Abstract
Objectives:
To assess the efficacy and tolerability of a fixed-dose combination of olmesartan and amlodipine in an unselected population of patients in primary care and to compare the results with recent randomized controlled trial evidence.
Methods:
A multicenter, noninterventional, noncontrolled observational study with 8241 hypertensive patients seen by 2187 physicians in daily practice. Blood pressure (BP) reduction, comorbid disease, pharmacotherapy, and tolerability were documented over a 12–18-week observational period.
Results:
Patients had a mean age of 62.8 ± 11.8 years (48.1% female), and 74.8% had at least one comorbid risk factor or condition. In total, 51.3% received olmesartan-amlodipine 20/5 mg, 30.6% received 40/5 mg, and 17.9% received 40/10 mg at baseline, mostly because of lack of efficacy on prior antihypertensive therapy (73.8%). BP at baseline was 161.8 ± 16.6/93.6 ± 10.2 mmHg (39.8% had Grade 2 hypertension), and the observed BP reduction was −29.0 ± 17.1/−13.5 ± 10.9 mmHg (P < 0.0001), with a significant correlation between BP at baseline and BP reduction (Spearman’s Rho −0.811 for systolic BP and −0.759 for diastolic BP). BP reduction appeared to be dependent on dose and prior antihypertensive therapy, but not on age, gender, body mass index, duration of hypertension, or the presence of diabetes. At the final visit, 69.4% (4.3% at baseline) were controlled (<140/90 mmHg). Adverse drug reactions were observed in 2.76% of the study population; 94.25% of these adverse drug reactions were judged as nonserious events, and 31.5% of all adverse drug reactions reported were peripheral edema.
Conclusion:
The fixed-dose olmesartan-amlodipine combination was effective and well tolerated in an unselected population of patients in primary care practice. These results confirm prior randomized controlled trial evidence.
Keywords: blood pressure, cardiovascular risk, antihypertensive treatment, observation
Introduction
The combination of two antihypertensive drugs is recommended by the European Society of Hypertension (ESH)/European Society of Cardiology (ESC)1,2 in patients with blood pressure (BP) not adequately controlled with antihypertensive monotherapy or as first-line therapy in patients at high risk. Blockers of the renin–angiotensin system, being the foundation of antihypertensive therapy in >50% of patients, are recommended to be combined with either thiazide diuretics (hydrochlorothiazide) or calcium channel blockers. Within this context, fixed-dose combinations offer the advantage of easy once-daily dosing which has been shown to improve patient compliance. While there was a preference for thiazide-type diuretics in older recommendations, recent data from the ACCOMPLISH (Avoiding Cardiovascular Events in Combination Therapy in Patients Living with Systolic Hypertension) study3 have resulted in a shift of this preference.2 In ACCOMPLISH, a fixed combination of an angiotensin-converting enzyme (ACE) inhibitor with hydrochlorothiazide was compared with a respective combination incorporating amlodipine, resulting in a significant reduction of cardiovascular morbidity and mortality with the amlodipine combination.
In response to a rising demand for highly effective and tolerable fixed-dose combinations with amlodipine, a combination of olmesartan-amlodipine was developed that was able to reduce BP by −30.1/−19.0 mmHg in doses up to 40/10 mg in a recent study.4 Almost maximal BP reduction was achieved as early as four weeks after treatment initiation and was sustained throughout the eight-week study period. Tolerability was good, with a reduction of incidence of edema, known to be increased with amlodipine monotherapy at high doses. Fixed-dose combinations of antihypertensive drugs improve patient compliance which, in turn, is associated with a reduction in hospitalization and cardiovascular events.5,6
These results prompted us to question whether the efficacy and safety results seen in this double-blind randomized clinical trial would be preserved in an open-label, noninterventional, observational study in primary care. This comparison is of particular importance because patients with unusual characteristics (eg, very old/young, very obese/slim, those with abundant concomitant medication) are usually excluded from randomized clinical trials, but are not infrequently encountered in primary care practice.
Patients and methods
SERVE (DE-SEV-02-08-DE) was a noninterventional, noncontrolled, prospective study in primary care practice in Germany. It was part of a European project including 15,000 patients. Participating practices were sampled at random from specialists in general medicine, internal medicine, cardiology, and practitioners from all regions defined by the Institute of Medical Statistics. The study was conducted according to local laws and regulations (§67 (6) Arzneimittelgesetz, AMG) and the FSA Code (Freiwillige Selbstkontrolle für die Arzneimittelindustrie e.V.), and was duly notified to the federal authorities (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) and the federal panel doctors’ association (Kassenärztliche Bundesvereinigung, KBV). Ethics committee approval and written patient informed consent were obtained.
Patients
Patients showing primary arterial hypertension and in whom previous pharmacotherapy did not reach target BP recommended by the ESH were identified. Beyond these criteria, physicians were free to select patients within the scope of drug licensing, ie, no other inclusion and exclusion criteria were defined. They were also allowed to adjust the dosage of the drug and of any concomitant medication throughout the duration of the study according to patients’ needs.
Recording of data
At the enrolment visit, demographic data and data relating to the presence of hypertension (BP, heart rate, target BP achievement, and duration of hypertension) were obtained. Patient history including further concomitant cardiovascular risk factors and disease (metabolic syndrome, diabetes mellitus, stable angina pectoris, left ventricular hypertrophy, myocardial infarction, stroke/transitory ischemic attack, peripheral arterial disease, reduction of kidney or liver function, and smoking) was documented, along with disease duration. With respect to pharmacotherapy, previous/concomitant antihypertensive therapy and concomitant non antihypertensive medication were recorded. Finally, the reason for switching to the fixed combination of olmesartan-amlodipine was documented.
At the optional checkup visits at 4–6 and 8–12 weeks and the final visit at 12–18 weeks, adverse events, changes in BP, target BP achievement, and physician assessment of patient compliance, effectiveness, and tolerability (very good, good, satisfactory, insufficient) were obtained.
Physicians were asked to document patients who were being treated with olmesartan-amlodipine (20/5 mg, 40/5 mg, 40/10 mg) because of inadequately controlled primary arterial hypertension. The documentation forms were recorded by Christine Franzen Consulting, a Clinical Research Organization in Stolberg, Germany. Data were examined for their plausibility in all documentation forms. Five percent of the participating physicians underwent monitoring of their documentation by fax. The patients were recorded in pseudonymized form, ie, only the age and gender of the patient were recorded, and all data collected during the observation period were treated confidentially. Data were electronically stored in accordance with data protection provisions. An honorarium for the documentation of eligible patients was paid in accordance with the official scale of physicians’ fees (Gebührenordnung für Ärzte [GOÄ]).
Adverse drug reactions
Adverse drug reactions were explicitly asked about at each follow-up visit and recorded in detail (eg, nature, date, duration, result, and causal relationship with therapy). All physicians were asked to report serious adverse events to the pharmaceutical manufacturer immediately, which in turn notified the BfArM in accordance with legal requirements.
Statistical analysis
The statistical analysis was descriptively conducted, exploratively assessed, and then produced in tabular and graphic form. For categoric data, absolute and relative frequencies and, for continuous variables, the average and standard deviation were calculated. SAS software (v. 9.2 SAS Institute, Cary, NC) was used for the statistical analyses. The Spearman’s Rho was used for rank correlations (relationship of initial BP to reduction in BP). In order to be able to record rare adverse drug reactions, the number of cases was set at 8000 patients, which allowed adverse drug reactions up to an incidence of 0.1% to be recorded with a probability of almost 100% and adverse drug reactions with an incidence of 0.01% and a probability of 55.1%.
Results
Patient characteristics
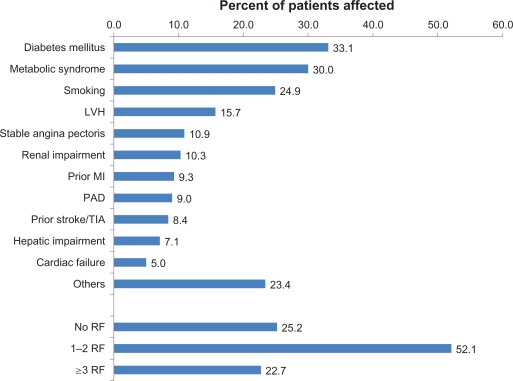
A total of 8241 patients were identified by 2187 physicians between January and December 2009, of whom 8237 had received an olmesartan-amlodipine fixed combination (safety population). Patients had a mean age of 62.8 ± 11.8 years (48.1% female). All but one patient had arterial hypertension (99.99%), and 47.5% for more than five years. See Table 1 for further details of patient characteristics. A total of 74.8% of patients had at least one cardiovascular risk factor or had comorbid cardiovascular disease (mean number 1.9 ± 2.3, Figure 1), with diabetes mellitus (33.1%), metabolic syndrome (30.0%), and smoking (24.9%) being the most frequent.
Table 1.
Characteristics of patient safety population
| Data available | n | Value | |
|---|---|---|---|
| Age (mean ± SD, years) | 8237 | 62.8 ± 11.8 | |
| Age ≥ 65 years | 8237 | 3841 | 46.6 |
| Female (%) | 8237 | 3966 | 48.1 |
| BMI (mean ± SD; kg/m2) | 8157 | 29.0 ± 4.7 | |
| Obesity (%) | 8157 | 2835 | 34.8 |
| Waist circumference | |||
| Men (% ≥ 102 cm) | 3570 | 2119 | 59.4 |
| Women (% ≥ 88 cm) | 3242 | 2567 | 79.2 |
| Hypertension (%) | 8237 | 8236 | 99.9 |
| >5 years (%) | 8009 | 3801 | 47.5 |
Abbreviations: SD, standard deviation; BMI, body mass index.
Figure 1.
Comorbid cardiovascular risk factors and disease.
Abbreviations: LVH, left ventricular hypertrophy; MI, myocardial infarction; PAD, peripheral arterial disease; TIA, transient ischemic attack; RF, risk factors.
Antihypertensive treatment at baseline and during follow-up
In total, 7511/8235 (91.2%) of patients had received any prior antihypertensive therapy. An olmesartan-amlodipine fixed-dose combination was frequently introduced in preference to ACE inhibitors, calcium channel blockers, and angiotensin receptor blockers (Table 2). After initiation, 51.3% received olmesartan-amlodipine 20/5 mg, 30.6% received 40/5 mg, and 17.9% received 40/10 mg. Most patients (73.8%) were switched because of lack of efficacy and 17.3% because of lack of tolerability on prior therapy, 14.2% because of insufficient compliance, and 17.4% were switched from a free combination to a fixed combination of both agents (13.8% guideline recommendation, 2.6% missing; multiple answers possible).
Table 2.
Antihypertensive therapy (safety population; multiple answers possible)
| Data available |
Baseline |
After initiation |
|||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Beta-blockers | 8237 | 3431 | 41.7 | 2463 | 29.9 |
| Diuretics | 8237 | 2561 | 31.1** | 1661 | 20.2 |
| ACE inhibitors | 8237 | 3567 | 43.3 | 534 | 6.5 |
| Calcium channel blockers | 8237 | 3314 | 40.2** | 511 | 6.2 |
| Amlodipine | 8237 | 2360 | 28.7 | 343 | 4.2 |
| Angiotensin receptor blockers | 8237 | 3155 | 38.3 | 514 | 6.2 |
| Olmesartan | 8237 | 1288 | 15.6 | 277 | 3.4 |
| Others* | 8237 | 1286 | 15.6 | 922 | 11.2 |
| Olmesartan-amlodipine 20/5 | 8237 | n.a. | n.a. | 4232 | 51.3 |
| Olmesartan-amlodipine 40/5 | 8237 | n.a. | n.a. | 2526 | 30.6 |
| Olmesartan-amlodipine 40/10 | 8237 | n.a. | n.a. | 1479 | 17.9 |
Notes:
Central and peripheral acting antiadrenergic drugs/alpha-blockers/vasodilators/others;
1935 patients received fixed-dose combinations of renin angiotensin system blocking agents with either diuretics or calcium channel blockers.
Abbreviations: ACE, angiotensin-converting enzyme; n.a., not applicable.
The mean duration of exposure was 114.1 ± 34.5 days (median 110 [interquartile range 93–128]). Patient compliance was regarded by the physicians to be very good in 66.7% or good in 28.0% (total of 94.7%), but some patients were switched by the treating physicians between dose combinations (Table 3). In total, 75.1% received their initial fixed-dose combination until study end, 24.9% being either up- or downtitrated.
Table 3.
Shift table for added antihypertensive treatment at baseline and for cumulative changes at the last documented visit (safety population)
|
Olmesartan-amlodipine 20/5 mg at last documented visit |
Olmesartan-amlodipine 40/5 mg at last documented visit |
Olmesartan-amlodipine 40/10 mg at last documented visit |
Total (baseline)* |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Olmesartan-amlodipine 20/5 mg at baseline | 2877 | 37.0 | 817 | 10.5 | 301 | 3.8 | 3955 | 51.4 |
| Olmesartan-amlodipine 40/5 mg at baseline | 132 | 1.7 | 1736 | 22.3 | 538 | 6.9 | 2406 | 31.0 |
| Olmesartan-amlodipine 40/10 mg at baseline | 50 | 0.6 | 77 | 0.9 | 1233 | 15.8 | 1360 | 17.5 |
| Total (follow-up) | 3059 | 39.4 | 2630 | 33.8 | 2072 | 26.6 | ||
Note:
Add up to 99.9% because of rounding.
BP at baseline and during follow-up
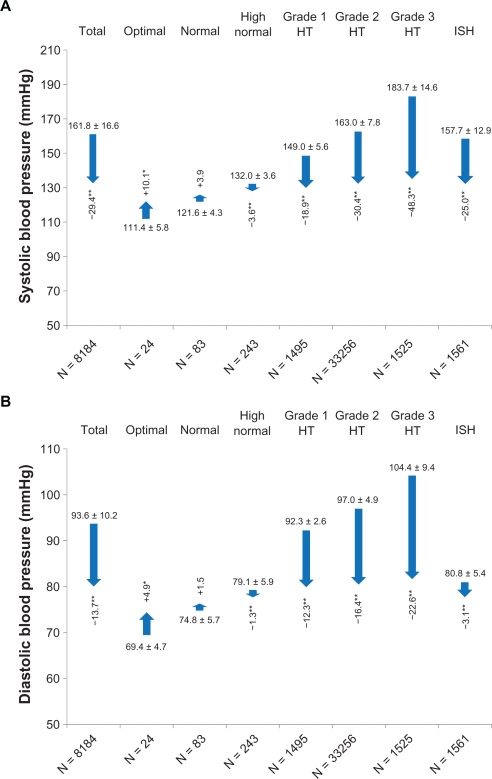
Mean BP at baseline was 161.8 ± 16.6/93.6 ± 10.2 mmHg (pulse pressure 68.2 ± 14.7), the majority being classified to have ESH/ESC Grade 2 hypertension (39.8%); 18.3% and 18.6% had Grade 1 or Grade 3 hypertension, respectively, and 19.1% had isolated systolic hypertension. There was an overall mean BP reduction of 29.0 ± 17.1/13.5 ± 10.9 mmHg (P < 0.0001 versus baseline; pulse pressure 15.7 ± 15.0, P < 0.0001 versus baseline), that increased dependent on initial BP classification (Figure 2). BP reduction was most pronounced in patients with Grade 3 hypertension (−48.3 ± 16.9/−22.6 ± 11.4 mmHg, P < 0.0001 versus baseline). Accordingly, there was a clear correlation between systolic BP reduction and systolic BP at baseline (Spearman’s Rho −0.811), diastolic BP reduction and diastolic BP at baseline (Spearman’s Rho −0.759), and pulse pressure reduction and pulse pressure at baseline (Spearman’s Rho −0.804).
Figure 2.
Blood pressure lowering with respect to blood pressure category at baseline.
Notes: *P < 0.01; **P < 0.0001.
Abbreviations: HT, hypertension; ISH, isolated systolic hypertension.
BP reduction at the final visit was also dependent on the dose employed (Table 4). Using olmesartan-amlodipine 20/5 mg, a mean BP reduction of −27.6 ± 16.3 mmHg/−13.2 ± 10.6 mmHg was achieved, that was increased up to −31.0 ± 18.8 mmHg/−14.1 ± 11.7 mmHg with olmesartan-amlodipine 40/10 mg.
Table 4.
|
Δ Systolic BP |
Δ Diastolic BP |
|||||||
|---|---|---|---|---|---|---|---|---|
| SERVE | Pvalue versus baseline | SERVE subgroup** | COACH | SERVE | Pvalue versus baseline | SERVE subgroup** | COACH | |
| BP at baseline | 161.8 ± 16.6 | 166.3 ± 15.4 | 163.8 ± 16.1 | 93.6 ± 10.2 | 108.0 ± 5.9 | 101.6 ± 5.2 | ||
| Olmesartan-amlodipine 20/5 mg | −27.6 ± 16.3 (n = 3254) | <0.0001 | −31.9 ± 15.4 (n = 989) | −23.6 ± 14.9 (n = 160) | −13.2 ± 10.6 (n = 3254) | <0.0001 | −19.5 ± 8.1 (n = 989) | −14.0 ± 9.1 (n = 160) |
| Olmesartan-amlodipine 40/5 mg | −29.5 ± 16.5 (n = 2749) | <0.0001 | −33.0 ± 15.2 (n = 766) | −25.4 ± 14.7 (n = 157) | −13.5 ± 10.5 (n = 2749) | <0.0001 | −18.8 ± 8.1 (n = 766) | −15.5 ± 8.2 (n = 157) |
| Olmesartan-amlodipine 40/10 mg | −31.0 ± 18.8 (n = 2083) | <0.0001 | −35.0 ± 18.0 (n = 467) | −30.1 ± 15.9 (n = 161) | −14.1 ± 11.7 (n = 2083) | <0.0001 | −19.8 ± 9.7 (n = 467) | −19.0 ± 8.9 (n = 161) |
Note:
Assignment to dose was based on the previous visit.
SERVE patients complying with the COACH in- and exclusion criteria.
Abbreviation: BP, blood pressure.
Patient characteristics, including age, body mass index, duration of hypertension (except for patients with a short duration in which BP lowering was enhanced) and the presence of diabetes had no substantial influence on the efficacy of olmesartan-amlodipine. In contrast, efficacy was nominally higher in patients without previous/concomitant antihypertensive medication than in those with prior medication (−34.3 ± 17.4 versus −28.9 ± 16.9 mmHg systolic and −17.5 ± 11.0 versus −13.3 ± 10.7 mmHg diastolic).
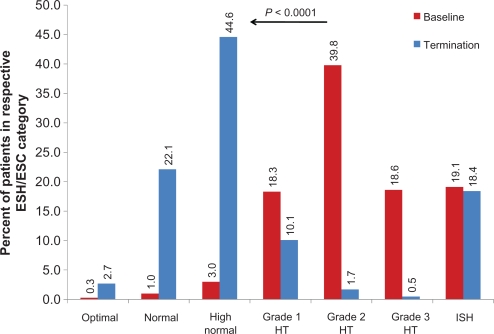
At the final visit (usually 12–18 weeks after enrolment), there was a strong shift in the ESH/ESC categorization (Figure 3), with 69.4% of patients (4.3% at baseline) having only high normal BP (18.4% isolated systolic hypertension; 19.1% at baseline). Patients with isolated systolic hypertension at the final visit usually had had moderate to severe hypertension at baseline, while patients with isolated systolic hypertension at baseline usually were categorized as being high-normal at the final visit.
Figure 3.
Blood pressure categorization according to European Society of Hypertension and European Society of Cardiology.
Note: P value for the comparison of classification at the termination visit versus baseline.
Abbreviations: HT, hypertension; ISH, isolated systolic hypertension.
Tolerability
Adverse drug reactions occurred in a total of 227 patients during the study, representing 2.76% of all included patients (Table 5). Of all adverse drug reactions, 213 were assessed as nonserious and 13 as serious by the reporting physicians. The status of seriousness was not assessable due to a lack of data in one case. Within this study, three deaths have been reported. None of them was related to the study medication according to the judgment of the treating physicians.
Table 5.
Number of patients with an adverse drug reaction in the observation period
| Total population (n = 8237) | O20, A5 (patients, n) | O40, A5 (patients, n) | O40, A10 (patients, n) | Total (patients, n) |
| Patients without ADR | 4141 | 2472 | 1400 | 8013 |
| Patients with ADR | 91 | 54 | 79 | 226* |
| Serious | 5 | 4 | 3 | 13 |
| Not serious | 86 | 50 | 76 | 213 |
| Change in dose* | O20, A5 (n, % of patients with ADRs) | O40, A5 (n, % of patients with ADRs) | O40, A10 (n, % of patients with ADRs) | Total (n, % of patients with ADRs) |
| Not changed | 3 (1.33) | 4 (1.78) | 4 (1.78%) | 11 (4.89%) |
| Drug withdrawn | 86 (38.22) | 49 (21.78) | 68 (30.22%) | 204 (90.67%)** |
| Dose reduced | 1 (0.44) | 1 (0.44) | 6 (2.67%) | 8 (3.56%) |
| Dose increased | 1 (0.44) | 0 (0) | 0 (0%) | 1 (0.44%) |
| Unknown | 0 (0) | 0 (0) | 1 (0.44%) | 1 (0.44%) |
Notes:
Data from one patient not included;
One patient with unknown dose.
Abbreviation: ADRs, adverse drug reactions.
Table 6 shows an overview of all reported events, clustered and coded according to MedDRA (12.0; MedDRA MSSO, Berlin, Germany). In total, 338 events were reported, the largest number (147) within the system organ class “General disorders and administration site conditions” harboring the lowest level term “peripheral edemas”; 31.5% of all reported adverse drug reactions were peripheral edema in 111/8237 patients. These results are in agreement with the physicians’ assessment of tolerability which was “very good” (70.7%) or “good” (25.6%) in the majority of treated patients.
Table 6.
Adverse drug reactions coded according to MedDRA® Version 12.0
| Total population (n = 8237) MedDRA®primary system organ classes (SOC) | O20, A5 | O40, A5 | O40, A10 | Total |
|---|---|---|---|---|
| Total number of ADRs, n (%) | 140 (41.42) | 76 (22.49) | 121 (35.80%) | 338 (100%)* |
| Cardiac disorders | 2 (0.59) | 2 (0.59) | 3 (0.89%) | 7 (2.07%) |
| Ear and labyrinth disorders | 1 (0.30) | 1 (0.30) | 1 (0.30%) | 3 (0.89%) |
| Eye disorders | 2 (0.59) | 0 (0) | 3 (0.89%) | 5 (1.48%) |
| Gastrointestinal disorders | 18 (5.33) | 12 (3.55) | 6 (1.78%) | 36 (10.65%) |
| General disorders and administration site conditions | 45 (13.31) | 36 (10.65) | 65 (19.23%) | 147 (43.49%)* |
| Hepatobiliary disorders | 1 (0.30) | 0 (0) | 0 (0%) | 1 (0.30%) |
| Immune system disorders | 1 (0.30) | 0 (0) | 0 (0%) | 1 (0.30%) |
| Infections and infestations | 0 (0) | 0 (0) | 1 (0.30%) | 1 (0.30%) |
| Investigations | 3 (0.89) | 2 (0.59) | 0 (0%) | 5 (1.48%) |
| Metabolism and nutrition disorders | 2 (0.59) | 0 (0) | 0 (0%) | 2 (0.59%) |
| Musculoskeletal and connective tissue disorders | 4 (1.18) | 3 (0.89) | 8 (2.37%) | 15 (4.44%) |
| Nervous system disorders | 27 (7.99) | 4 (1.18) | 8 (2.37%) | 39 (11.54%) |
| Psychiatric disorders | 3 (0.89) | 1 (0.30) | 9 (2.66%) | 13 (3.85%) |
| Reproductive system and breast disorders | 2 (0.59) | 0 (0%) | 2 (0.59%) | 4 (1.18%) |
| Respiratory, thoracic and mediastinal disorders | 8 (2.37) | 4 (1.18) | 2 (0.59%) | 14 (4.14%) |
| Skin and subcutaneous tissue disorders | 13 (3.85) | 4 (1.18) | 7 (2.07%) | 24 (7.10%) |
| Vascular disorders | 8 (2.37) | 7 (2.07) | 6 (1.78%) | 21 (6.21%) |
Note:
One patient each with unknown dose.
Abbreviation: ADRs, adverse drug reactions.
Discussion
There is substantial cardiovascular risk conferred by the presence of arterial hypertension, which has been shown to be reduced in patients with adequate BP control using antihypertensive medication. A fixed-dose combination of olmesartan-amlodipine, which was documented to be effective and well tolerated in previous randomized, controlled trials,4,7–12 has been observed under clinical practice conditions in the present study for a 12–18 week period resulting in the documentation of a strong BP lowering with high tolerability.
Blood pressure lowering in perspective
The results of SERVE conducted in the real-life situation include a strong reduction of BP in hypertensive patients by 29.0 ± 17.1/13.5 ± 10.9 mmHg (P < 0.0001 versus baseline) with a clear correlation between BP at baseline and BP reduction (Spearman’s Rho −0.811 for systolic BP and −0.759 for diastolic BP) and a dependency on dose and prior antihypertensive therapy, but not on age, gender, body mass index, duration of hypertension, or the presence of diabetes. After a usual treatment duration of 12–18 weeks, 69.4% of patients managed to attain the high-normal BP level.
The study results at the final visit (Table 4) should be interpreted in comparison with the results of the recent randomized, controlled COACH (Counseling Older Adults to Control Hypertension) trial4 based on the respective dose level of the fixed combination. COACH had a follow-up of eight weeks. Mean systolic BP reductions achieved are nominally higher in SERVE than in COACH, with differences being more pronounced in the lower dose range (olmesartan-amlodipine 20/5 mg). On the other hand, the reduction of diastolic BP was stronger in COACH than in SERVE. There are a variety of reasons that might account for the observed differences in BP reduction, even beyond differences in patient characteristics outlined in Table 7. First and foremost, mean BP at baseline was 161.8/93.6 mmHg in SERVE and 163.6/101.5 mmHg in COACH, indicating similar systolic but substantial differences in diastolic BP. This would argue for a stronger reduction of diastolic BP in COACH, because the fall in diastolic BP is strongly related to baseline BP. Second, the values obtained in SERVE have been obtained on the background of a variety of different drugs. The fixed combination could be added to an existing antihypertensive drug therapy and even used in treatment-naïve patients (which would not have been on-label). Third, the comparison is based on pharmacotherapy at baseline, and most of the patients were uptitrated and others were downtitrated. This also might result in a bias of the overall results towards higher BP reductions, in particularly at low doses.
Table 7.
| COACH (Randomized, controlled trial) | SERVE (Noninterventional study) | |
|---|---|---|
| Inclusion | Men or women ≥ 18 years Seated DBP ≥ 95 mmHg and ≤120 mmHg; difference ≤10 mmHg between 2 measurements |
Men or women ≥ 18 years Essential hypertension uncontrolled |
| Exclusion | DBP > 120 mmHg | |
| History of cardiovascular disease | Cardiogenic shock, acute MI (<4 weeks), unstable angina | |
| Uncontrolled diabetes | Severely reduced liver function and biliary duct obstruction | |
| Smoking > one pack of cigarettes | ||
| Laboratory values or systemic disease considered clinicallysignificant by the investigator | ||
| Taking any medication that could interfere with the objectives of the study | ||
| Pregnancy and nursing | 2nd and 3rd trimester of pregnancy |
Abbreviations: DBP, diastolic blood pressure; MI, myocardial infarction; COACH.
While a detailed comparison of BP-lowering efficacy on a patient basis between SERVE and COACH is beyond the scope of the present analysis, the results strongly suggest that BP observed in clinical trials is similar to that found in primary care, and point to the possibility of adjusting BP effectively with the fixed-dose combination with the benefit of the known advantages of fixed-dose combinations, such as convenient use and increased patient compliance.
Tolerability of fixed-dose combination
The adverse event profile of olmesartan has been analyzed in placebo-controlled trials over two years of treatment, with more than 3000 patients showing good tolerability.13,14 On the other hand, dihydropyridine calcium channel blockers, such as amlodipine, have been associated with peripheral edema, which is likely to result from preferential arteriolar vasodilation and an increase in the pressure gradient between the arteriolar and venular capillaries, leading to exudation of interstitial fluid.15,16
Data from the recent randomized controlled COACH trial4 testing amlodipine up to 10 mg and/or olmesartan up to 40 mg versus placebo suggest an amelioration of peripheral edema with the combined use of amlodipine and olmesartan. While 36.8% of patients on 10 mg amlodipine (18.5% with 40 mg olmesartan) experienced edema, the incidence was reduced to 23.5% in patients receiving a combination at the same doses. This is thought to be the result of lower precapillary resistance, normalized intracapillary pressure, and reduced fluid exudation with the use of the angiotensin receptor blocker.15,17
In this observational study, 2.76% of patients had adverse drug reactions, of which about one-third (31.5% of the aforementioned 2.76%) were peripheral edema, suggesting a substantially lowered risk of edema in clinical practice, or a lower vigilance of physicians in primary care for this side effect, questioning its clinical relevance. Differences in the incidence of edema may also be somewhat related to the overall treatment duration. While an incidence rate of 23.5% was reported for the first randomized, controlled eight-week phase of COACH,4 an incidence rate of only 14.5% was reported for the 44-week open-label extension.8 It may also be related to a certain degree of underreporting in trials such as SERVE, in which physicians were not specifically asked whether edema had been present or not, as opposed to COACH in which there were specific questions addressing this issue.
Limitations
The present study has the inherent limitations of noninterventional studies, including lack of a control group and no randomization. Therefore, the BP lowering reported may be a higher estimate than the true effect. On the other hand, the study documented a substantial BP reduction across a wide range of different patients, of which a proportion would not qualify for a randomized controlled trial such as COACH, because of comorbid disease and concomitant pharmacotherapy (Table 4). Therefore, this kind of study is of particular importance when targeting a high external validity, and reflects actual clinical practice to a far greater extent than randomized trials.
Conclusion
The fixed-dose combination of olmesartan and amlodipine was effective and well tolerated in an unselected population of patients in primary care practice. These results confirm prior randomized controlled trial evidence.
Acknowledgments
The efforts of participating physicians, their staff, and the patients who participated in this study are acknowledged.
Footnotes
Disclosure
The study was funded by Daiichi Sankyo Deutschland GmbH. PB, RK, and RES received research funding and lecture honoraria from Daiichi Sankyo. WPW, TS, and WE are employees of Daiichi Sankyo Deutschland GmbH. EMF is an employee of Daiichi Sankyo Europe GmbH. The preparation of the manuscript was funded by Daiichi Sankyo Deutschland GmbH.
References
- 1.Mancia G, de Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force document. Blood Press. 2009;18:308–347. doi: 10.3109/08037050903450468. [DOI] [PubMed] [Google Scholar]
- 3.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 4.Chrysant SG, Melino M, Karki S, Lee J, Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther. 2008;30:587–604. doi: 10.1016/j.clinthera.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 6.Mathes J, Kostev K, Gabriel A, Pirk O, Schmieder RE. Relation of the first hypertension-associated event with medication, compliance and persistence in naive hypertensive patients after initiating monotherapy. Int J Clin Pharmacol Ther. 2010;48:173–183. doi: 10.5414/cpp48173. [DOI] [PubMed] [Google Scholar]
- 7.Barrios V, Brommer P, Haag U, Calderon A, Escobar C. Olmesartan medoxomil plus amlodipine increases efficacy in patients with moderate-to-severe hypertension after monotherapy: A randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig. 2009;29:427–439. doi: 10.2165/00044011-200929070-00001. [DOI] [PubMed] [Google Scholar]
- 8.Chrysant SG, Oparil S, Melino M, Karki S, Lee J, Heyrman R. Efficacy and safety of long-term treatment with the combination of amlodipine besylate and olmesartan medoxomil in patients with hypertension. J Clin Hypertens (Greenwich) 2009;11:475–482. doi: 10.1111/j.1751-7176.2009.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mourad JJ, Le Jeune S. Effective systolic blood pressure reduction with olmesartan medoxomil/amlodipine combination therapy: Post hoc analysis of data from a randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig. 2009;29:419–425. doi: 10.2165/00044011-200929060-00005. [DOI] [PubMed] [Google Scholar]
- 10.Oparil S, Lee J, Karki S, Melino M. Subgroup analyses of an efficacy and safety study of concomitant administration of amlodipine besylate and olmesartan medoxomil: Evaluation by baseline hypertension stage and prior antihypertensive medication use. J Cardiovasc Pharmacol. 2009;54:427–436. doi: 10.1097/FJC.0b013e3181bad74e. [DOI] [PubMed] [Google Scholar]
- 11.Volpe M, Brommer P, Haag U, Miele C. Efficacy and tolerability of olmesartan medoxomil combined with amlodipine in patients with moderate to severe hypertension after amlodipine monotherapy: A randomized, double-blind, parallel-group, multicentre study. Clin Drug Investig. 2009;29:11–25. doi: 10.2165/0044011-200929010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Chrysant SG, Lee J, Melino M, Karki S, Heyrman R. Efficacy and tolerability of amlodipine plus olmesartan medoxomil in patients with difficult-to-treat hypertension. J Hum Hypertens. 2010 Feb 18; doi: 10.1038/jhh.2010.5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puchler K, Laeis P, Stumpe KO. Blood pressure response, but not adverse event incidence, correlates with dose of angiotensin II antagonist. J Hypertens Suppl. 2001;19:S41–S48. doi: 10.1097/00004872-200106001-00006. [DOI] [PubMed] [Google Scholar]
- 14.Schindler C, Ferrario CM. Olmesartan for the treatment of arterial hypertension. Future Cardiol. 2008;4:357–372. doi: 10.2217/14796678.4.4.357. [DOI] [PubMed] [Google Scholar]
- 15.Pedrinelli R, Dell’Omo G, Mariani M. Calcium channel blockers, postural vasoconstriction and dependent oedema in essential hypertension. J Hum Hypertens. 2001;15:455–461. doi: 10.1038/sj.jhh.1001201. [DOI] [PubMed] [Google Scholar]
- 16.Messerli FH. Vasodilatory edema: A common side effect of antihypertensive therapy. Am J Hypertens. 2001;14:978–979. doi: 10.1016/s0895-7061(01)02178-1. [DOI] [PubMed] [Google Scholar]
- 17.Weir MR. Incidence of pedal edema formation with dihydropyridine calcium channel blockers: Issues and practical significance. J Clin Hypertens (Greenwich) 2003;5:330–335. doi: 10.1111/j.1524-6175.2003.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]