Abstract
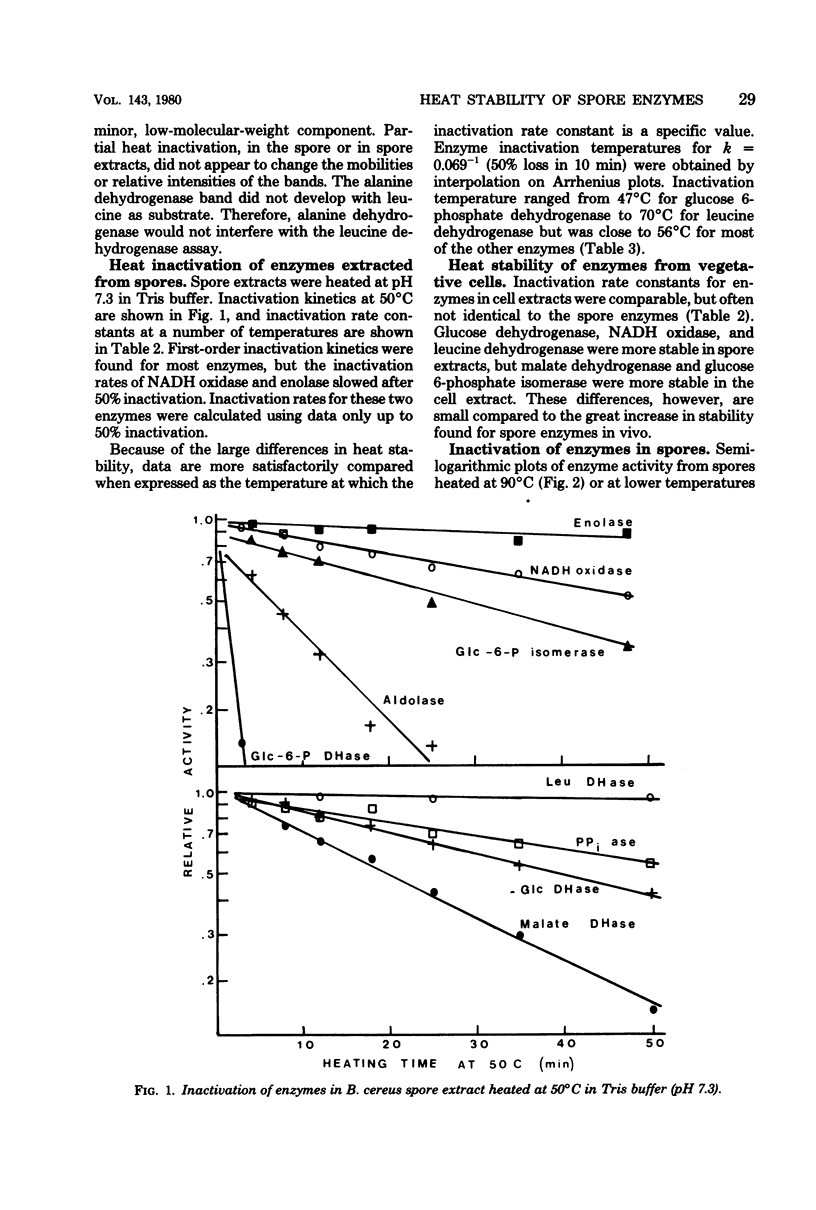
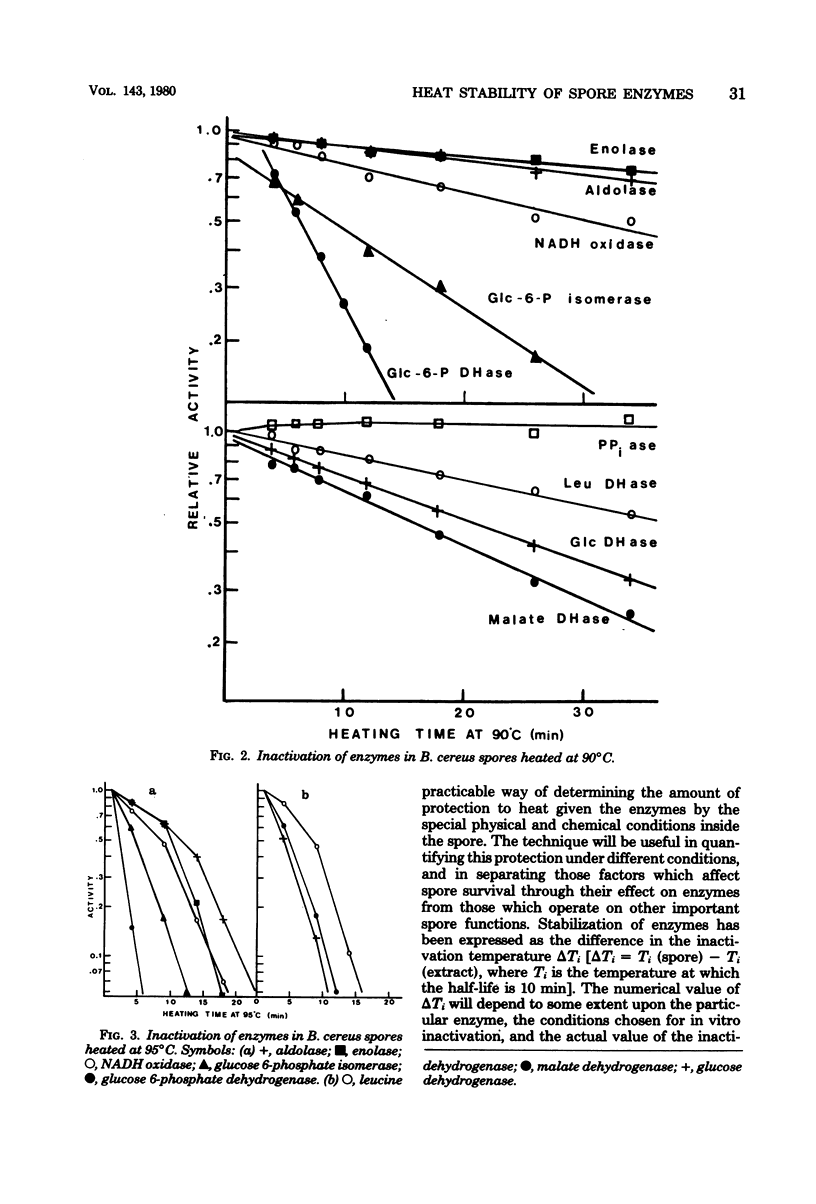
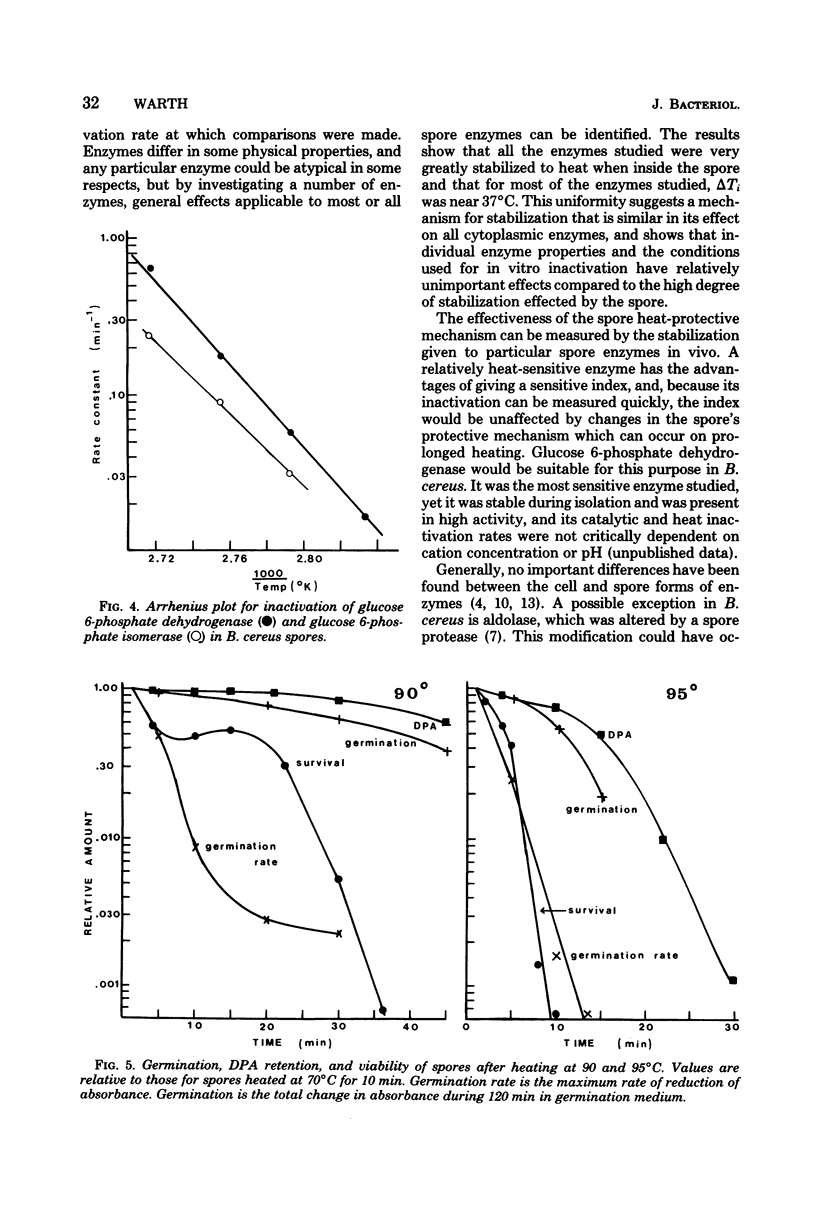
Inactivation rates for nine enzymes extracted from Bacillus cereus spores were measured at several temperatures, and the temperature at which each enzyme had a half-life of 10 min (inactivation temperature) was determined. Inactivation temperatures ranged from 47 degrees C for glucose 6-phosphate dehydrogenase to 70 degrees C for leucine dehydrogenase, showing that spore enzymes were not unusually heat stable. Enzymes extracted from vegetative cells of B. cereus had heat stabilities similar to the respective enzymes from spores. When spores were heated and the enzymes were subsequently extracted and assayed, inactivation temperatures for enzymes within the spore ranged from 86 degrees C for glucose 6-phosphate dehydrogenase to 96 degrees C for aldolase. The internal environment of the spore raised the inactivation temperature of most enzymes by approximately 38 degrees C. Loss of dipicolinic acid from spores was initially slow compared with enzyme inactivation but increased rapidly with longer heating. Viability loss was faster than loss of most enzyme activities and faster than dipicolinic acid release.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. M. Heat injury of bacterial spores. Adv Appl Microbiol. 1978;23:245–261. doi: 10.1016/s0065-2164(08)70072-8. [DOI] [PubMed] [Google Scholar]
- GREEN J. H., SADOFF H. L. COMPARISION OF SOLUBLE REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE OXIDASES FROM CELLS AND SPORES OF CLOSTRIDIUM BOTULINUM. J Bacteriol. 1965 Jun;89:1499–1505. doi: 10.1128/jb.89.6.1499-1505.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- Muramatsu N., Noso Y. Purification and characterization of glucose-6-phosphate isomerase from Bacillus stearothermophilus. Arch Biochem Biophys. 1971 May;144(1):245–252. doi: 10.1016/0003-9861(71)90475-9. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Celikkol E., Engelbrecht H. L. Conversion of bacterial aldolase from vegetative to spore form by a sporulation-specific protease. Proc Natl Acad Sci U S A. 1970 Jul;66(3):844–849. doi: 10.1073/pnas.66.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring T. G., Wold F. The purification and characterization of Escherichia coli enolase. J Biol Chem. 1971 Nov 25;246(22):6797–6802. [PubMed] [Google Scholar]
- Tesone C., Torriani A. Protease associated with spores of Bacillus cereus. J Bacteriol. 1975 Oct;124(1):593–594. doi: 10.1128/jb.124.1.593-594.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tono H., Kornberg A. Biochemical studies of bacterial sporulation. 3. Inorganic pyrophosphatase of vegetative cells and spores of Bacillus subtilis. J Biol Chem. 1967 May 25;242(10):2375–2382. [PubMed] [Google Scholar]
- Ujita S., Shiroza T., Kimura K. Fructose 1,6-bisphosphate aldolases from vegetative cells and spores of some bacilli. J Biochem. 1978 Feb;83(2):503–510. doi: 10.1093/oxfordjournals.jbchem.a131937. [DOI] [PubMed] [Google Scholar]
- Warth A. D. Molecular structure of the bacterial spore. Adv Microb Physiol. 1978;17:1–45. doi: 10.1016/s0065-2911(08)60056-9. [DOI] [PubMed] [Google Scholar]
- Warth A. D. Relationship between the heat resistance of spores and the optimum and maximum growth temperatures of Bacillus species. J Bacteriol. 1978 Jun;134(3):699–705. doi: 10.1128/jb.134.3.699-705.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



