Abstract
Background:
The aim of this study was to evaluate the effect of levosimendan on mortality in cardiogenic shock (CS) after ST elevation myocardial infarction (STEMI).
Methods and results:
Data were obtained prospectively from the SCAAR (Swedish Coronary Angiography and Angioplasty Register) and the RIKS-HIA (Register of Information and Knowledge about Swedish Heart Intensive Care Admissions) about 94 consecutive patients with CS due to STEMI. Patients were classified into levosimendan-mandatory and levosimendan-contraindicated cohorts. Inotropic support with levosimendan was mandatory in all patients between January 2004 and December 2005 (n = 46). After the SURVIVE and REVIVE II studies were presented, levosimendan was considered contraindicated and was not used in consecutive patients between December 2005 and December 2006 (n = 48). The cohorts were similar with respect to pre-treatment characteristics and concomitant medications. There was no difference in the incidence of new-onset atrial fibrillation, in-hospital cardiac arrest and length of stay at the coronary care unit. There was no difference in adjusted mortality at 30 days and at one year.
Conclusion:
The use of levosimendan neither improves nor worsens mortality in patients with CS due to STEMI. Well-designed randomized clinical trials are needed to define the role of inotropic therapy in the treatment of CS.
Keywords: shock, myocardial infarction, inotropic agents, heart failure, pharmacology
Introduction
Cardiogenic shock (CS) complicates 7%–10% of acute myocardial infarctions (MI) and is the leading cause of death in patients hospitalized for MI, with mortality rates of up to 60%.1–3 There is a scarcity of scientific data defining the role of inotropic and other therapy in the treatment of CS. Levosimendan is a novel inotropic drug with vasodilator effects that is used in the treatment of CS.4 In 2003, Sweden was one of the first countries to approve levosimendan (Simdax®) for an indication in acute heart failure (AHF). Since then, this agent has been increasingly used in Swedish hospitals, not only for AHF but also for “off-label” indications5,6 including treatment of CS,7 despite that levosimendan’s efficacy and safety in these patients has not been properly assessed. Two randomized clinical trials have recently demonstrated no survival benefit for levosimendan over dobutamine8 (SURVIVE) or placebo9–13 (REVIVE II) in patients with AHF. On the contrary, the results have created the legitimate concern as to whether levosimendan might actually increase mortality, because increased incidences of hypotension and malignant ventricular arrhythmia, and more deaths were reported in REVIVE II with levosimendan compared with placebo.11–13 Because patients with CS are particularly sensitive to arrhythmias and worsening hypotension, it is essential to gather all available information about the effects of this drug in CS. Therefore, the aim of this study was to evaluate the effects of levosimendan on short-term and long-term mortality rates in CS.
Patients and methods
Patient characteristics
All consecutive patients presenting with CS due to ST elevation myocardial infarction (STEMI) between January 2004 and December 2006 were enrolled in this registry study. Shock was defined by the SHOCK trial criteria14 as follows: presence of hypotension (a systolic blood pressure of <90 mmHg for at least 30 minutes or the need for supportive measures to maintain a systolic blood pressure of >90 mmHg) and end-organ hypoperfusion (cool extremities or a urine output of <30 mL per hour, and a heart rate of >60 beats per minute). Data were obtained from the SCAAR15 (Swedish Coronary Angiography and Angioplasty Register) and RIKS-HIA16 (Register of Information and Knowledge about Swedish Heart Intensive Care Admissions) registries and from patient charts. The SCAAR and RIKS-HIA registries contain detailed data (patient’s characteristics, treatment data, outcome, etc.) on consecutive patients from all hospitals that perform coronary interventions and provide intensive coronary care in Sweden. The registries are sponsored by the Swedish Health Authorities. The technology is developed and administered by the Uppsala Clinical Research Center. Since 2001, SCAAR and RIKS-HIA have been Internet-based with data recorded online through a Web interface. For the purpose of this study, we extracted and analyzed data reported only from our hospital, ie, Sahlgrenska University Hospital (SU). The SU is situated in Gothenburg and is the largest hospital in Scandinavia, providing specialized health care for ∼1.5 million inhabitants in the Västra Götaland region of western Sweden. There are seven additional primary hospitals in this region. The treatment strategy for CS at Västra Götaland is based on the European Society of Cardiology (ESC)/American Heart Association (AHA)/American College of Cardiology (ACC)17,18 recommendations. The most important priority in the health care chain for CS patients is to provide expeditious primary percutaneous coronary intervention (PCI) with around-the-clock availability. Ninety-four consecutive patients who fulfilled the inclusion criteria were enrolled between January 2004 and December 2006. The patients were transported for acute angiography and PCI from the Gothenburg area (n = 47) as well as from areas covered by the 7 primary hospitals in Västra Götaland (n = 47) according to the local regional guidelines. A successful angioplasty was defined as following: no more than 50% post-intervention stenosis, an improvement of at least 20% in the degree of stenosis, and a flow of thrombolysis in myocardial infarction (TIMI) grade II or III.14 The completeness of revascularization was defined as following: no more than 10% of the left ventricular myocardium is supplied by the untreated vessel/vessels with significant (>60%) diameter stenosis.19
Two cohorts of patients (levosimendan-mandatory and levosimendan-contraindicated) were defined based on the two distinct treatment strategies that were established and implemented at our clinic during this period. These two cohorts were compared in terms of clinical characteristics and 30-day mortality and at 1-year. The study was approved by the ethics committee.
Treatment strategy I
The first cohort (n = 46) consisted of patients enrolled between January 2004 and December 2005. During this period, levosimendan was used as the “first choice” agent to treat CS. According to the local regional guidelines, all patients with CS due to STEMI must be urgently transported to SU for primary PCI. In the catheterization laboratory (cath lab), introduction of an intraaortic balloon pump (IABP) and levosimendan were part of the mandatory treatment algorithm. These guidelines were based on our interpretation of the available data at the time, and were largely influenced by the results from the SHOCK,14 LIDO,20 RUSSLAN,21 CASINO,22 and OPTIME-HF23 clinical studies. Consequently, levosimendan was regarded as being superior to the traditional inotropes like dopamine, dobutamine, and milrinone. The patients could also receive combination therapies such as levosimendan plus noradrenalin or levosimendan plus dopamine or dobutamine if monotherapy with levosimendan did not result in hemodynamic stability, although polypharmacy was generally discouraged. The choice of the additional catecholamine agent was left at the discretion of the attending physician. Treatment with levosimendan could be commenced in the cath lab before, during, or after the revascularization procedure starting with an IV bolus of 12 μg/kg followed by a continuous infusion of 0.1 μg/kg/min for 24 hours or 48 hours. An attempt to discontinue inotropic support with noradrenalin, dopamine, and dobutamine (if used with levosimendan) was recommended as soon as the patient achieved hemodynamic stability for >12 hours. Stability was defined as systolic blood pressure >110 mmHg, mean arterial pressure (MAP) > 60 mmHg, cardiac index of 2.2 L/min/m2, good peripheral perfusion, and urine output >50 mL per hour. Levosimendan infusion was allowed to continue for either 24 hours or 48 hours, even if hemodynamic stability was achieved. Cardiac output, cardiac index and mixed venous oxygen saturation were monitored continuously with pulmonary artery catheter.
Treatment strategy II
After presentation of the data from the SURVIVE8 and REVIVE-II9 studies at the AHA meeting in 2005, we revised our guidelines. Based on the new data, levosimendan was excluded from the treatment algorithm due to concern about the increased incidence of hypotension and arrhythmias observed in these studies that might increase mortality in patients with CS. Consequently, none of the patients enrolled between December 2005 and December 2006 received levosimendan (n = 48). The new recommendation was to apply a restrictive approach regarding the use of all inotropic agents, ie, the routine use of inotropes was strongly discouraged. Inotropic support was recommended only if the reperfusion therapy, optimal hydration therapy and IABP support did not improve the patient’s hemodynamic status. The hemodynamic and clinical goals were systolic blood pressure >110 mmHg, MAP > 60 mmHg, cardiac index of 2.2 L/min/m2, good peripheral perfusion, and urine output >50 mL per hour. This approach is different from the previous strategy because all patients in the levosimendan-mandatory group received levosimendan early in the clinical course on a routine basis. The choice of appropriate agent (ie, dopamine, noradrenalin, dobutamine, or milrinone) was left to the discretion of the attending physicians. Standard doses and up-titration schemes were used as recommended by the responsible pharmacological companies. An attempt to discontinue inotropic support was recommended as soon as patient had achieved hemodynamic stability, defined as systolic blood pressure >110 mmHg and urine output >50 mL per hour.
Statistics
Continuous variables are expressed as the means ± standard deviations and categorical variables as percentages. Comparisons between continuous variables were performed using Student’s t-test. Pearson’s Chi-square or Fisher’s exact tests were used to compare categorical variables. Normality of the variables was tested with the Kolmogorov-Smirnov test. Cox-regression with a forward stepwise model was used to adjust for the differences between the two cohorts. The following variables were included in the model: age, gender, hypertension, smoking, IABP, treatment with levosimendan, success of revascularization, and completeness of revascularization. All statistical analyses were performed using SPSS® software (version 17.0.2, SPSS Inc., Chicago, IL, USA). Statistical significance was considered at P < 0.05 (for two-tailed hypothesis).
Results
Patients
The cohorts were similar with respect to pre-treatment characteristics and concomitant medications (Tables 1 and 2).
Table 1.
Patient characteristics I
| Levosimendan mandatory n = 46 | Levosimendan contraindicated n = 48 | P-value | |
|---|---|---|---|
| Age (mean ± SD) | 65 ± 12.1 | 67 ± 10.8 | 0.39 |
| Female, n (%) | 11 (23.9) | 14 (29.2) | 0.56 |
| Hypertension, n (%) | 17 (37.0) | 20 (41.7) | 0.64 |
| Diabetes, n (%) | 10 (21.7) | 14 (29.2) | 0.41 |
| Previous MI, n (%) | 9 (19.6) | 8 (16.7) | 0.71 |
| Previous CABG, n (%) | 0 (0.0) | 2 (4.2) | 0.16 |
| Previous PCI, n (%) | 2 (4.3) | 4 (8.3) | 0.43 |
| Insulin, n (%) | 3 (6.5) | 6 (12.5) | 0.32 |
| Anti-diabetic po, n (%) | 5 (10.9) | 2 (4.2) | 0.22 |
| Beta-blockade, n (%) | 11 (23.9) | 11 (22.9) | 0.91 |
| ACE, n (%) | 9 (19.6) | 5 (10.4) | 0.21 |
| ARB, n (%) | 3 (6.5) | 4 (8.3) | 0.74 |
| Statin, n (%) | 7 (15.2) | 4 (8.3) | 0.29 |
| ASA, n (%) | 13 (28.3) | 9 (18.8) | 0.28 |
| Warfarin, n (%) | 0 (0.0) | 3 (6.3) | 0.09 |
Abbreviations: ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; CABG, coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention; po, per oral.
Table 2.
Angiography and revascularization
| Levosimendan mandatory n =46 | Levosimendan contraindicated n = 48 | P-value | |
|---|---|---|---|
| Angiography findings | |||
| One-vessel disease, n (%) | 11 (23.9) | 9 (18.8) | 0.53 |
| Multi-vessel disease, n (%) | 26 (56.5) | 32 (66.7) | 0.44 |
| Left main disease, n (%) | 9 (19.6) | 7 (14.5) | 0.51 |
| Number of treated segments | |||
| LM, n (%) | 2 (1.9) | 8 (6.6) | 0.11 |
| LAD, n (%) | 48 (45.3) | 42 (34.7) | 0.13 |
| LCx, n (%) | 24 (22.6) | 29 (23.9) | 0.88 |
| RCA, n (%) | 32 (30.2) | 42 (34.7) | 0.48 |
| Saphenous graft, n (%) | 0 (0.0) | 1 (0.1) | 0.31 |
| Compl. revasc., n (%) | 23 (50.0) | 19 (39.6) | 0.31 |
| Success. proc., n (%) | 43 (93.5) | 41 (85.4) | 0.20 |
Abbreviations: Compl. revasc., completeness of revascularization; LAD, left anterior descending artery; LCx, left circumflex artery; LM, left main; RCA, right coronary artery; Success. proc., successful procedure.
Treatments
The data are summarized in Tables 2 and 3. After the initial evaluation with coronary angiography, 92 patients (98%) underwent acute PCI intervention and 2 patients (2%) (from the levosimendan-mandatory group) underwent acute coronary bypass surgery (P = 0.24). The most frequently treated vessel was the left anterior descending artery (LAD) and right coronary artery (RCA) in both cohorts, respectively (Table 2). Complete revascularization was achieved in approximately half of all admitted patients and was similar in both groups (Table 2). The procedural success was generally high, and the procedure was deemed unsuccessful in only a few cases (Table 2).
Table 3.
Patient characteristics II
| Levosimendan mandatory n = 46 | Levosimendan contraindicated n = 48 | P-value | |
|---|---|---|---|
| IABP, n (%) | 34 (73.9) | 39 (81.3) | 0.46 |
| Resuscitation, n (%) | 1 (2.2) | 1 (2.1) | 0.97 |
| Inotropy, n (%) | 46 (100) | 26 (54.2) | 0.01 |
| Thrombolytics, n (%) | 2 (4.3) | 1 (2.1) | 0.61 |
| GP IIb/IIIa, n (%) | 44 (0.96) | 48 (100) | 0.49 |
| ICU days, (median ± IQR) | 7 (3–15) | 7 (3–14) | 0.54 |
| AV-block, n (%) | 3 (6.5) | 4 (8.3) | 0.72 |
| Atrial fib., n (%) | 8 (17.4) | 8 (16.7) | 0.93 |
Abbreviations: Atrial fib., atrial fibrillation; AV-block, AV block II or III; GP IIb/IIIa, glycoprotein IIb/IIIa receptor inhibitors; IABP, intraaortic balloon pump counterpulsation; ICU, intensive care unit.
The use of thrombolytic therapy prior to arrival in the cath lab was low (Table 3). All patients who underwent PCI revascularization were treated with a glycoprotein IIb/IIIa receptor (GP IIa/IIIb) inhibitor, which was started in the cath lab and continued in the intensive care unit (ICU). The length of stay in the ICU was similar between the groups. There was no difference in the incidence of new-onset atrial fibrillation or atrioventricular (AV) block (Table 3). Drug eluting stents were used only in a few patients. The use of inotropic therapy was almost halved (P < 0.001) in the second cohort, reflecting adherence to the change in the treatment guidelines (Table 3). The number of in-hospital cardiac arrests and resuscitation procedures was low, and was similar in both groups. A majority of patients were treated with bare metal stents (97.3% in the levosimendan mandatory group and 95.9% in the levosimendan contraindicated group, P = 0.58). Only a minority of patients were treated with drug-eluting stents (2.4% and 4.1%, P = 0.58 in the levosimendan mandatory group and the levosimendan contraindicated group respectively). There was no difference in average stent length (18.4 mm and 18.9 mm, P = 0.66) as well as in stent diameter (3.4 mm and 3.5 mm, P = 0.79) in the levosimendan-mandatory group and the levosimendan-contraindicated group respectively. There was no difference in the treatment with continuous positive airway pressure (CPAP) between the groups (40% vs 41%, P = 0.9).
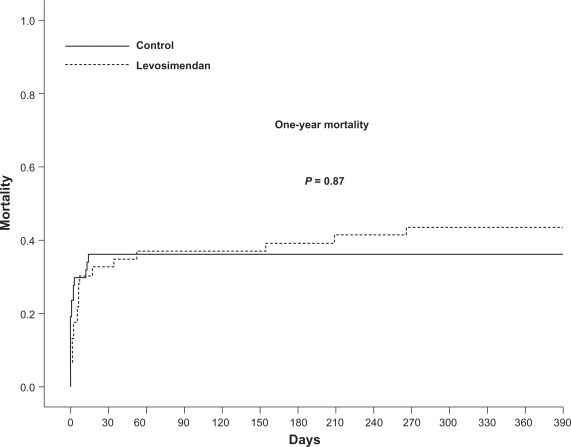
Mortality
The patient follow-up was 100%. The majority of deaths occurred within 30 days post-MI, and included 15 (32.6%) patients in the first cohort and 17 (35.4%) in the second cohort (Figure 1). After 30 days, five additional patients died in the levosimendan-mandatory group while no deaths occurred in the levosimendan-contraindicated group. There was no difference in the adjusted 30-day mortality (hazard ratio [HR] 0.97; confidence interval [CI] 0.53–1.78; P = 0.93) and 1-year mortality (HR 1.05; CI 0.57–1.92; P = 0.87) between the groups (Figure 1). In the Cox proportional hazard regression, age, procedural success, and completeness of revascularization were independent predictors of mortality (Table 4).
Figure 1.
Kaplan-Meier curve for 1-year mortality. There was no difference in the unadjusted or adjusted mortality rates between the two cohorts at 30 days and at 1 year. The majority of patients died within 30 days post-myocardial infarction.
Table 4.
Cox proportional-hazard regression
| HR* | CI | P-value | |
|---|---|---|---|
| Age | 1.05 | 1.01–1.08 | 0.01 |
| Compl. revasc | 0.49 | 0.24–0.98 | 0.04 |
| Success. proc. | 0.38 | 0.15–0.98 | 0.04 |
| Levosimendan | 1.3 | 0.66–2.23 | 0.52 |
Risk of death according to treatment assignment and prognostic variables.
Abbreviations: CI, 95% confidence interval; Compl. revasc., completeness of revascularization; HR, hazard ratio; Success. proc., successful procedure.
Discussion
Cardiogenic shock is a serious complication of acute myocardial infarction. Prompt and successful revascularization is the only documented treatment strategy that reduces mortality.14 No firm scientific evidence has been presented to support the use of inotropic agents in the treatment of CS. In this study, we compared two treatment strategies from every-day clinical practice at a large university clinic. The most important result is that the use of levosimendan in CS has neutral effects on short-term and long-term mortality under the conditions described in the study.
Levosimendan is an inotropic agent approved in some countries for a treatment of AHF.24 It has been proposed that the positive inotropic effect of this substance is primarily mediated through calcium sensitization of troponin-C, and that this action does not increase myocardial oxygen consumption for a given inotropic effect when compared with traditional inotropic agents such as β-adrenergic receptor agonists (eg, dopamine, dobutamine) and phosphodiesterase (PDE) inhibitors (eg, milrinone).25 Levosimendan was introduced on the market during an era of increased awareness regarding the negative effects of inotropic agents on mortality in chronic heart failure (CHF) patients. This paradigm-shift was the result of compelling evidence from large randomized clinical trials showing that neurohormonal blockade (and therefore negative inotropy) improves the biology of the failing heart and prolongs life.26,27 Consequently, the advent of a pharmacological agent with the above-mentioned pharmacodynamic profile was very attractive, and levosimendan was enthusiastically accepted by many clinicians. However, this initial enthusiasm has been dampened considerably by accumulating evidence from experimental and clinical studies. Disappointingly, levosimendan acts much like traditional inotropic agents in terms of PDE inhibition,28,29 increased intracellular calcium concentration,30 and increased oxygen demands.31 Levosimendan has been reported to have cardioprotective effects25,32–35 in vitro and in animal models, although the significance of these ancillary pharmacodynamic properties for clinical end-points has not been clearly demonstrated. Indeed, the similarity between the traditional inotropes and levosimendan has been unambiguously demonstrated in two large clinical trials: SURVIVE8 and REVIVE II.9 Interestingly, the latter study has not been published in its entirety, although the trial data was first presented in 2005. Only two studies with levosimendan in CS have reported survival rates.36,37 Our findings reinforce data from a small randomized study comparing levosimendan with dobutamine on long-term survival. In this study, levosimendan and dobutamine had similar effects on survival at one-year.37 The absence of a meaningful clinical benefit (ie, morbidity, mortality) and high cost of levosimendan (eg, 10 times the cost of dopamine), in our opinion does not justify routine use of this agent for the “on-label” indication in AHF and even less so for the “off-label” indications, such as CS.
The strength of our study is the relatively large number of patients included, and that it was conducted at a large clinic with a well-organized and consistent prehospitalization and hospital care system. This ensures that a uniform approach was used for all patients, thus minimizing the effects of random variables caused by the absence of randomization.
Limitations
This was an observational study, and as such the results should be viewed primarily as hypothesis-building. Although we compensated for the differences in patient characteristics using standard statistical modeling, the inability to adjust for unknown confounders is inherent to observational studies. The use of levosimendan was not associated with any apparent adverse effects. However, levosimendan was part of the treatment concept together with revascularization and IABP. We cannot, therefore, extend our conclusions to the CS patients who were treated differently. It is possible that the use of IABP might have masked some of the adverse effects of levosimendan, such as hypotension, arrhythmias, and increased mortality.
Conclusion
Levosimendan neither decreases nor increases mortality in patients with CS. There is a compelling need to define the role of inotropic agents and other therapeutic interventions in the treatment of CS. This goal can only be achieved by well designed and conducted randomized trials.
Acknowledgments
The study was supported by grants from the Swedish Heart and Lung Foundation, Swedish Research Council, Gothenburg Medical Society, and the Swedish Medical Society. The authors express their gratitude to all individuals and authorities involved in the SCAAR and RIKS-HIA registries.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Goldberg RJ, Samad NA, Yarzebski J, Gurwitz J, Bigelow C, Gore JM. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med. 1999;340:1162–1168. doi: 10.1056/NEJM199904153401504. [DOI] [PubMed] [Google Scholar]
- 2.Hasdai D, Holmes DR, Jr, Califf RM, et al. Cardiogenic shock complicating acute myocardial infarction: Predictors of death. Gusto investigators. Global utilization of streptokinase and tissue-plasminogen activator for occluded coronary arteries. Am Heart J. 1999;138:21–31. doi: 10.1016/s0002-8703(99)70241-3. [DOI] [PubMed] [Google Scholar]
- 3.Hasdai D, Topol EJ, Califf RM, Berger PB, Holmes DR., Jr Cardiogenic shock complicating acute coronary syndromes. Lancet. 2000;356:749–756. doi: 10.1016/S0140-6736(00)02640-4. [DOI] [PubMed] [Google Scholar]
- 4.Lehtonen L. Levosimendan: A promising agent for the treatment of hospitalized patients with decompensated heart failure. Curr Cardiol Rep. 2000;2:233–243. doi: 10.1007/s11886-000-0074-6. [DOI] [PubMed] [Google Scholar]
- 5.Pinto BB, Rehberg S, Ertmer C, Westphal M. Role of levosimendan in sepsis and septic shock. Curr Opin Anaesthesiol. 2008;21:168–177. doi: 10.1097/ACO.0b013e3282f43c56. [DOI] [PubMed] [Google Scholar]
- 6.Tasouli A, Papadopoulos K, Antoniou T, et al. Efficacy and safety of perioperative infusion of levosimendan in patients with compromised cardiac function undergoing open-heart surgery: Importance of early use. Eur J Cardiothorac Surg. 2007;32:629–633. doi: 10.1016/j.ejcts.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Christoph A, Prondzinsky R, Russ M, et al. Early and sustained haemodynamic improvement with levosimendan compared to intraaortic balloon counterpulsation (IABP) in cardiogenic shock complicating acute myocardial infarction. Acute Card Care. 2008;10:49–57. doi: 10.1080/17482940701358564. [DOI] [PubMed] [Google Scholar]
- 8.Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: The survive randomized trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006;8:105–110. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Delaney A, Bradford C, McCaffrey J, Bagshaw SM, Lee R. Is there a place for levosimendan in the intensive care unit. Crit Care Resusc. 2007;9:290–292. [PubMed] [Google Scholar]
- 11.Landmesser U, Drexler H. Update on inotropic therapy in the management of acute heart failure. Curr Treat Options Cardiovasc Med. 2007;9:443–449. doi: 10.1007/s11936-007-0039-9. [DOI] [PubMed] [Google Scholar]
- 12.Lehtonen L, Poder P. The utility of levosimendan in the treatment of heart failure. Ann Med. 2007;39:2–17. doi: 10.1080/07853890601073346. [DOI] [PubMed] [Google Scholar]
- 13.Teerlink JR. Overview of randomized clinical trials in acute heart failure syndromes. Am J Cardiol. 2005;96:59G–67G. doi: 10.1016/j.amjcard.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. Shock investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 15.Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 16.Stenestrand U, Lindback J, Wallentin L. Long-term outcome of primary percutaneous coronary intervention vs prehospital and in-hospital thrombolysis for patients with ST-elevation myocardial infarction. JAMA. 2006;296:1749–1756. doi: 10.1001/jama.296.14.1749. [DOI] [PubMed] [Google Scholar]
- 17.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (Esicm) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 18.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 19.Cowley MJ, Vandermael M, Topol EJ, et al. Is traditionally defined complete revascularization needed for patients with multivessel disease treated by elective coronary angioplasty? Multivessel angioplasty prognosis study (MAPS) group. J Am Coll Cardiol. 1993;22:1289–1297. doi: 10.1016/0735-1097(93)90532-6. [DOI] [PubMed] [Google Scholar]
- 20.Follath F, Cleland JG, Just H, et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the lido study): A randomised double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/s0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
- 21.Moiseyev VS, Poder P, Andrejevs N, et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (russlan) Eur Heart J. 2002;23:1422–1432. doi: 10.1053/euhj.2001.3158. [DOI] [PubMed] [Google Scholar]
- 22.Cleland JG, Ghosh J, Freemantle N, et al. Clinical trials update and cumulative meta-analyses from the American College of Cardiology: WATCH, SCD-HeFT, DINAMIT, CASINO, INSPIRE, STRATUS-US, RIO-Lipids and cardiac resynchronisation therapy in heart failure. Eur J Heart Fail. 2004;6:501–508. doi: 10.1016/j.ejheart.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 24.Parissis JT, Rafouli-Stergiou P, Paraskevaidis I, Mebazaa A. Levosimendan: From basic science to clinical practice. Heart Fail Rev. 2009;14(4):265–275. doi: 10.1007/s10741-008-9128-4. [DOI] [PubMed] [Google Scholar]
- 25.Pollesello P, Papp Z. The cardioprotective effects of levosimendan: Preclinical and clinical evidence. J Cardiovasc Pharmacol. 2007;50:257–263. doi: 10.1097/FJC.0b013e3180986230. [DOI] [PubMed] [Google Scholar]
- 26.Effect of metoprolol cr/xl in chronic heart failure: Metoprolol cr/xl randomised intervention trial in congestive heart failure (merit-hf) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 27.The cardiac insufficiency bisoprolol study II (CIBIS-II): A randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 28.Hasenfuss G, Pieske B, Castell M, Kretschmann B, Maier LS, Just H. Influence of the novel inotropic agent levosimendan on isometric tension and calcium cycling in failing human myocardium. Circulation. 1998;98:2141–2147. doi: 10.1161/01.cir.98.20.2141. [DOI] [PubMed] [Google Scholar]
- 29.Hasenfuss G, Pieske B, Kretschmann B, Holubarsch C, Alpert NR, Just H. Effects of calcium sensitizers on intracellular calcium handling and myocardial energetics. J Cardiovasc Pharmacol. 1995;26(Suppl 1):S45–S51. [PubMed] [Google Scholar]
- 30.Ajiro Y, Hagiwara N, Katsube Y, Sperelakis N, Kasanuki H. Levosimendan increases L-type CA(2+) current via phosphodiesterase-3 inhibition in human cardiac myocytes. Eur J Pharmacol. 2002;435:27–33. doi: 10.1016/s0014-2999(01)01569-2. [DOI] [PubMed] [Google Scholar]
- 31.Todaka K, Wang J, Yi GH, et al. Effects of levosimendan on myocardial contractility and oxygen consumption. J Pharmacol Exp Ther. 1996;279:120–127. [PubMed] [Google Scholar]
- 32.Kersten JR, Montgomery MW, Pagel PS, Warltier DC. Levosimendan, a new positive inotropic drug, decreases myocardial infarct size via activation of K(ATP) channels. Anesth Analg. 2000;90:5–11. doi: 10.1097/00000539-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Maytin M, Colucci WS. Cardioprotection: A new paradigm in the management of acute heart failure syndromes. Am J Cardiol. 2005;96:26G–31G. doi: 10.1016/j.amjcard.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Tritapepe L, De Santis V, Vitale D, et al. Levosimendan pre-treatment improves outcomes in patients undergoing coronary artery bypass graft surgery. Br J Anaesth. 2009;102:198–204. doi: 10.1093/bja/aen367. [DOI] [PubMed] [Google Scholar]
- 35.Zangrillo A, Biondi-Zoccai G, Mizzi A, et al. Levosimendan reduces cardiac troponin release after cardiac surgery: A meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth. 2009;23:474–478. doi: 10.1053/j.jvca.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Fuhrmann JT, Schmeisser A, Schulze MR, et al. Levosimendan is superior to enoximone in refractory cardiogenic shock complicating acute myocardial infarction. Crit Care Med. 2008;36:2257–2266. doi: 10.1097/CCM.0b013e3181809846. [DOI] [PubMed] [Google Scholar]
- 37.Samimi-Fard S, Garcia-Gonzalez MJ, Dominguez-Rodriguez A, Abreu-Gonzalez P. Effects of levosimendan versus dobutamine on long-term survival of patients with cardiogenic shock after primary coronary angioplasty. Int J Cardiol. 2008;127:284–287. doi: 10.1016/j.ijcard.2007.04.143. [DOI] [PubMed] [Google Scholar]