrine neoplasms associated with pancreatic duct stenosis and accompanied by marked dilatation of the main pancreatic duct and/or marked atrophy of upstream pancreas, and we sought to determine the pathophysiologic mechanism for this finding.
Serotonin production by endocrine neoplasms of the small intestine has been associated with significant fibrosis and even stenosis of the bowel (4–7), leading us to speculate that serotonin associated fibrosis may be producing pancreatic duct stenosis and upstream pancreatic duct dilatation/pancreatic atrophy in the six cases we observed. Serotonin is produced by a small number of pancreatic endocrine neoplasms (8), and the expression of serotonin can be demonstrated by immunolabeling the neoplastic tissue (9).
In this study, we reviewed the histology and serotonin immunoreactivity of pancreatic endocrine neoplasms with or without associated pancreatic duct stenosis, as defined by ductal dilatation and/or pancreatic atrophy found at CT, to determine if serotonin produced by pancreatic endocrine neoplasms is associated with pancreatic duct stenosis.
Materials and Methods
We obtained appropriate institutional approval for this Health Insurance Portability and Accountability Act–compliant study. The requirement for informed consent was waived. CT scans of patients with a pancreatic endocrine neoplasm that were performed between June 1, 2007, and February 27, 2009, were reviewed. We observed a series of patients with a distinctive finding on CT images of the pancreas. Six patients with pancreatic endocrine neoplasms whose preoperative imaging revealed pancreatic duct stenosis with marked pancreatic duct dilatation and/or marked atrophy of upstream pancreatic atrophy were included in our study. These patients each had a focal narrowing of the pancreatic duct associated with a mass lesion of the pancreatic parenchyma. The clinical records and gross findings for these six patients were reviewed. The size and location of the pancreatic mass did not seem to adequately account for the degree of narrowing of the duct. In two patients, the mass was extremely subtle. A tissue microarray containing 47 pancreatic endocrine neoplasms was used as a control. The clinical records and available CT reports for the control cases were reviewed for any pancreatic duct stenosis or dilatation or pancreatic atrophy. Histologic findings and immunoreactivity to serotonin were evaluated for both study and control cases.
CT Technique
CT images were obtained by using 64–detector row CT scanners (Somatom Sensation or Definition; Siemens Medical Solutions, Malvern, Pa). A detector collimation setting of 64 × 0.6 mm was used. The data were reconstructed at 0.75-mm section thickness at 0.5-mm intervals for multiplanar reformation and three-dimensional imaging. For diagnostic reading, 3- or 5-mm section thickness and 3- or 5-mm reconstruction intervals were used. We acquired dual-phase images 25 seconds after the start of intravenous contrast material injection for the arterial phase and 55–60 seconds after injection for the early portal venous phase. Iohexol (100–120 mL, Omnipaque 350; GE Healthcare, Princeton, NJ) was injected through a peripheral venous line (3–4 mL/sec). For dedicated evaluation of the pancreas, after fasting for at least 2–3 hours, each patient typically ingested 500–750 mL of water 20–30 minutes prior to the CT study and an additional 250 mL of water immediately prior to imaging.
Image Analysis
CT studies were reviewed by two radiologists (S.S.S. and S.K., with 25 and 12 years experience with abdominal CT imaging, respectively). Each radiologist reviewed the images independently, and then a consensus was reached. The images were reviewed on a picture archiving and communication system (Advanced Visualization, version 5.30.7.26; Emageon, Birmingham, Ala). Three-dimensional images were created on a workstation (Multi-Modality Workplace; Siemens Medical Solutions, Malvern, Pa) by using software (InSpace; Siemens Medical Solutions) by one (S.K.) of the two radiologists.
Based on the consensus findings, measurements were performed by one (S.K.) of the two radiologists by using the picture archiving and communication system. The following findings were recorded: (a) size of the pancreatic mass when the mass was visualized, (b) degree of contrast enhancement (based on measurement of CT attenuation in regions of interest in the mass and in the normal pancreatic parenchyma on arterial and venous phase images, each mass was classified as hyperenhanced or hypoenhanced relative to normal pancreatic parenchyma), (c) the largest diameter of the pancreatic duct, and (d) the presence of pancreatic atrophy. The pancreatic duct was considered to be dilated when its diameter was equal to or greater than 3 mm. Severity of dilatation was graded as follows: 3–6 mm was considered minimal dilatation; 6–9 mm, moderate; and greater than 9 mm, marked. The degree of atrophy was expressed as marked when the anteroposterior diameter of the pancreatic parenchyma (excluding the pancreatic duct) was smaller than 9 mm, moderate when it was at least 9 mm but smaller than 12 mm, and minimal when it was at least 12 mm but smaller than 15 mm.
Histopathologic Correlation
The six patients included in our study had their neoplasms surgically resected, either by pancreatoduodenectomy (Whipple procedure) or distal pancreatectomy at our hospital between 2007 and 2009. The gross descriptions and histologic slides of the surgically resected pancreata from these six patients were reviewed by two pathologists (C.S. and R.H.H., with 5 and more than 20 years experience, respectively). Each case was reviewed by both pathologists, and a consensus was reached.
Forty-seven pancreatic endocrine neoplasm cases in our tissue bank were used as controls. These controls were resected either by pancreatodudoenectomy (Whipple procedure) or distal pancreatectomy at our hospital between 1993 and 2001. The images were not available for these controls. However, reports for imaging performed at our institution (n = 22) or clinical records describing imaging findings for patients who underwent imaging at outside institutions (n = 22) were available for review for 44 of the 47 controls. Formalin-fixed paraffin-embedded blocks of tumor from these controls were used to create a tissue microarray.
Immunohistochemical studies were performed on samples from the six patients with pancreatic duct stenosis and on sections from the tissue microarray containing the 47 control pancreatic endocrine neoplasms. Sections (5 µm) were prepared from formalin-fixed paraffin-embedded tissue blocks and immunolabeled for serotonin (Zymed, San Francisco, Calif) at a 1:200 dilution by using an automated stainer (BenchMark XT; Ventana Medical Systems, Tucson, Ariz) after steam-mediated antigen retrieval (10).
Statistical Analysis
Serotonin immunoreactivity of the six pancreatic endocrine neoplasms with associated pancreatic duct stenosis and of the 47 control pancreatic endocrine neoplasms was compared by using the Fisher exact test.
Results
Clinical Presentations and Radiologic Findings
CT images in four patients demonstrated that the severity of pancreatic duct dilatation and/or pancreatic atrophy was out of proportion to the size of the tumor (Table 1). Specifically, only two of the tumors (patients 2 and 3) measured 2.5 cm or larger. The other four tumors were all 1.5 cm or smaller, with no discrete pancreatic mass identified on CT images in patients 5 and 6. Four of the neoplasms arose in the head of the gland; one, in the body; and one, in the tail. None of the six patients had carcinoid syndrome or other hormone-related syndromes other than diabetes mellitus.
Table 1.
Demographics, Clinical Symptoms, and CT Findings of Study Cases
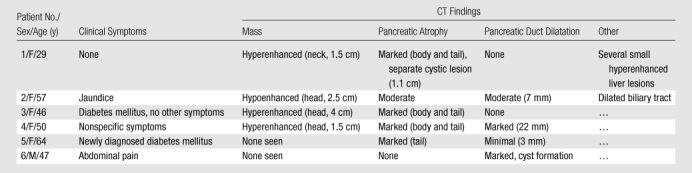
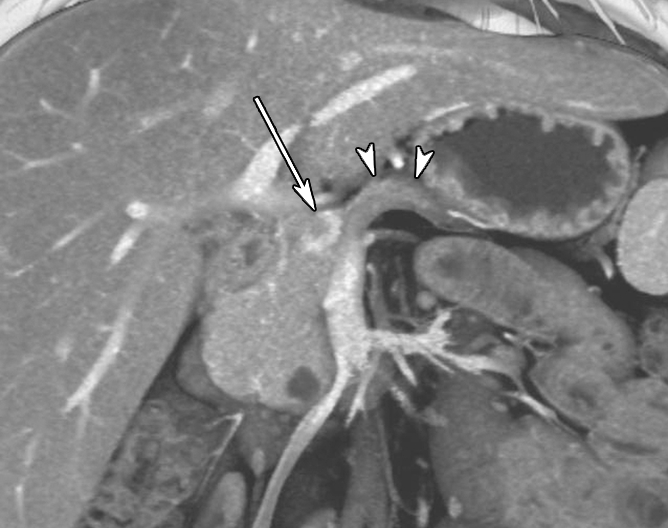
The demographics, clinical symptoms, and CT findings of the six patients are summarized in Table 1. Patient 1 was a 29-year-old woman who was incidentally found to have a hyperenhanced 1.5-cm mass in the pancreatic neck, along with marked atrophy of the body and tail (Fig 1). In addition, a separate cystic lesion (1.1 cm) was seen in the inferior aspect of the head of the gland. Several small hyperenhanced lesions were also seen in the liver. Patient 2, a 57-year-old woman, presented with obstructive jaundice. CT revealed a pancreatic head mass (2.5 cm), which was slightly hypoenhanced relative to normal pancreatic parenchyma, with dilatation of both the common bile and pancreatic ducts. Patient 3, a 46-year-woman who had a long-standing history of insulin-dependent diabetes mellitus, was also incidentally discovered to have a hyperenhanced mass (4 cm) in the pancreatic head. There was also marked atrophy of the pancreatic tail. Patient 4 was a 50-year-old woman who presented with abdominal discomfort. CT revealed a hyperenhanced pancreatic head mass (1.5 cm), marked atrophy of the body and tail, and a markedly dilated pancreatic duct measuring up to 22 mm (Fig 2). Patient 5, a 64-year-old woman, presented with newly diagnosed insulin-dependent diabetes mellitus. CT revealed marked atrophy of the pancreatic tail accompanied by minimal dilatation of the pancreatic duct in the pancreatic tail (Fig 3). There was no discrete mass seen in the pancreas. Patient 6 was a 47-year-old man who presented with abdominal pain. CT revealed a markedly dilated pancreatic duct; however, the tumor was not seen on CT images, and this case was preoperatively misinterpreted as being a cystic neoplasm.
Figure 1a:
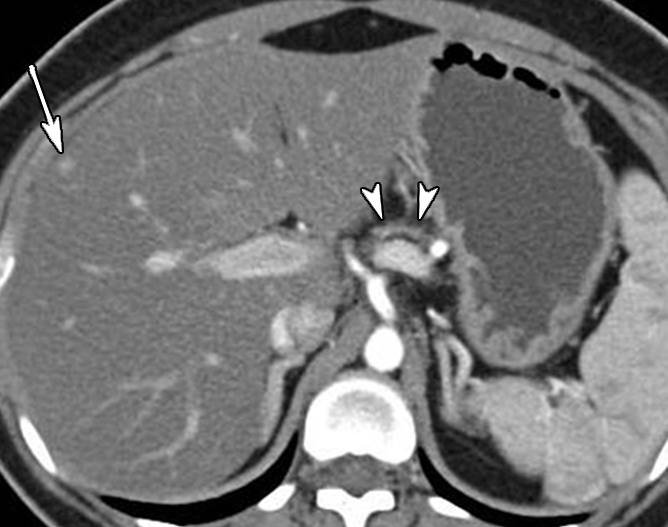
Contrast material–enhanced CT images in patient 1 (29-year-old woman). (a) Axial arterial phase image shows marked atrophy of the pancreatic body (arrowheads) (anteroposterior dimension = 5 mm). Arrow = small enhanced liver mass (metastasis). (b) Venous phase anterior volume-rendered image shows enhanced mass (arrow) with upstream pancreatic atrophy (arrowheads). A separate small cystic lesion (pathologic finding: oligocystic serous cystadenoma) is incidentally shown in the inferior aspect of the pancreatic head.
Figure 2:
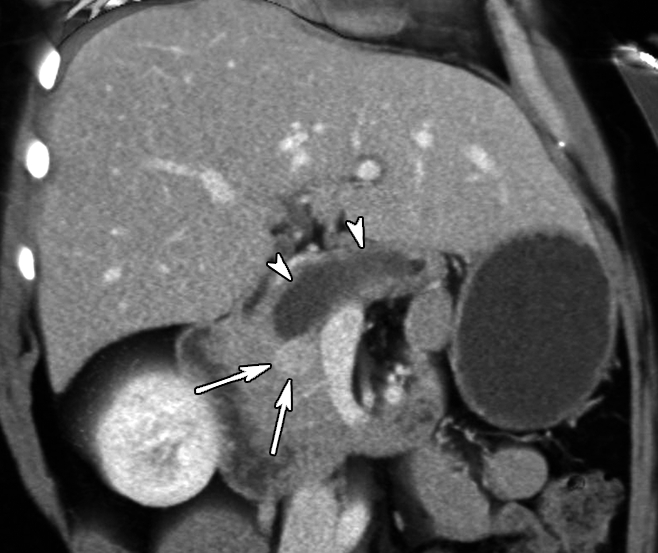
Venous phase contrast-enhanced oblique anterior volume-rendered CT image in patient 4 (57-year-old woman) shows enhanced mass (arrows) with marked main pancreatic duct dilatation (arrowheads).
Figure 3a:
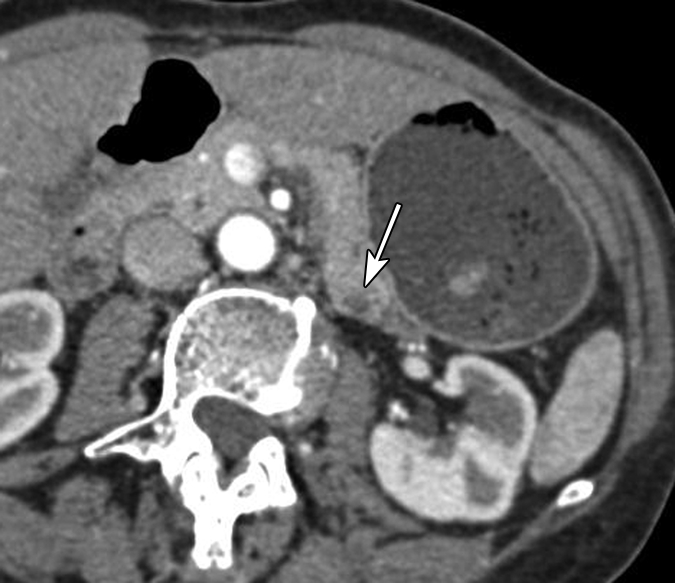
(a) Oblique axial arterial phase, (b) axial venous phase, and (c) oblique coronal CT images in patient 5 (64-year-old woman) show minimally dilated main pancreatic duct (arrowheads) in a pancreatic tail with an atrophic truncated appearance. No discrete mass is shown. There is a subtle hypoenhanced area (arrows) in the pancreatic tail.
Figure 1b:
Contrast material–enhanced CT images in patient 1 (29-year-old woman). (a) Axial arterial phase image shows marked atrophy of the pancreatic body (arrowheads) (anteroposterior dimension = 5 mm). Arrow = small enhanced liver mass (metastasis). (b) Venous phase anterior volume-rendered image shows enhanced mass (arrow) with upstream pancreatic atrophy (arrowheads). A separate small cystic lesion (pathologic finding: oligocystic serous cystadenoma) is incidentally shown in the inferior aspect of the pancreatic head.
Figure 3b:
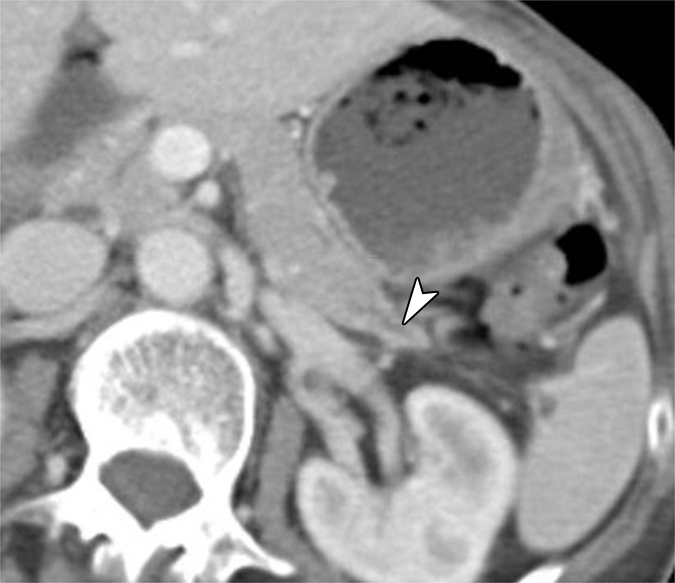
(a) Oblique axial arterial phase, (b) axial venous phase, and (c) oblique coronal CT images in patient 5 (64-year-old woman) show minimally dilated main pancreatic duct (arrowheads) in a pancreatic tail with an atrophic truncated appearance. No discrete mass is shown. There is a subtle hypoenhanced area (arrows) in the pancreatic tail.
Figure 3c:
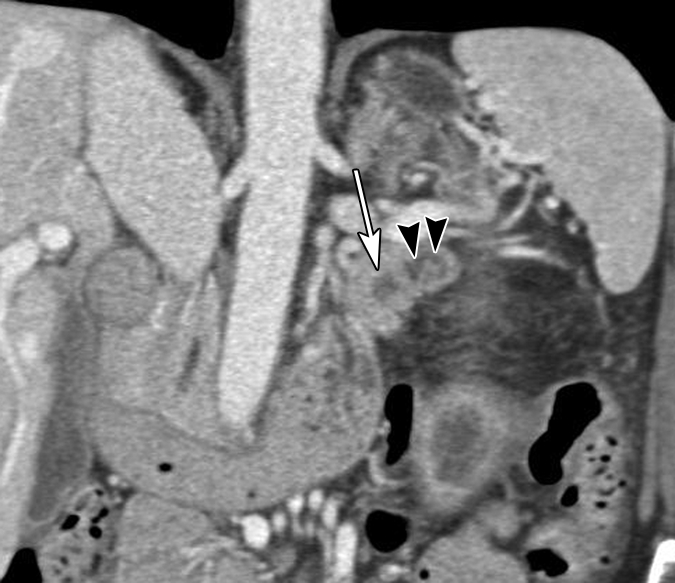
(a) Oblique axial arterial phase, (b) axial venous phase, and (c) oblique coronal CT images in patient 5 (64-year-old woman) show minimally dilated main pancreatic duct (arrowheads) in a pancreatic tail with an atrophic truncated appearance. No discrete mass is shown. There is a subtle hypoenhanced area (arrows) in the pancreatic tail.
Forty-seven well-differentiated pancreatic endocrine neoplasms were used as controls. Either imaging reports or clinical records were available for review from 44 of these 47 controls. Among these 44 controls, one patient had an ampullary adenoma with high-grade dysplasia diagnosed at endoscopy. The specimen obtained after Whipple resection revealed an incidental 1.1-cm endocrine tumor in the head of the pancreas. However, it was not certain if this patient had dilatation of the pancreatic duct. There were four controls whose pancreatic duct was reported to be dilated or obstructed: One had CT images obtained at our institution that showed a small neoplasm associated with upstream pancreatic duct dilatation; one had endoscopic retrograde cholangiopancreatography at an outside institution, which revealed a tumor at the ampulla blocking the pancreatic duct, and a surgical specimen that revealed a 2.3-cm neuroendocroine tumor of the pancreas; one had a 7-cm mass in the head of the pancreas, with a mass-effect stenosis of the main pancreatic duct and upstream dilation at endoscopic retrograde cholangiopancreatography; and one had minimal pancreatic duct dilatation just upstream of a 6-cm mass in the head of the pancreas. In addition, there were two control patients whose pancreatic duct was not reported to be dilated but in whom there was atrophy of the upstream pancreas. One had a 3-cm mass in the head of the pancreas, with atrophy of the body and tail, and the other had a 2.2-cm mass in the neck of the pancreas, with abrupt transition to completely fatty replaced tissue with only minimal residual pancreatic tissue visualized in upstream body and tail.
Gross and Histologic Findings
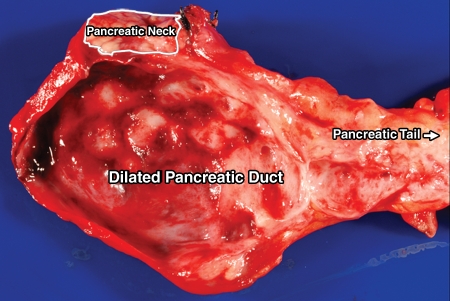
Grossly, all six study pancreatic endocrine neoplasms were solitary and solid masses. While pancreatic endocrine neoplasms in patients 2 and 4 had a sharp border, the tumors in patients 1, 3, 5, and 6 were ill defined (Table 2). The infiltrative growth pattern in these four cases was unusual for pancreatic endocrine neoplasms, which made it difficult to differentiate the neoplasms grossly from a ductal adenocarcinoma. None of the neoplasms extended into the peripancreatic soft tissue. In all the cases, the main pancreatic duct was narrowed adjacent to the neoplasm. The pancreatic duct upstream from the tumor was markedly dilated in three cases (patients 2, 4, and 6). Patient 6 had a small barely visible tumor in the body of the pancreas; however, a stricture was present in the adjacent pancreatic duct, and the pancreatic duct upstream from the tumor was markedly dilated (Fig 4).
Table 2.
Pathologic Findings in Study Cases
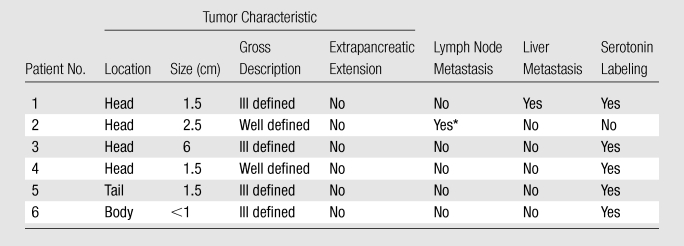
Metastatic pancreatic endocrine neoplasm in peripancreatic lymph nodes.
Figure 4:
Image of the gross specimen from patient 6 (47-year-old man) shows a marked dilated pancreatic duct that mimicks a cystic lesion. Mass is indistinct.
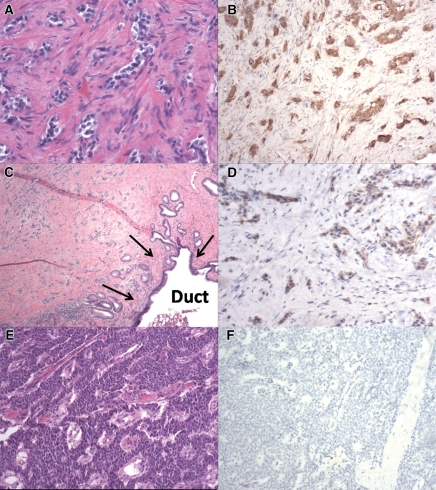
Microscopically, all six neoplasms were well differentiated and composed of small to medium cells with moderate to abundant eosinophilic to amphophilic finely granular cytoplasm (Fig 5). The neoplastic cells were arranged in nests or trabeculae. Four cases (patients 1, 3, 4, and 6) were associated with striking stromal fibrosis (Fig 5, A and C). In these cases, the fibrosis extended into the adjacent periductal soft tissue, causing stenosis of the main pancreatic duct (Fig 5, C). Representative H-E–stained photomicrographs from patients 3 and 6 are shown in Figure 5, A and C. Most of the sample from patient 5 had only moderate stromal fibrosis; however, as the neoplasm approached the main pancreatic duct, the fibrosis became more prominent. The neoplasm in patient 2 was extremely hypercellular with minimal stromal fibrosis (Fig 5, E), but this neoplasm was immediately adjacent to the main pancreatic duct. The pancreatic duct obstruction seen in this case appeared to be caused by a mass effect rather than by the fibrosis seen in the other cases.
Figure 5:
Photomicrographs in patients 3 (A, B), 6 (C, D), and 2 (E, F). A, Sample shows well-differentiated endocrine neoplasm with marked stromal fibrosis. (Hematoxylin-eosin [H-E] stain.) B, D, Samples show strong serotonin immunoreactivity of neoplastic cells, with no labeling in stromal cells. C, Sample from downstream dilatation of the pancreatic duct shows well-differentiated endocrine neoplasm with marked stromal fibrosis extending into a main pancreatic duct (arrows). (H-E stain.) E, Sample shows a hypercellular well-differentiated endocrine neoplasm with minimal stromal fibrosis. (H-E stain.) F, Sample shows negative serotonin immunoreactivity of neoplastic cells. (Original magnification in A, ×200; in B, D, E, F, ×100; in C, ×20.)
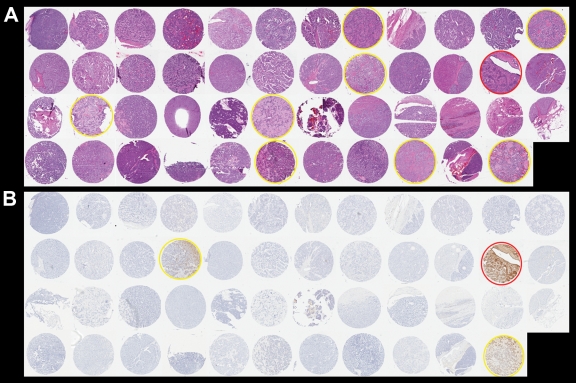
H-E staining of the 47 control pancreatic endocrine neoplasms is shown in Figure 6, A. Thirty-eight of these control neoplasms were hypercellular with minimal stromal fibrosis. Eight had some stromal hyalinization but no extensive fibrosis. Only one of the 47 controls displayed prominent stromal fibrosis with angulated nests of neoplastic cells infiltrating the fibrotic stroma (circled in red in Figure 6, A; Fig 7a). This morphologic appearance is similar to that observed in patients 1, 3, 4, and 6.
Figure 6:
Photomicrographs in 47 controls. A, Tissue microarray shows 47 well-differentiated endocrine neoplasms. Yellow circle = hyalinized stroma, red circle = prominent stromal fibrosis. (H-E stain.) B, Immunohistochemical labeling shows partial serotonin immunoreactivity (yellow circle) in two cases and strong serotonin immunoreactivity (red circle) in one case. (Original magnification in A and B, ×200.)
Figure 7a:
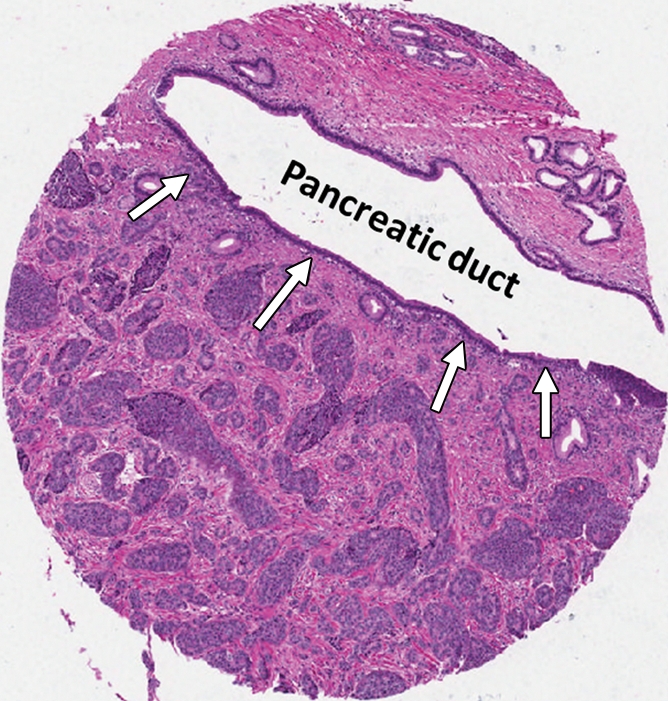
Photomicrographs of the control case with marked stromal fibrosis at higher magnification. (a) Well-differentiated endocrine neoplasm with prominent stromal fibrosis. Note marked stromal fibrosis extending into pancreatic duct (arrows). (H-E stain.) (b) Serotonin labeling shows strong immunoreactivity. (Original magnification in a and b, ×200.)
Figure 7b:
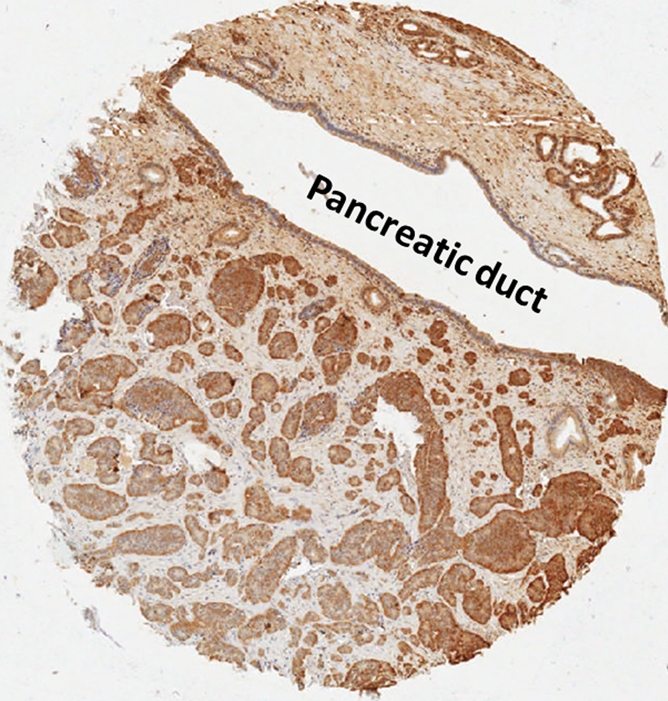
Photomicrographs of the control case with marked stromal fibrosis at higher magnification. (a) Well-differentiated endocrine neoplasm with prominent stromal fibrosis. Note marked stromal fibrosis extending into pancreatic duct (arrows). (H-E stain.) (b) Serotonin labeling shows strong immunoreactivity. (Original magnification in a and b, ×200.)
Immunohistochemical Labeling for Serotonin
Immunohistochemical labeling for serotonin was positive in five (83%) of the six study cases, four of which demonstrated strong and diffuse labeling. Interestingly, the four cases with strong and diffuse labeling also showed marked stromal fibrosis (Fig 5, B and D). In contrast, the case with negative serotonin findings (patient 2) was extremely hypercellular with minimal fibrosis (Fig 5, E and F). The neoplasm in patient 5 had patchy serotonin labeling, and it was the case with moderate but not prominent fibrosis. Therefore, it appears that the degree of serotonin expression correlates with the severity of fibrosis seen in the tumor.
The 47 control pancreatic endocrine neoplasms were also analyzed for serotonin immunoreactivity; three of them (6%) had positive results (Fig 6, B). Two of the three had only focal serotonin labeling. Interestingly, the control sample with strong and diffuse serotonin labeling also demonstrated a pattern of prominent stromal fibrosis. The H-E staining and serotonin labeling of this control are shown in Figure 7 at higher magnification. This neoplasm was small but adjacent to a pancreatic duct. The extension of fibrosis into the periductal tissue was apparent on the H-E–stained sample (Fig 7a), which could have caused the upstream pancreatic duct dilatation seen on CT images in this control patient. None of the control pancreatic endocrine neoplasms without positive serotonin immunoreactivity demonstrated stromal fibrosis.
Compared with the control group, we observed that serotonin expression was significantly more commonly seen in the pancreatic endocrine neoplasms associated with pancreatic duct obstruction (P < .01, Fisher exact test). These findings suggest that local serotonin production by the neoplasm may be associated with stromal fibrosis that, in turn, leads to pancreatic duct stenosis.
Discussion
In our study, we demonstrated that four pancreatic endocrine neoplasms were associated with prominent stromal fibrosis and that this fibrosis can extend to involve the main pancreatic duct, producing ductal stenosis and upstream dilatation of the duct and/or upstream pancreatic atrophy. A strong and diffuse immunoreactivity for serotonin was seen in all cases with this pattern of stromal fibrosis. In contrast, strong serotonin expression was usually not observed in neoplasms with minimal fibrosis.
Pancreatic endocrine neoplasms that express serotonin (ie, carcinoid tumors) account for only a small portion of pancreatic endocrine neoplasms. Compared with other well-differentiated endocrine neoplasms of the pancreas, pancreatic carcinoid tumors are associated with a higher rate of malignant behavior (9). The poor prognosis with these neoplasms may, in part, be because they are usually diagnosed at an advanced stage, after distant metastases have occurred and the patient has developed the carcinoid syndrome. Early detection and diagnosis can improve survival. The problem is that the mass lesion is often asymptomatic and indistinct at early stages. In this study, we demonstrated that other associated radiologic changes can potentially aid in the early diagnosis of pancreatic carcinoid tumors. For example, the neoplasm in two cases (patients 5 and 6) was subtle or inapparent on CT images; only the marked dilation of the upstream pancreatic duct or marked atrophy of the upstream pancreas was visible. These findings prompted exploratory surgery, and these tumors were resected at an early, presumably curable, stage. Therefore, marked pancreatic duct dilatation, pancreatic atrophy, and other secondary changes can suggest the presence of a pancreatic endocrine neoplasm even when a distinct mass is not seen.
Isolated reports of an association of pancreatic carcinoid tumor with dilation of the pancreatic duct have been described before. Nagai et al (11) reported a case of pancreatic carcinoid tumor with obstructive pancreatitis. Endoscopic retrograde pancreatography revealed main pancreatic duct stenosis and dilation of the upstream pancreatic duct. Similar radiologic findings were also observed by Takaji et al (12); they reported that three of four cases showed main pancreatic duct dilation upstream of the tumor. Nagai et al (11) suggested that the pancreatic carcinoid tumor obstructing the pancreatic duct might have arisen from argentaffin cells located in the main pancreatic duct. However, no clear relationship between pancreatic endocrine neoplasms and pancreatic duct stenosis has been described.
Wilson et al (8) performed immunohistochemical studies for serotonin in two cases of primary carcinoid tumors of the pancreas with the carcinoid syndrome. They found that the percentage of neoplastic cells that were immunoreactive for serotonin was 20% in one case and 60% in the other, and prominent stromal fibrosis was noted in one of the two cases. In our study, we observed that serotonin immunoreactivity correlated with the degree of stromal fibrosis and that stromal fibrosis, in turn, may explain the duct stenosis observed. Serotonin has been previously implicated in fibrosis. Carcinoid tumors of the midgut, in which serotonin is the predominant hormone secreted by the neoplastic cells, are almost always associated with extensive fibrosis (13). In addition, serotonin has been shown to stimulate fibroblast mitosis in cell cultures (14). However, other researchers have observed that methysergide, a nonselective serotonin antagonist, can also occasionally cause retroperitoneal fibrosis in patients with the carcinoid syndrome (15). Recently, serotonin has been shown to play a crucial role in the progression of liver fibrosis by enhancing production of transforming growth factor β by selective activation of the 5-HT2A serotonin receptors (16). In our study, we clearly demonstrated the correlation of cellular serotonin expression by the neoplastic cells with the development of fibrosis. Although we cannot rule out the possibility that this is an unrelated phenomenon, our findings still suggest that serotonin can be used as a marker for the development of stromal fibrosis in the pancreas.
Pancreatic duct dilatation and/or pancreatic atrophy were present in tumors with minimal stromal fibrosis in one of the six study cases and some of the control cases. However, the secondary changes seen in most of these cases can be explained by the tumor size or location. To confirm that serotonin is the molecule responsible for stromal fibrosis seen in some pancreatic endocrine neoplasms, more studies, especially at a cellular level, will be needed.
One of the limitations of our study was that we were unable to obtain the actual CT images for many of the control cases. We addressed this by reviewing the available CT reports and clinical records; however, we cannot rule out the possibility that subtle features may not have been completely reported. In addition, one of the study cases was referred from another institution, so CT images of this patient were not available for us to review.
In summary, a small portion of pancreatic endocrine neoplasms produce serotonin. The expression of serotonin may be associated with stromal fibrosis, and fibrosis, in turn, can cause stenosis of the pancreatic duct. In clinical practice, imaging findings of pancreatic duct stenosis that is out of proportion to the size of the causative hyperenhanced mass or that does not have an associated distinct mass are suspicious for a pancreatic neoplasm. Pancreatic atrophy and pancreatic ductal stenosis should arouse suspicion of a pancreatic endocrine neoplasm.
Advances in Knowledge.
Some pancreatic endocrine neoplasms are associated with prominent stromal fibrosis, which can extend to involve the main pancreatic duct, producing ductal stenosis and upstream dilatation of the duct and/or upstream pancreatic atrophy.
Strong and diffuse immunoreactivity for serotonin was seen in cases with prominent stromal fibrosis, but strong serotonin expression was not observed in neoplasms with minimal fibrosis.
The expression of serotonin may be associated with stromal fibrosis in some pancreatic endocrine neoplasms, which, in turn, causes pancreatic duct stenosis.
Implication for Patient Care.
Clinicians should be aware that small pancreatic endocrine neoplasms can produce upstream ductal dilatation and/or pancreatic atrophy out of proportion to the size of the tumor.
Received January 6, 2010; revision requested February 22; revision received March 16; accepted April 6; final version accepted May 19.
Supported by Michael Rolfe Pancreatic Cancer Foundation.
Funding: This research was supported by a National Cancer Institute Specialized Programs of Research Excellence grant in gastrointestinal cancer (grant CA62924).
Authors stated no financial relationship to disclose.
Abbreviation:
- H-E
- hematoxylin-eosin
References
- 1.Jensen RT. Pancreatic neuroendocrine tumors: overview of recent advances and diagnosis. J Gastrointest Surg 2006;10(3):324–326 [DOI] [PubMed] [Google Scholar]
- 2.Capelli P, Martignoni G, Pedica F, et al. Endocrine neoplasms of the pancreas: pathologic and genetic features. Arch Pathol Lab Med 2009;133(3):350–364 [DOI] [PubMed] [Google Scholar]
- 3.Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci 2004;1014:13–27 [DOI] [PubMed] [Google Scholar]
- 4.Horton KM, Hruban RH, Yeo C, Fishman EK. Multi-detector row CT of pancreatic islet cell tumors. RadioGraphics 2006;26(2):453–464 [DOI] [PubMed] [Google Scholar]
- 5.Druce M, Rockall A, Grossman AB. Fibrosis and carcinoid syndrome: from causation to future therapy. Nat Rev Endocrinol 2009;5(5):276–283 [DOI] [PubMed] [Google Scholar]
- 6.Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD. Current status of gastrointestinal carcinoids. Gastroenterology 2005;128(6):1717–1751 [DOI] [PubMed] [Google Scholar]
- 7.Lesurtel M, Soll C, Graf R, Clavien PA. Role of serotonin in the hepato-gastrointestinal tract: an old molecule for new perspectives. Cell Mol Life Sci 2008;65(6):940–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson RW, Gal AA, Cohen C, DeRose PB, Millikan WJ. Serotonin immunoreactivity in pancreatic endocrine neoplasms (carcinoid tumors). Mod Pathol 1991;4(6):727–732 [PubMed] [Google Scholar]
- 9.Mao C, el Attar A, Domenico DR, Kim K, Howard JM. Carcinoid tumors of the pancreas: status report based on two cases and review of the world’s literature. Int J Pancreatol 1998;23(2):153–164 [DOI] [PubMed] [Google Scholar]
- 10.Leong ASY, Cooper K, Leong FJWM. Manual of diagnostic antibodies for immunohistology. 2nd ed Cambridge, England: Cambridge University Press, 2002 [Google Scholar]
- 11.Nagai E, Yamaguchi K, Hashimoto H, Sakurai T. Carcinoid tumor of the pancreas with obstructive pancreatitis. Am J Gastroenterol 1992;87(3):361–364 [PubMed] [Google Scholar]
- 12.Takaji R, Matsumoto S, Mori H, et al. Carcinoid tumors of the pancreas: dynamic CT and MRI features with pathological correlation. Abdom Imaging 2009;34(6):753–758 [DOI] [PubMed] [Google Scholar]
- 13.Warner TF, O’Reilly G, Lee GA. Mesenteric occlusive lesion and ileal carcinoids. Cancer 1979;44(2):758–762 [DOI] [PubMed] [Google Scholar]
- 14.Lee SL, Wang WW, Lanzillo JJ, Fanburg BL. Serotonin produces both hyperplasia and hypertrophy of bovine pulmonary artery smooth muscle cells in culture. Am J Physiol 1994;266(1 pt 1):L46–L52 [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Saibil F, Kassim O, McKee J. Retroperitoneal fibrosis caused by carcinoid tumour. Q J Med 1985;56(219):367–375 [PubMed] [Google Scholar]
- 16.Li T, Weng SG, Leng XS, et al. Effects of 5-hydroxytamine and its antagonists on hepatic stellate cells. Hepatobiliary Pancreat Dis Int 2006;5(1):96–100 [PubMed] [Google Scholar]