Abstract
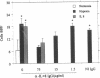
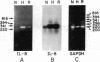
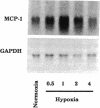
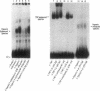
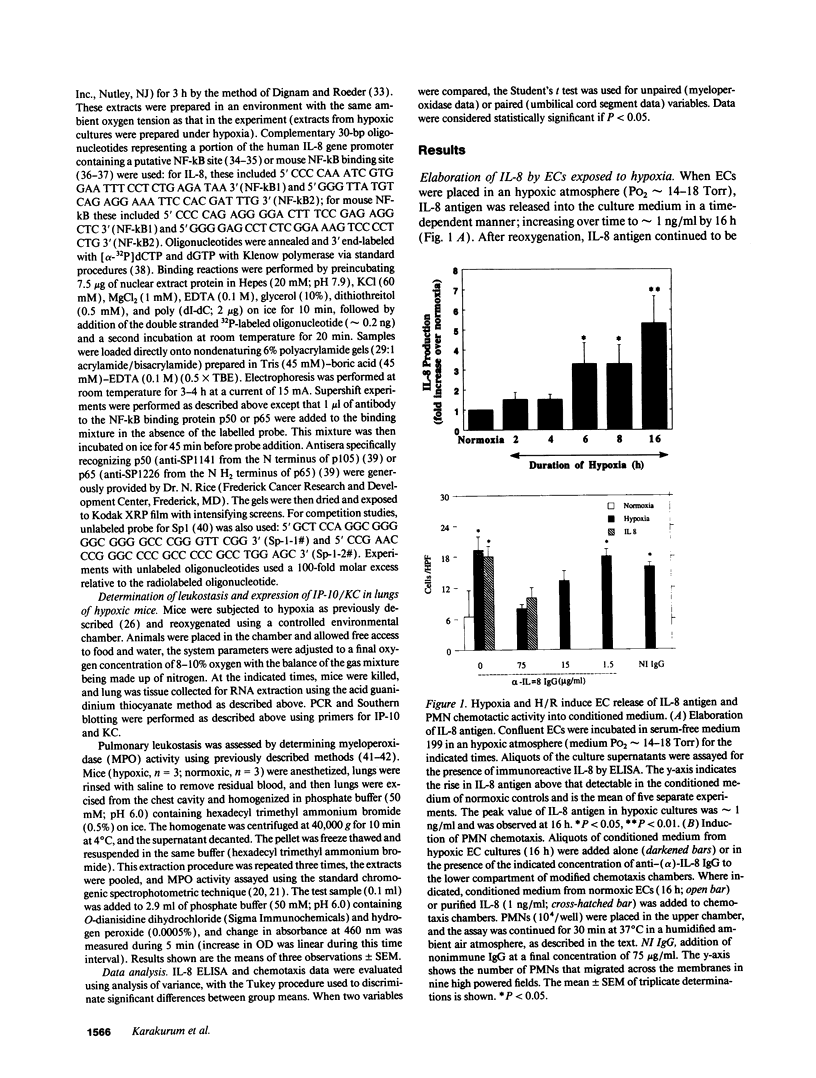
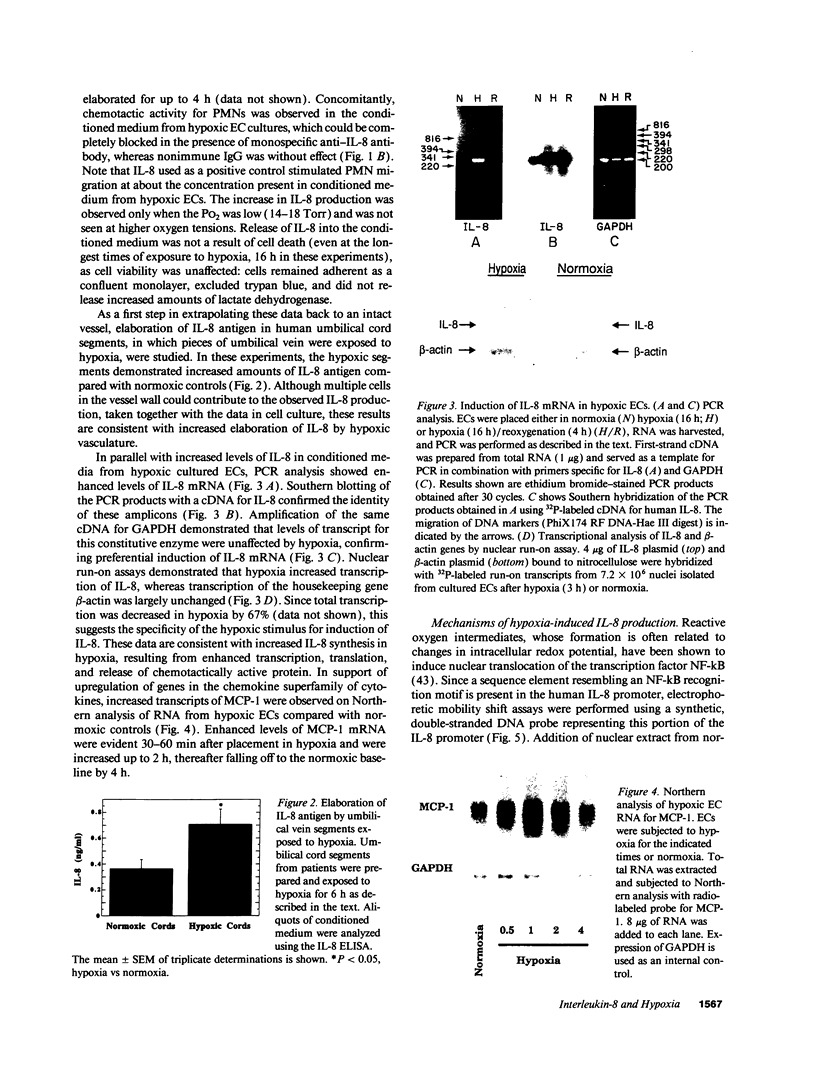
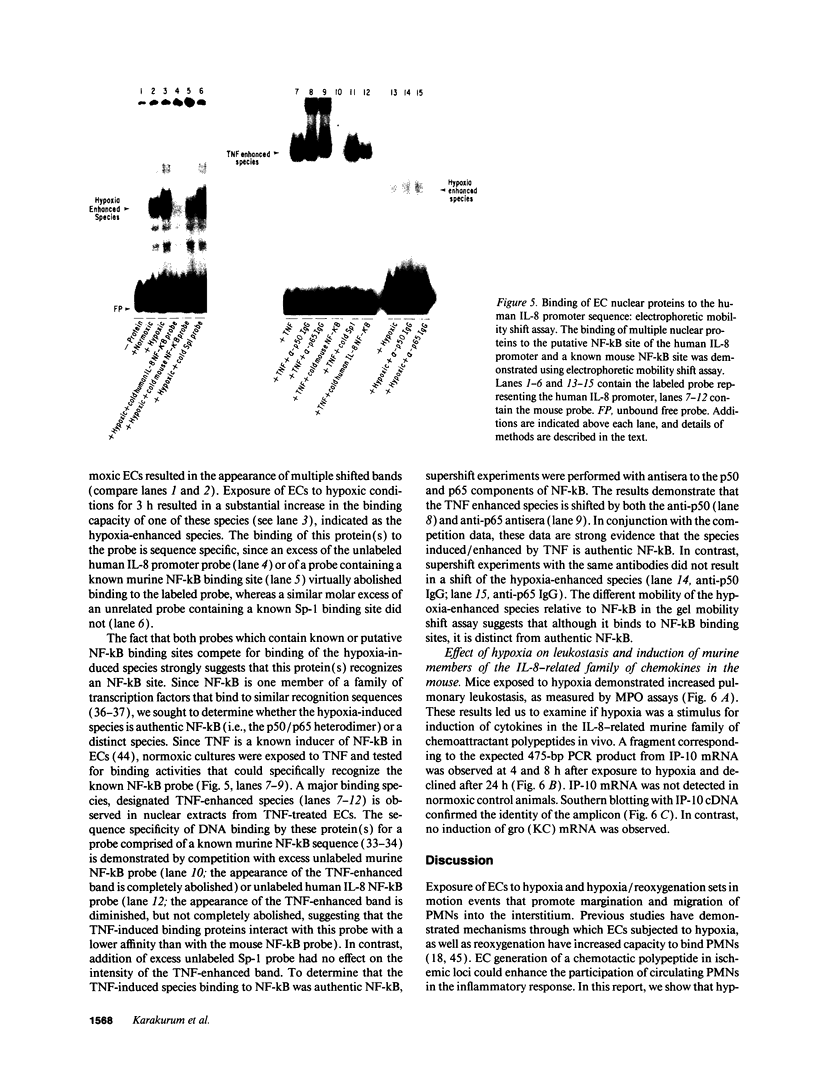
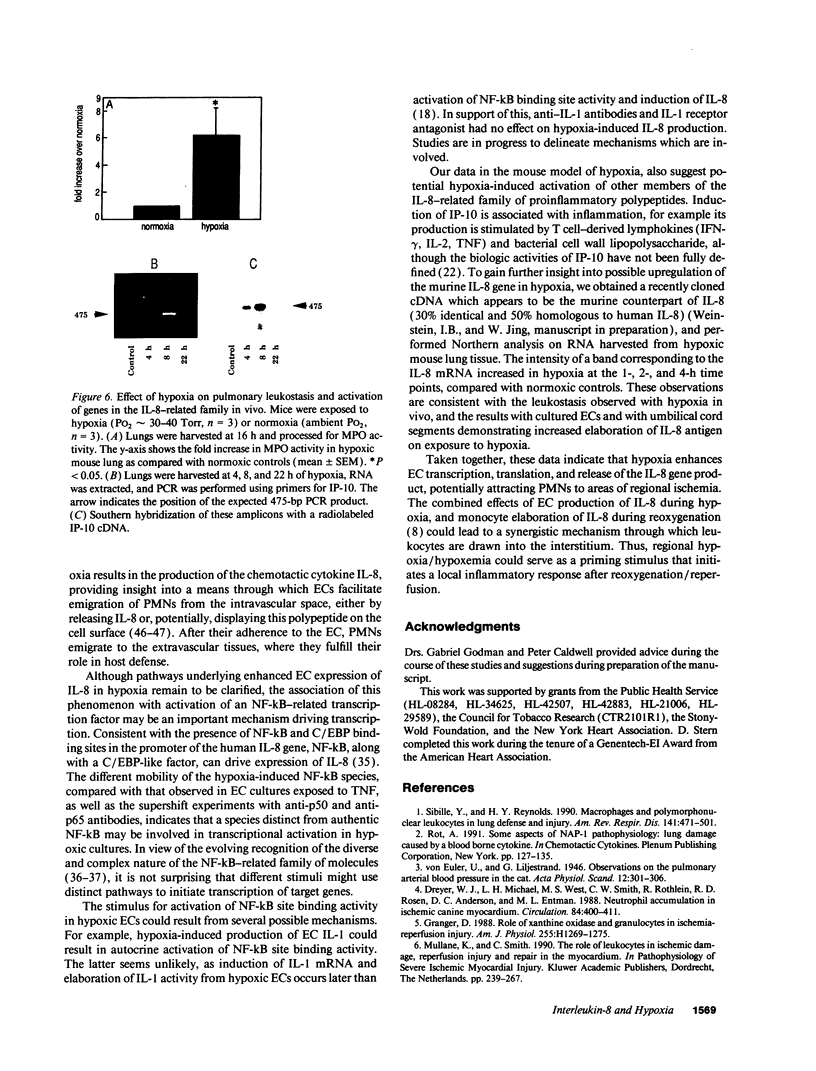
Because leukocyte-mediated tissue damage is an important component of the pathologic picture in ischemia/reperfusion, we have sought mechanisms by which PMNs are directed into hypoxic tissue. Incubation of human endothelial cells (ECs) in hypoxia, PO2 approximately 14-18 Torr, led to time-dependent release of IL-8 antigen into the conditioned medium; this was accompanied by increased chemotactic activity for PMNs, blocked by antibody to IL-8. Production of IL-8 by hypoxic ECs occurred concomitantly with both increased levels of IL-8 mRNA, based on polymerase chain reaction analysis, and increased IL-8 transcription, based on nuclear run-on assays. Northern analysis of mRNA from hypoxic ECs also demonstrated increased levels of mRNA for macrophage chemotactic protein-1, another member of the chemokine superfamily of proinflammatory cytokines. IL-8 gene induction was associated with the presence of increased binding activity in nuclear extracts from hypoxic ECs for the NF-kB site. Studies with human umbilical vein segments exposed to hypoxia also demonstrated increased elaboration of IL-8 antigen compared with normoxic controls. In mice exposed to hypoxia (PO2 approximately 30-40 Torr), there was increased pulmonary leukostasis, as evidenced by increased myeloperoxidase activity in tissue homogenates. In parallel, increased levels of transcripts for IP-10, a murine homologue in the chemokine family related to IL-8, were observed in hypoxic lung tissue. Taken together, these data suggest that hypoxia constitutes a stimulus for leukocyte chemotaxis and tissue leukostasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G. M., Freytag S. O. Synergistic activation of a human promoter in vivo by transcription factor Sp1. Mol Cell Biol. 1991 Apr;11(4):1935–1943. doi: 10.1128/mcb.11.4.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbs C. F., Cregor M. D., Turek J. J., Badylak S. F. Endothelial superoxide production in the isolated rat heart during early reperfusion after ischemia. A histochemical study. Am J Pathol. 1991 Nov;139(5):1069–1080. [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carré P. C., Mortenson R. L., King T. E., Jr, Noble P. W., Sable C. L., Riches D. W. Increased expression of the interleukin-8 gene by alveolar macrophages in idiopathic pulmonary fibrosis. A potential mechanism for the recruitment and activation of neutrophils in lung fibrosis. J Clin Invest. 1991 Dec;88(6):1802–1810. doi: 10.1172/JCI115501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford M. H., Grover F. L., Kolb W. P., McMahan C. A., O'Rourke R. A., McManus L. M., Pinckard R. N. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988 Dec;78(6):1449–1458. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- Detmers P. A., Lo S. K., Olsen-Egbert E., Walz A., Baggiolini M., Cohn Z. A. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990 Apr 1;171(4):1155–1162. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer W. J., Michael L. H., West M. S., Smith C. W., Rothlein R., Rossen R. D., Anderson D. C., Entman M. L. Neutrophil accumulation in ischemic canine myocardium. Insights into time course, distribution, and mechanism of localization during early reperfusion. Circulation. 1991 Jul;84(1):400–411. doi: 10.1161/01.cir.84.1.400. [DOI] [PubMed] [Google Scholar]
- Filep J., Hermán F., Braquet P., Mózes T. Increased levels of platelet-activating factor in blood following intestinal ischemia in the dog. Biochem Biophys Res Commun. 1989 Jan 31;158(2):353–359. doi: 10.1016/s0006-291x(89)80055-5. [DOI] [PubMed] [Google Scholar]
- Goldblum S. E., Wu K. M., Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol (1985) 1985 Dec;59(6):1978–1985. doi: 10.1152/jappl.1985.59.6.1978. [DOI] [PubMed] [Google Scholar]
- Granger D. N. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988 Dec;255(6 Pt 2):H1269–H1275. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S., Ogawa S., Kuwabara K., Brett J., Leavy J. A., Ryan J., Koga Y., Plocinski J., Benjamin W., Burns D. K. Synthesis and release of interleukin 1 by reoxygenated human mononuclear phagocytes. J Clin Invest. 1992 Sep;90(3):1007–1015. doi: 10.1172/JCI115913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Ibbotson G., Russell J., Wallace J. L., Granger D. N. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am J Physiol. 1990 Aug;259(2 Pt 1):G300–G305. doi: 10.1152/ajpgi.1990.259.2.G300. [DOI] [PubMed] [Google Scholar]
- Lo S. K., Everitt J., Gu J., Malik A. B. Tumor necrosis factor mediates experimental pulmonary edema by ICAM-1 and CD18-dependent mechanisms. J Clin Invest. 1992 Mar;89(3):981–988. doi: 10.1172/JCI115681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi B. R., Mullane K. M. Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol. 1986;26:201–224. doi: 10.1146/annurev.pa.26.040186.001221. [DOI] [PubMed] [Google Scholar]
- Metinko A. P., Kunkel S. L., Standiford T. J., Strieter R. M. Anoxia-hyperoxia induces monocyte-derived interleukin-8. J Clin Invest. 1992 Sep;90(3):791–798. doi: 10.1172/JCI115953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery K. F., Osborn L., Hession C., Tizard R., Goff D., Vassallo C., Tarr P. I., Bomsztyk K., Lobb R., Harlan J. M. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6523–6527. doi: 10.1073/pnas.88.15.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrucchio G., Alloatti G., Mariano F., de Paulis R., Comino A., Emanuelli G., Camussi G. Role of platelet-activating factor in the reperfusion injury of rabbit ischemic heart. Am J Pathol. 1990 Jul;137(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Mahe Y., Matsushima K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990 Dec 5;265(34):21128–21133. [PubMed] [Google Scholar]
- Mukaida N., Shiroo M., Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989 Aug 15;143(4):1366–1371. [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Ogawa S., Gerlach H., Esposito C., Pasagian-Macaulay A., Brett J., Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990 Apr;85(4):1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratych R. E., Chuknyiska R. S., Bulkley G. B. The primary localization of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987 Aug;102(2):122–131. [PubMed] [Google Scholar]
- Rice N. R., MacKichan M. L., Israël A. The precursor of NF-kappa B p50 has I kappa B-like functions. Cell. 1992 Oct 16;71(2):243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Rot A. Some aspects of NAP-1 pathophysiology: lung damage caused by a blood-borne cytokine. Adv Exp Med Biol. 1991;305:127–135. doi: 10.1007/978-1-4684-6009-4_15. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Engler R. L. Granulocytes as active participants in acute myocardial ischemia and infarction. Am J Cardiovasc Pathol. 1987 Jan;1(1):15–30. [PubMed] [Google Scholar]
- Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991 Aug;10(8):2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shlafer M., Brosamer K., Forder J. R., Simon R. H., Ward P. A., Grum C. M. Cerium chloride as a histochemical marker of hydrogen peroxide in reperfused ischemic hearts. J Mol Cell Cardiol. 1990 Jan;22(1):83–97. doi: 10.1016/0022-2828(90)90974-7. [DOI] [PubMed] [Google Scholar]
- Shreeniwas R., Koga S., Karakurum M., Pinsky D., Kaiser E., Brett J., Wolitzky B. A., Norton C., Plocinski J., Benjamin W. Hypoxia-mediated induction of endothelial cell interleukin-1 alpha. An autocrine mechanism promoting expression of leukocyte adhesion molecules on the vessel surface. J Clin Invest. 1992 Dec;90(6):2333–2339. doi: 10.1172/JCI116122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille Y., Reynolds H. Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990 Feb;141(2):471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Granger D. N., Anderson D. C., Rothlein R., Lane C., Kvietys P. R. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am J Physiol. 1992 Jun;262(6 Pt 2):H1891–H1898. doi: 10.1152/ajpheart.1992.262.6.H1891. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]