Abstract
Purpose
To estimate the agreement of confocal scanning laser tomograph (CSLT) Topographic Change Analysis (TCA) with assessment of stereophotographs and standard automated perimetry (SAP) for detecting glaucomatous progression and to identify factors associated with agreement between methods.
Design
Observational cohort study.
Participants
246 eyes of 167 glaucoma patients, glaucoma suspects and ocular hypertensives.
Methods
CSLT series (n≥ 4 tests, mean follow-up 4 years), stereophotographs and SAP results were included in the analysis. The number of progressors by guided progression analysis (GPA, “likely progression”), progressors by masked stereophotographs assessment and progressors by TCA as determined for three parameters related to the number of progressed superpixels within the disc margin was determined. Agreement between progression by each TCA parameter, stereophotographs and GPA was assessed using the Kappa test. Analysis of variance (ANOVA) with post-hoc analysis was applied to identify baseline factors including image quality (standard deviation of the mean topography), disc size and disease severity (pattern standard deviation (PSD) and cup area) associated with agreement/non-agreement between methods.
Main Outcome Measures
Agreement in assessing glaucomatous progression between the methods including factors associated with agreement/non-agreement between methods.
Results
Agreement between progression by TCA and progression by stereophotographs and/or GPA was generally poor regardless of the TCA parameter and specificity cut-offs applied. For the parameters with the strongest agreement, CSIZEdisc (cluster size in disc) and CAREAdisc (cluster area in disc), Kappa values were 0.16 (63.9%, agreement on 134 non-progressing eyes and 23 progressing eyes) and 0.15 (64.1%, agreement on 135 non-progressing eyes and 22 progressing eyes) at 99% cut-off. Most of the factors evaluated were not significantly associated with agreement/non-agreement between methods (all p>0.07). However, SAP PSD was greater in the progressors by stereophotography only group compared to the progressors by TCA only group (5.8±4.7 and 2.6±2.2, respectively p=0.003 for CSIZEdisc at 95% specificity and 5.4±4.6 and 2.5±2.3, respectively p=0.002 for CAREAdisc at 99% specificity).
Conclusions
Agreement for detection of longitudinal changes between TCA, stereophotography and SAP GPA is poor. Progressors by stereophotography only tended to have more advanced disease at baseline than progressors by TCA only.
Introduction
Establishing the presence of glaucomatous progression is challenging. First, the task is complicated by the fact that glaucoma is a slowly progressing disease and subtle changes occurring at the level of the disc and the retinal nerve fiber layer (RNFL) are difficult to detect. Second, because of instruments’ variability in structural and functional assessment, confirmation of progression is often required. Third, because risk factors associated with glaucomatous progression are not well established, it is difficult to identify patients at increased risk for disease progression that may require a closer follow-up.
Nevertheless, both structural and functional assessment of glaucomatous progression remain critical because studies have suggested that in some eyes, anatomic changes at the level of the optic disc can precede visual field loss detected by standard automated perimetry (SAP) while in other eyes, visual field loss is detected before structural change1–4. Early detection of progression could enable early therapeutic intervention aimed at preventing the occurrence of further visual field loss and visual impairment due to glaucoma5–7.
Confocal scanning laser tomography (CSLT), used to quantitatively assess optic disc topography in cross-sectional studies8–14, also allows quantification of changes in disc topographies over time by means of the Topographic Change Analysis (TCA)15 and hence the potential to assist clinicians in establishing and monitoring glaucomatous structural damage16–23.
However, it is unclear what constitutes a clinically significant amount of change identified by TCA. Most suggestions are based on the presence of changed clusters of some minimum size. In a previous report, Bowd et al. have suggested 0.90, 0.95 and 0.99 specificity cut-offs for various TCA parameters using 1000 permuted topographic series obtained from HRT images of 18 healthy eyes21. The permutation method is based on the assumption that in non-progressing eyes, the order of the exams is interchangeable, and that the change will be attributable to image variability.
The purpose of this study was to use these cut-offs to identify eyes progressed by TCA and to estimate the agreement between TCA and standardized assessment of glaucomatous progression in the Diagnostic Innovations in Glaucoma Study (DIGS). In addition, we sought to identify factors associated with agreement/non-agreement between the TCA, visual field and photographic methods for detection of glaucomatous progression.
Methods
Patients were prospectively evaluated at the Hamilton Glaucoma Center, University of California, San Diego, as part of the Diagnostic Innovations in Glaucoma Study (DIGS), a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma24. Informed written consent was obtained from all participants and all methods were approved by the University of California, San Diego, Institutional Review Board. The study adhered to the Declaration of Helsinki for research involving human subjects.
As part of DIGS, each study participant underwent a complete ophthalmologic examination including review of medical history, best-corrected visual acuity testing, slit-lamp biomicroscopy, intraocular pressure measurement, dilated stereoscopic fundus examination using a 78-D lens, gonioscopy, simultaneous stereoscopic optic disc photography (TRC-SS, Topcon Instruments Corporation of America, Paramus, NJ), and standard automated perimetry (SAP) using program 24-2 and the Swedish Interactive Threshold Algorithm (Humphrey visual field analyzer, Carl Zeiss Meditec, Dublin, CA) annually during the course of follow-up.
At study entry, all eyes had best-corrected visual acuity of 20/40 or better, sphere within ±5.0 diopters and cylinder within ±3.0 diopters and open angle at gonioscopy.
Participants were excluded if they had a history of intraocular surgery except for uncomplicated cataract or glaucoma surgery. Participants with secondary causes of elevated intraocular pressure (e.g., iridocyclitis, trauma), other intraocular eye disease, other diseases affecting the visual field (e.g., pituitary lesions, demyelinating diseases, HIV or AIDS, or diabetic retinopathy), or under medications known to affect visual field sensitivity were also excluded. To be included in the study, patients were required to have a follow-up greater than two years and at least four good-quality CSLT exams, in addition to sterophotographs of the optic disc and at least 5 reliable SAP fields (≤33% false positives, false negatives, fixation losses) each within 6 months of their first and most recent HRT examination.
For each patient, treatment aimed at lowering IOP was based on the clinicians’ recommendations.
Instrumentation
Confocal scanning laser tomography
The Heidelberg Retina Tomograph II (HRT, Heidelberg Engineering, Heidelberg, Germany) provides topographical measurements of the optic disc and peripapillary retina. Details on the instrument and its principle of use have been described elsewhere8. For each study eye, at least once a year, three scans were centered on the optic disc and a mean topography was automatically generated. Magnification errors were corrected using patient’s corneal curvature measurements. An experienced examiner outlined the optic disc margin on the mean topographic image while viewing simultaneous stereoscopic photographs of the optic disc. All images included in the analysis were carefully examined and reviewed for proper centering, focus, and illumination; all mean topography images had a standard deviation of <50 microns. Mean topography images with a standard deviation greater than 50 microns were discarded. HRT software version 1.5.9.0 or earlier was used for acquisition and analysis was performed with the recently released software version 3.0.
Topographic Change Analysis
The Topographic Change Analysis (TCA) is currently the primary method for assessing glaucomatous change using the CSLT. In brief, TCA describes significant and repeatable changes in the height of picture elements (so called “superpixels”) over the topographic map of the optic nerve, with red and green colours showing significantly changed elements (with red demonstrating retinal height depression and green demonstrating retinal height elevation) compared to baseline. TCA change summary parameters can be used to describe size and location of regions of change. In a previous report using these same participants, the performance of several TCA parameters for assessing glaucomatous progression has been described21. In this study, TCA progression was determined for the three best performing previously described parameters (i.e., CSIZEdisc, number of progressed superpixels in the largest cluster; CAREAdisc, area (mm2) of progressed superpixels in the largest cluster; and CVOLdisc, volume of progressed superpixels in the largest cluster; all three within the disc margin) at each of three previously determined specificity cut-offs (90%, defined as liberal criteria, 95%, defined as conservative criteria, 99%, defined as stringent criteria).
Stereophotography
Simultaneous stereophotographs were obtained annually after maximal pupil dilation using a Topcon camera. Baseline photos were taken on the date of HRT imaging for more than 50% of patients. Each stereophotograph was graded as glaucomatous or normal based on the presence or absence of neuroretinal rim thinning, RNFL thinning (focal or diffuse), or excavation and/or undermining of the cup characteristic of glaucoma. Also, cup/disk ratio asymmetry of >0.2 was noted, in which case the eye with the greater cup/disk was considered glaucomatous if the discs were similarly sized. To detect progressive glaucomatous optic neuropathy, the photograph closest to the baseline HRT date was selected and paired with the most recent follow-up photograph. Classifications were assigned as progression or no progression based on the presence or absence of increased rim thinning, excavation, or RNFL defects (new or enlarged). Each photograph was graded by two experienced graders using a stereoscopic viewer (Asahi Pentax StereoViewer II; Asahi Optical Co, Tokyo, Japan) and a standard fluorescent light-box. Graders were masked to patient identity, date, diagnosis, and other graders’ results. The temporal order of each progression pair was unmasked after final assessment. Disagreements in grading were resolved by adjudication by a third experienced grader.
Visual field abnormality criteria
A visual field was considered abnormal if repeatable abnormal SAP results on two consecutive exams were present with either a pattern standard deviation (PSD) greater than 5% of normal or a Glaucoma Hemifield Test (GHT) result of “outside normal limits” (both defined based on the instrument’s normative database). Glaucomatous progression by visual field was evaluated using the HFA Guided Progression Analysis (GPA). Progression by GPA was defined as a significant decrease from two baseline exams in the pattern deviation at three or more of the same test points on three consecutive tests, which is classified by the software as “Likely Progression”.
All visual fields were subjectively reviewed using standardized protocols by the VisFACT (Visual Field Assessment CenTer) housed at the Hamilton Glaucoma Center, University of California, San Diego for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia), and inattention25. None of the cases detected as progression by visual fields was discarded due to artifacts or to a non-glaucomatous progression appearance.
Statistical analysis
The kappa test was used to evaluate the agreement between progression by each TCA parameter, stereophotographs and GPA. ANOVA with Dunnett’s test for post-hoc analysis also was applied to identify baseline factors including image quality (standard deviation of the mean CSLT topography), disc size and disease severity (PSD and cup area) associated with agreement/non-agreement between methods. One random eye was selected for the analysis in case both eyes of the same subject were eligible for inclusion in the study. Two ANOVA models were constructed to include progressors by stereophotography and/or GPA only and progressors by stereophotography only separately for each model. For the Dunnett’s test, the mean of the group progressed by HRT TCA only was used as control mean.
A p value less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS (version 15.0; SPSS Inc., Chicago, IL), MedCalc (version 8.0.1.0 MedCalc Software, Belgium) and JMP version 6.1 (SAS Institute, Cary, North Carolina, USA) statistical software.
Results
A total of 246 eyes of 167 DIGS glaucoma patients, suspects and ocular hypertensives met the inclusion/exclusion criteria and were included in the analysis. 36 out of 246 (14.6%) were classified as progressors by masked stereophotograph assessment and/or GPA. 15 (6.1%) progressed by stereophotographs only, 18 (7.3%) progressed by GPA only and 3 (1.2%) progressed by both. The average (SD) follow-up time was 48.8 (13.8) months, with a minimum of 4 HRT images (range, 4–8). Table 1 summarizes the characteristics of progressed and non-progressed eyes.
Table 1.
Characteristics of progressed and non-progressed eyes from the Diagnostic Innovations in Glaucoma Study.
| Progressors (n=36 eyes) |
Non progressors (n=210 eyes) |
|
|---|---|---|
|
Age (years), mean (95% CI) |
70.4 (67.3–73.5) | 66.2 (64.2–68.2) |
|
HRT exams (n), median (range) |
5 (4–8) | 4 (4–8) |
|
HRT follow-up (y), median (range) |
4.1 (2.4–7.0) | 3.6 (1.6–7.4) |
|
SAP MD at baseline (dB), mean (95% CI) |
−3.6 (−5.4- −1.84) | −1.72 (−2.2- −1.3) |
|
SAP PSD at baseline (dB), mean (95% CI) |
4.2 (2.9–5.5) | 2.5 (2.2–2.8) |
|
Abnormal disc by photography only at baseline, n (%) |
12 (33.3) | 54 (25.7) |
|
Abnormal visual field only at baseline, n (%) |
4 (11.1) | 28 (13.3) |
|
Abnormal disc and abnormal visual field at baseline, n (%) |
15 (41.7) | 41 (19.5) |
|
Normal disc and normal visual field at baseline, n (%) |
5 (13.9) | 87 (41.4) |
Abbreviations:
CI: Confidence Intervals
HRT: Heidelberg Retina Tomograph
SAP: Standard automated perimetry
MD: Mean deviation
PSD: Pattern Standard Deviation
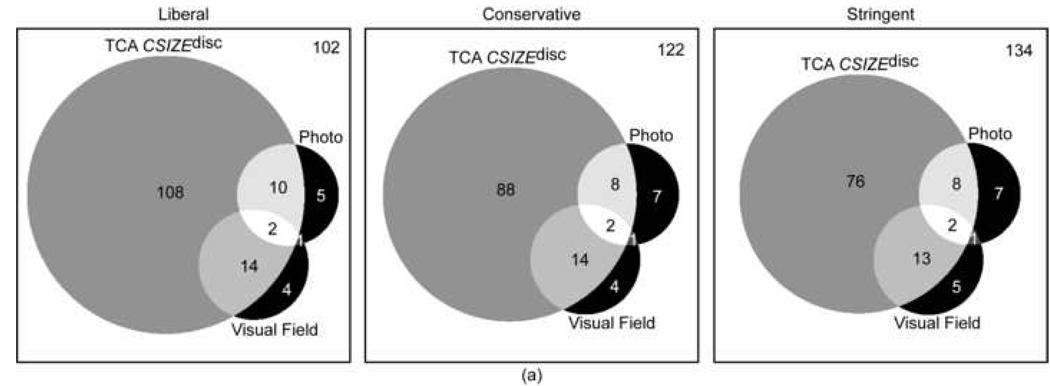
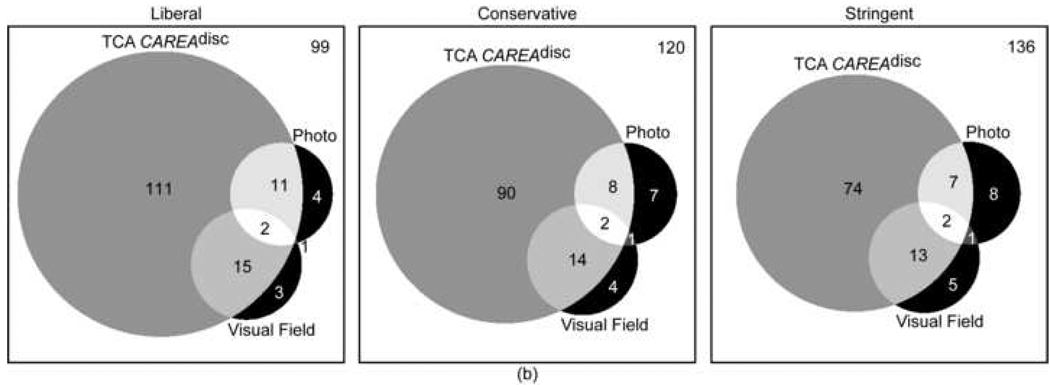
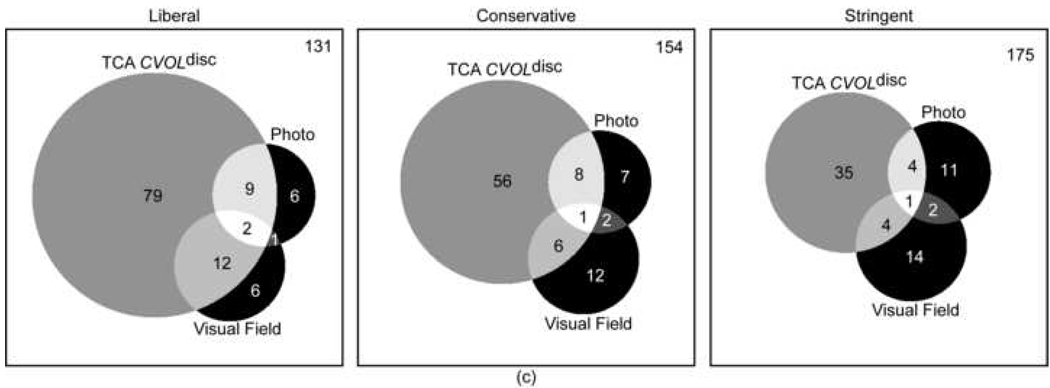
Agreement in assessing glaucomatous progression between progression by stereophotography and/or GPA and TCA was generally poor. This was true regardless of the TCA parameter and specificity cut-off applied. For the parameters with the strongest agreement, CSIZEdisc and CAREAdisc, Kappa values were 0.16 (63.9%, agreement on 134 non-progressing eyes and 23 progressing eyes) and 0.15 (64.1%, agreement on 135 non-progressing eyes and 22 progressing eyes) at 99% specificity cut-offs (stringent criteria). Table 2 shows the Kappa values and Figure 1 shows the corresponding Venn diagrams describing the level of agreement for progression and no progression as detected by stereophotographs and visual fields for each TCA parameter evaluated at various specificity criteria (i.e., liberal, conservative and stringent).
Table 2.
Percentages of agreement and kappas (standard error) for progression with the standard methods and for progression with the Topographic Change Analysis parameters.
| Agreement for progression (%) |
Agreement for no progression (%) |
Kappa (SE)* |
||
|---|---|---|---|---|
| CSIZEdisc | Liberal | 10.6 | 41.5 | 0.10 (0.04) |
| Conservative | 9.8 | 49.6 | 0.13 (0.05) | |
| Stringent | 9.4 | 54.5 | 0.16 (0.05) | |
| CAREAdisc | Liberal | 11.4 | 40.0 | 0.11 (0.04) |
| Conservative | 9.8 | 48.6 | 0.12 (0.05) | |
| Stringent | 9.0 | 55.1 | 0.15 (0.05) | |
| CVOLdisc | Liberal | 9.4 | 53.3 | 0.15 (0.05) |
| Conservative | 6.1 | 62.6 | 0.10 (0.06) | |
| Stringent | 3.7 | 71.1 | 0.08 (0.07) |
Calculation based on both eyes of the same patient included in the analysis. SE is slightly larger when one random eye only is included in the kappa calculation.
Note: Specificity cut-offs for TCA were set at 90% (liberal criteria), 95% (conservative criteria) and 99% (stringent criteria) specificity cut-offs.
The standard methods for progression are represented by stereophotography and/or SAP GPA.
Abbreviations:
TCA: Topographic Change Analysis
SE: Standard Error
CSIZEdisc: Number of progressed superpixels in the largest cluster
CAREAdisc: Area of progressed superpixels in the largest cluster
CVOLdisc: Volume of progressed superpixels in the largest cluster
SAP: Standard automated perimetry
GPA: Guided Progression Analysis
Figure 1.
a-b-c: Proportional Venn diagrams showing the agreement between progression TCA CSIZEdisc (a), TCA CAREAdisc (b) and TCA CVOLdisc (c) evaluated at 90% (liberal criteria), 95% (conservative criteria) and 99% (stringent criteria) specificity cut-offs and progression by stereophotography and visual field (SAP GPA). The number of eyes with all three tests stable is shown in the top right corner of each diagram.
Abbreviations:
TCA: Topographic Change Analysis
SAP: Standard Automated Perimetry
GPA: Guided Progression Analysis
CSIZEdisc: Number of progressed superpixels in the largest cluster
CAREAdisc: Area of progressed superpixels in the largest cluster
CVOLdisc: Volume of progressed superpixels in the largest cluster
Table 3 shows the results from ANOVA with post-hoc analysis used to evaluate baseline factors associated with agreement/non-agreement between methods. The results of the ANOVA models shown include the group progressed by stereophotography and/or GPA only for CSIZEdisc (conservative). A similar trend was found for TCA CAREAdisc and CVOLdisc (data not shown). In general, eyes that progressed by TCA, GPA and stereophotographic methods of analysis had more advanced disease at baseline, represented by a greater SAP MD and PSD, than eyes progressed by HRT TCA only, regardless of the TCA parameter and specificity criteria considered. Most of the factors evaluated were not significantly associated with agreement/non-agreement between methods (all p>0.07) (see Table 3). These results also were confirmed in a separate ANOVA model including the group of eyes progressed by stereophotography only (for example, mean (SD) PSD in eyes progressed by stereophotography only and eyes progressed by TCA only was 5.8 (4.7) and 2.6 (2.2), respectively p=0.001 for CSIZEdisc at 95% specificity and 5.4 (4.6) and 2.5 (2.3), respectively p=0.003 for CAREAdisc at 99% specificity). In addition, as shown in Table 3, eyes progressed by fields and photographic methods had a significantly greater percentage of HRT Moorfields Regression Analysis (MRA) global classification borderline or outside normal limits compared to eyes progressed by TCA only.
Table 3.
Results from analysis of variance with Dunnett’s test for post-hoc analysis for the HRT CSIZEdisc (conservative criteria) for all baseline parameters evaluated (95% confidence intervals in parenthesis).
| Progression by CSIZEdisc (conservative) only (N=56) |
Progression by stereophotography and/or SAP GPA only (N=11) |
Progression by more than one method (TCA, stereophotography and SAP GPA) (N=19) |
All methods no progression (N=81) |
P value |
|
|---|---|---|---|---|---|
| Age (years) | 58.7 (56.2– 61.2) |
63.5 (56.7–70.3) | 64.1 (59.3–68.9) | 60.7 (58.6– 62.8) |
0.17 |
|
Ancestry (% Caucasian) |
64.7 | 58.3 | 70.8 | 63.1 | 0.80† |
|
Disc area HRT (mm2) |
2.2 (2.1–2.3) | 2.1 (1.9–2.5) | 2.1 (1.9–2.3) | 2.0 (1.9–2.1) | 0.10 |
| IOP (mmHg) | 19.0 (17.9– 20.4) |
20.0 (16.6–23.5) | 18.3 (15.7–20.9) | 19.1 (17.9– 20.4) |
0.83 |
| Sphere (dpt) | −1.1 (−1.5- −0.7) | −1.1 (−2.1- −0.03) | −0.6 (−1.5- 0.2) | −0.75 (−1.1- − 0.5) |
0.51 |
|
Cylinder (dpt) |
0.6 (0.4–0.8) | 0.4 (0.1–0.8) | 0.5 (0.3–0.7) | 0.6 (0.5–0.7) | 0.24 |
| SAP MD (db) | −1.65 (−2.4– − 0.88) |
−1.63 (−3.35– −0.09) | −4.3 (−5.6– −3.0)* | −1.25 (−1.89 – −0.60 |
<0.00 1 |
|
SAP PSD (db) |
2.49 (1.94– 3.05) |
4.12 (2.92–5.25)* | 4.00 (3.04–4.95)* | 2.15 (1.68– 2.60) |
0.002 |
|
HRT cup area (mm2) |
0.83 (0.70– 0.96) |
1.06 (0.77–1.35) | 1.01 (0.79–1.23) |
0.67 (0.56– 0.78)* |
0.007 |
|
HRT Max Cup Depth (mm) |
0.69 (0.63– 0.74) |
0.71 (0.58–0.83) | 0.71 (0.61–0.80) | 0.62 (0.57– 0.67) |
0.16 |
|
HRT linear cup to disk ratio |
0.58 (0.53– 0.62) |
0.67 (0.57–0.76)* | 0.66 (0.60–0.74)* | 0.55 (0.51– 0.58) |
0.005 |
|
MRA Classification (% BL or ONL) |
60.8 | 90.8 | 84.2 | 53.75 | 0.008† |
|
Standard Deviation topography (µm) |
17.4 (15.0– 19.7) |
24.1(18.8–29.4) | 18.4 (14.4–22.4) | 19.2 (17.3– 21.1) |
0.14 |
Note: For the Dunnett’s test, the mean of the group progressed by HRT TCA
CSIZEdisc only was used as control mean.
Mean significantly different compared to the control
P value based on chi-square
Abbreviations:
HRT: Heidelberg Retina Tomograph
CSIZEdisc: Number of progressed superpixels in the largest cluster
SAP: Standard automated perimetry
GPA: Guided Progression Analysis
HRT: Heidelberg Retina Tomograph
IOP: Intraocular pressure
MD: Mean deviation
PSD: Pattern standard deviation
MRA: Moorfields Regression Analysis
BL: border line
ONL: outside normal limits
TCA: Topographic Change Analysis
Discussion
These results show that agreement for detection of longitudinal changes between TCA, stereophotography and/or SAP GPA is generally poor, suggesting that these tests may provide independent information on disease progression.
Our results indicate that eyes that progressed by SAP GPA and/or by stereophotography only, had more advanced disease at baseline compared to eyes progressed by TCA only. The other factors evaluated, including age or disc size, did not explain the lack of agreement between methods.
Although agreement between TCA and stereophotography tended to be worse than agreement between TCA and GPA visual field progression, the small difference was not statistically significant. It should also be noted that, in this study, for TCA, only change within the disc margin was considered, while stereophotographs evaluation included the peripapillary RNFL.
Previous studies have shown that TCA is a promising analysis technique for providing automated and sophisticated detection of change over time in HRT images16–23. It is important to note that TCA by itself is not designed to identify changes in comparison to population changes, but rather a significant change that is greater than the variability of the measurements. In this study, we applied previously derived specificity cut-offs TCA determined at a high specificity based on the amount of change identified in a longitudinal series of scans of healthy eyes21.
In fact, any imaging device used to identify glaucomatous structural change should ideally be able to discriminate between “true change” or disease progression and measurement variability. However, change can also be due to aging or other factors not currently established.
Unfortunately, it is unclear what constitutes “true change” in glaucoma. Glaucoma is a disease characterized by a progressive loss of retinal ganglion cells associated with an irreversible loss of visual function and clinicians currently rely on the identification of characteristic changes in the optic disc and the visual fields indicative of glaucomatous progression.
However, several studies have shown that the agreement between structural and functional methods for the detection of progression is moderate at best16–19, 26. For example, Chauhan et al. reported 27% of glaucomatous eyes progressing by both TCA and SAP, while 40% and 4% progressed by TCA and SAP only, respectively16. Strouthidis et al. found 42 eyes progressing with HRT only and 40 eyes progressing with SAP only in 198 ocular hypertensives eyes using HRT Rim Area and SAP change based on pointwise linear regression at a less stringent strategy (i.e., with HRT specificity ranging from 88.1 to 90.5%)26. Our findings also suggest that with the current methods used to detect progression, only a limited percentage of eyes will show concomitant changes in structure and function. Choosing different criteria (e.g., more liberal criteria as opposed to more stringent criteria) for detecting change using TCA in general did not influence the agreement between methods. These results are not surprising. Detectable structural and functional changes may not in fact be concurrent. Although it is plausible that structural loss may precede functional loss because of the redundancy in the system, it is also possible that loss of function may be the result of retinal ganglion cells impairment with no detectable structural loss. Moreover, because SAP GPA and HRT TCA require confirmation of change at follow-up visits, it is conceivable that change was detected at one visit but was unstable, causing the agreement for progression between methods to be suboptimal.
In general, TCA detected more eyes as progressing when compared to any other method used. It is unclear whether these eyes represent true disease progression or false positive results. Theoretically, when considering how the cut-offs were derived, the change detected by TCA should reflect “true” change albeit not necessarily related to the presence of the disease. Moreover, it remains to be established whether these changes may lead to subsequent loss of vision and therefore should be considered clinically relevant. Longer follow-up may help determine whether TCA can identify early disease progression before the occurrence of any detectable change in stereophotographs or SAP.
It is important to identify factors that can be responsible for agreement/disagreement between TCA, stereophotographs assessment and GPA for detecting glaucomatous progression. If we know what influences the ability of a specific method to detect progression, then clinicians could ensure that their patients are managed using the method most likely to detect change. In this study, we evaluated several baseline factors, such as optic disc size, image quality and disease severity in an attempt to determine whether eyes that progressed by TCA only, for example, had different characteristics compared to eyes progressed by other methods. Most of the factors evaluated, such as age or ancestry, were not significantly different among the groups. Differences in image quality of baseline HRT topographies also were not significant, possibly confirming that the changes detected by TCA are not the result of measurements’ variability, but rather they may represent a true “biological” effect. IOP measurements at baseline were not significantly different among groups. Although treatment may have lowered baseline IOP levels in some cases, these results suggest that progression detected by TCA may occur at every level of baseline IOP.
However, the results of this analysis showed that eyes progressed by GPA and/or by stereophotography only, had more advanced disease (i.e., a greater PSD at baseline, for example) at baseline than eyes progressed by TCA only.
One possible explanation for the finding is that, when judging progression using stereophotography, one may look more carefully for glaucomatous change in eyes with more advanced disease at baseline than in eyes with earlier glaucomatous damage. Alternatively, the change in eyes with more advanced damage may be in areas of the optic disc that are more variable (around the steep areas of the cup or blood vessels) and therefore be more difficult to detect with TCA. For example, of the 6 eyes that progressed by stereophotography only and not by TCA CSIZEdisc at 90% specificity cut-off, 4 eyes showed the presence of red superpixels in areas of the cup or areas adjacent to the vessels where change was detected on masked stereophotography assessment. However, the change with TCA was not significant for the method to identify the eyes as progressed. Although the standard deviation of the topography was not significantly associated with differences in diagnostic accuracy between TCA and stereophotographs or GPA, this measure reflects the mean standard deviation of the entire image, and not the variability of a local area. Moreover, the standard deviation of the topography was consistently higher in eyes with progression by stereophotography or GPA only compared to progression by TCA only, regardless the TCA parameter and specificity cut-off evaluated (e.g., for TCA CSIZEdisc with conservative criteria, the standard deviation was 24.1 microns and 17.4 microns in eyes progressed by stereophotography and/or GPA only and by TCA only, respectively). It may be that the standard deviation of the specific local region of change is relatively large but does not result in an increase of the overall standard deviation of the entire topography. In these cases, the localized TCA change detected may not have reached significance, as the variability was high in this area.
In conclusion, when TCA parameters were used to identify glaucomatous progression in the DIGS population, the agreement between TCA and stereophotographs and/or GPA assessment of progression was poor. Clinicians attempting to establish glaucomatous progression should not rely on a single method for the identification of glaucomatous progression. With longer follow-up, however, it is possible that the changes detected by TCA only or stereophotography or SAP only will at some point be detected by all techniques, leading to better agreement between methods. Progressors by stereophotography tended to have more advanced disease at baseline than progressors by TCA only. Future studies will likely provide further explanations for the lack of agreement between methods used to detect glaucomatous progression.
Acknowledgments
Grant Support: NIH EY011008, NIH EY008208 and participant incentive grants in the form of glaucoma medication at no cost from Alcon Laboratories Inc., Allergan, Pfizer Inc., and SANTEN Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure:
Carl Zeiss Meditec: FAM (F, R) PAS (F), RNW (F), LMZ (F)
Haag-Streit: PAS (F)
Heidelberg Engineering: MB (F), FAM (F), RNW (F), LMZ (F)
Lace Elettronica: CB (F)
Optovue: LMZ (F), RNW (F, C)
Reichert Instruments: FAM (R)
Welch-Allyn: PAS (F)
REFERENCES
- 1.Tuulonen A, Airaksinen PJ. Initial glaucomatous optic disk and retinal nerve fiber layer abnormalities and their progression. Am J Ophthalmol. 1991;111:485–490. doi: 10.1016/s0002-9394(14)72385-2. [DOI] [PubMed] [Google Scholar]
- 2.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 3.Lin SC, Singh K, Jampel HD, et al. Ophthalmic Technology Assessment Committee Glaucoma Panel. Optic nerve head and retinal nerve fiber layer analysis: a report by the American Academy of Ophthalmology. Ophthalmology. 2007;114:1937–1949. doi: 10.1016/j.ophtha.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ocular Hypertension Treatment Study Group, European Glaucoma Prevention Study Group. Validated prediction model for the development of primary open-angle glaucoma in individuals with ocular hypertension. Ophthalmology. 2007;114:10–19. doi: 10.1016/j.ophtha.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 6.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 7.McKean-Cowdin R, Wang Y, Wu J, et al. Los Angeles Latino Eye Study Group. Impact of visual field loss on health-related quality of life in glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:941–948. doi: 10.1016/j.ophtha.2007.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinreb RN. Laser scanning tomography to diagnose and monitor glaucoma. Curr Opin Ophthalmol. 1993;4:3–6. [PubMed] [Google Scholar]
- 9.Mikelberg FS, Parfitt CM, Swindale NV, et al. Ability of the Heidelberg retina tomograph to detect early glaucomatous field loss. J Glaucoma. 1995;4:242–247. [PubMed] [Google Scholar]
- 10.Zangwill LM, van Horn S, de Souza Lima M, et al. Optic nerve head topography in ocular hypertensive eyes using confocal scanning laser ophthalmoscopy. Am J Ophthalmol. 1996;122:520–525. doi: 10.1016/s0002-9394(14)72112-9. [DOI] [PubMed] [Google Scholar]
- 11.Uchida H, Brigatti L, Caprioli J. Detection of structural damage from glaucoma with confocal laser image analysis. Invest Ophthalmol Vis Sci. 1996;37:2393–2401. [PubMed] [Google Scholar]
- 12.Hatch WV, Flanagan JG, Etchells EE, et al. Laser scanning tomography of the optic nerve head in ocular hypertension and glaucoma. Br J Ophthalmol. 1997;81:871–876. doi: 10.1136/bjo.81.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wollstein G, Garway-Heath DF, Hitchings RA. Identification of early glaucoma cases with the scanning laser ophthalmoscope. Ophthalmology. 1998;105:1557–1563. doi: 10.1016/S0161-6420(98)98047-2. [DOI] [PubMed] [Google Scholar]
- 14.Zangwill LM, Bowd C, Berry CC, et al. Discriminating between normal and glaucomatous eyes using the Heidelberg Retina Tomograph, GDx Nerve Fiber Analyzer, and Optical Coherence Tomograph. Arch Ophthalmol. 2001;119:985–993. doi: 10.1001/archopht.119.7.985. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan BC, Blanchard JW, Hamilton DC, LeBlanc RP. Technique for detecting serial topographic changes in the optic disc and peripapillary retina using scanning laser tomography. Invest Ophthalmol Vis Sci. 2000;41:775–782. [PubMed] [Google Scholar]
- 16.Chauhan BC, McCormick TA, Nicolela MT, LeBlanc RP. Optic disc and visual field changes in a prospective longitudinal study of patients with glaucoma: comparison of scanning laser tomography with conventional perimetry and optic disc photography. Arch Ophthalmol. 2001;119:1492–1499. doi: 10.1001/archopht.119.10.1492. [DOI] [PubMed] [Google Scholar]
- 17.Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005;24:333–354. doi: 10.1016/j.preteyeres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Patterson AJ, Garway-Heath DF, Strouthidis NG, Crabb DP. A new statistical approach for quantifying change in series of retinal and optic nerve head topography images. Invest Ophthalmol Vis Sci. 2005;46:1659–1667. doi: 10.1167/iovs.04-0953. [DOI] [PubMed] [Google Scholar]
- 19.Kourkoutas D, Buys YM, Flanagan JG, et al. Comparison of glaucoma progression evaluated with Heidelberg retina tomograph II versus optic nerve head stereophotographs. Can J Ophthalmol. 2007;42:82–88. [PubMed] [Google Scholar]
- 20.Kalaboukhova L, Fridhammar V, Lindblom B. Glaucoma follow-up by the Heidelberg retina tomograph--new graphical analysis of optic disc topography changes. Graefes Arch Clin Exp Ophthalmol. 2006;244:654–662. doi: 10.1007/s00417-005-0107-3. [DOI] [PubMed] [Google Scholar]
- 21.Bowd C, Balasubramanian M, Weinreb RN, et al. Performance of confocal scanning laser tomography Topographic Change Analysis (TCA) for assessing glaucomatous progression. Invest Ophthalmol Vis Sci. 2009;50:691–701. doi: 10.1167/iovs.08-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vizzeri G, Weinreb RN, Martinez de la Casa JM, et al. Clinicians agreement in establishing glaucomatous progression using the Heidelberg retina tomograph. Ophthalmology. 2009;116:14–24. doi: 10.1016/j.ophtha.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chauhan BC, Hutchison DM, Artes PH, et al. Optic disc progression in glaucoma: comparison of confocal scanning laser tomography to optic disc photographs in a prospective study. Invest Ophthalmol Vis Sci. 2009;50:1682–1691. doi: 10.1167/iovs.08-2457. [DOI] [PubMed] [Google Scholar]
- 24.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–1360. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 25.Sample PA, Girkin CA, Zangwill LM, et al. ADAGES Study Group. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127:1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strouthidis NG, Scott A, Peter NM, Garway-Heath DF. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity and agreement. Invest Ophthalmol Vis Sci. 2006;47:2904–2910. doi: 10.1167/iovs.05-1584. [DOI] [PubMed] [Google Scholar]