Abstract
Heparin-induced thrombocytopenia (HIT) is an immunoglobulin-mediated serious complication of heparin therapy characterized by thrombocytopenia and high risk for venous and arterial thrombosis: HIT and thrombosis syndrome (HITTS). Argatroban, a direct thrombin inhibitor, is indicated as the anticoagulant for the treatment and prophylaxis of thrombosis in patients with HIT and in patients undergoing percutaneous coronary intervention (PCI) who have HIT. The aim of this review is to examine the pharmacological characteristics and the clinical efficacy and safety of this drug in adults with HIT, including those undergoing PCI. Briefly, 2 prospective multicenter, nonrandomized, open-label studies evaluated the efficacy and safety of argatroban as an anticoagulant in patients with HIT or HITTS. Both studies showed that the incidence of the primary efficacy end point, a composite of all-cause death, all-cause amputation, or new thrombosis, was reduced in argatroban-treated patients vs control subjects with HIT or HITTS. In both studies, bleeding rates were similar between the groups. Argatroban was evaluated as the anticoagulant therapy in 3 prospective, multicenter, open-label studies in HIT patients who underwent PCI. The studies were similar in design with respect to patient inclusion and exclusion criteria, the argatroban dosing regimen, and primary efficacy outcomes. The investigators performed a pooled analysis of these studies, which showed that most (≥95%) patients achieved a satisfactory outcome from the procedure and adequate anticoagulation (coprimary end points).
Keywords: argatroban, thrombocytopenia, thrombosis
Introduction
Heparin-induced thrombocytopenia (HIT) is an immunoglobulin-mediated serious complication of heparin therapy characterized by thrombocytopenia and high risk for venous and arterial thrombosis: HIT and thrombosis syndrome (HITTS).1 Two distinct types of HIT can occur: nonimmune and immune-mediated HIT. Nonimmune HIT, which occurs more frequently, is characterized by a mild decrease in the platelet count and is not harmful. The second type, immune-mediated HIT, occurs much less frequently but is dangerous. Immune-mediated HIT causes much lower platelet counts. Paradoxically, despite a very low platelet count, patients who suffer from HIT are at risk for major clotting problems.2
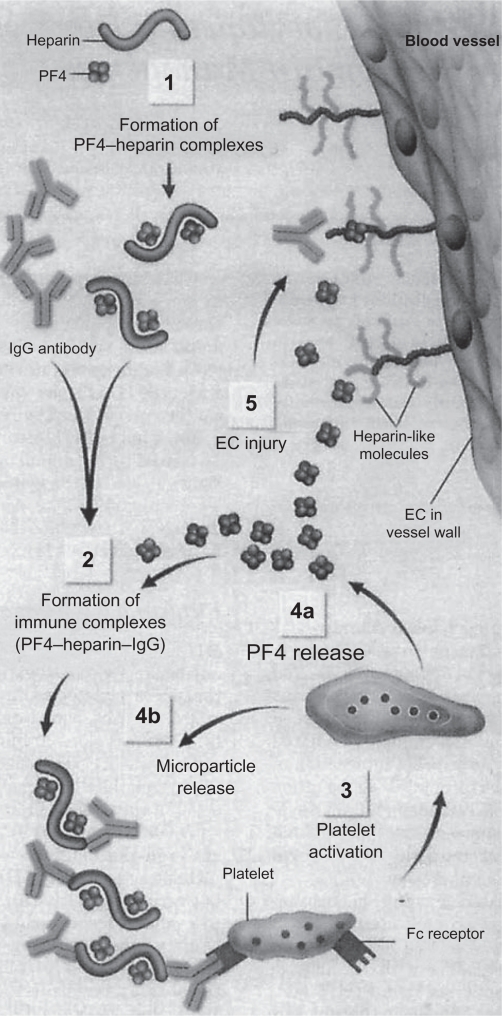
Immune-mediated HIT usually occurs from 5 to 14 days after first beginning heparin therapy. However, there are exceptions, with HIT infrequently developing either early (after a recent previous exposure to heparin) or late after heparin exposure.2 Heparin has high affinity for platelet factor 4 (PF4), a positively charged protein found in platelet α-granules and on some cell surfaces including platelets and endothelial cells. When heparins and PF4 bind, PF4 undergoes a conformational change, exposing neoepitopes that act as immunogens and lead to the generation of heparin–PF4 antibodies.3 The condition of HIT is caused by such antibodies, most frequently IgG, binding to the heparin–PF4 complex. The heparin–PF4 antibodies (sometimes called “HIT antibodies”) in the resultant multimolecular immune complex activate platelets via their Fc receptors, causing the release of prothrombotic platelet-derived microparticles, platelet consumption, and thrombocytopenia.3 The microparticles in turn promote excessive thrombin generation, frequently resulting in thrombosis. The antibody–antigen complexes also interact with monocytes, leading to tissue factor production, and thus antibody-mediated endothelial injury may occur. Both of these latter processes may contribute further to thrombosis.3 The pathogenesis of HIT is summarized in Figure 1.
Figure 1.
Pathogenesis of HIT: Heparin binds with PF4, which exposes neoepitopes on PF4 and leads to antibody production (1). Heparin–PF4–IgG immune complexes form (2), and IgG in the multimolecular complex triggers platelet activation via binding to Fc receptors (3). Activated platelet releases additional PF4 (4a) and prothrombotic platelet microparticles (4b), which enhance coagulation reactions. Thrombotic risk is further promoted by the binding of PF4 to heparin-like molecules (heparin sulfate molecules) on EC, contributing to antibody-mediated endothelial injury (5).
Reprinted with permission from Jang IK, Hursting MJ. When heparins promote thrombosis: review of heparin-induced thrombocytopenia. Circulation. 2005;111: 2671–2683.3 Copyright © 2005 Wolters Kluwer Health.
Abbreviations: HIT, heparin-induced thrombocytopenia; PF4, platelet factor 4; EC, endothelial cells.
For patients who are receiving heparin or have received heparin within the previous 2 weeks, the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (Eighth Edition) recommend investigating for a diagnosis of HIT if the platelet count falls by >50% and/or a thrombotic event occurs between days 5 and 14 (inclusive) following the initiation of heparin, even if the patient was no longer receiving heparin therapy when the thrombosis or thrombocytopenia occurred.4 Thrombocytopenia is common in hospitalized patients receiving unfractionated heparin (UFH), yet only a minority have HIT. A clinical scoring system may be useful for identifying those with HIT. Table 1 summarizes a clinical scoring system (4 T’s) for estimating the pretest probability of HIT based on its characteristic features (thrombocytopenia, timing, thrombosis) and the absence of other explanation(s).1
Table 1.
Estimating the pretest probability of HIT: the “4 T’s”
|
Points (0, 1, or 2 for each of 4 categories: maximum possible score = 8) |
|||
|---|---|---|---|
| 2 | 1 | 0 | |
| Thrombocytopenia | >50% platelet fall to nadir ≥20 | 30%–50% platelet fall or nadir 10–19 | <30% platelet fall or nadir <10 |
| Timinga of onset of platelet fall (or other sequelae of HIT) | 5–10 d or ≤1 d with recent heparin (past 30 d) | >10 d or timing unclear or <1 d with recent heparin (past 30–100 d) | <4 d (no recent heparin) |
| Thrombosis or other sequaele | Proven new thrombosis; skin necrosis; or acute systemic reaction after intravenous UFH bolus | Progressive or recurrent thrombosis; erythematous skin lesions; suspected thrombosis (not proven) | None |
| Other causes of platelet fall | None evident | Possible | Definite |
First day of immunizing heparin exposure considered day 0.
Reprinted with permission from Warkentin TE. Heparin-induced thrombocytopenia: diagnosis and management. Circulation. 2004;110:e454–e458.1 Copyright © 2004 Wolters Kluwer Health.
Notes: Pretest probability score: 6–8 indicates high; 4–5, intermediate; and 0–3, low.
Abbreviations: HIT, heparin-induced thrombocytopenia; UFH, unfractionated heparin.
Two types of assays for HIT, washed platelet activation assays and commercial PF4 enzyme immunoassays, are sensitive in detecting clinically relevant HIT antibodies; thus, a negative test generally rules out HIT. However, because weak (nonpathogenic) antibodies can also be detected (especially by enzyme immunoassays), a positive test does not necessarily confirm HIT, especially if the test is only weakly positive and suggests an alternative explanation.1 It is important to interpret the test in the appropriate clinical context.
Argatroban, a direct thrombin inhibitor, is indicated as the anticoagulant for the treatment and prophylaxis of thrombosis in patients with HIT. The aim of this review is to examine the pharmacological characteristics and the clinical efficacy and safety of this drug in adults with HIT, including those undergoing percutaneous coronary intervention (PCI).
Argatroban: pharmacological characteristics, dosage, and monitoring therapy
Argatroban is a synthetic direct thrombin inhibitor derived from L-arginine that reversibly binds to the thrombin active site. Argatroban exerts its anticoagulant effects by inhibiting thrombin-catalyzed or thrombin-induced reactions, including fibrin formation; activation of coagulation factors V, VIII, and XIII; activation of protein C; and platelet aggregation. Argatroban is capable of inhibiting the action of both free and clot-associated thrombin.5 When argatroban is administered by continuous infusion, immediately upon the initiation of infusion, anticoagulant effects are produced as its plasma concentrations begin to rise. Steady-state levels of both drug and anticoagulant effects are typically attained within 1–3 hours and are maintained until the infusion is discontinued or the dosage adjusted. The argatroban dose dependently increases the activated partial thromboplastin time (aPTT), the activated clotting time (ACT), the prothrombin time, the international normalized ratio (INR), and the thrombin time in healthy volunteers and cardiac patients.5
In adult patients with HIT or HITTS and without hepatic impairment, after discontinuation of heparin therapy and after determining baseline aPTT, the recommended initial dosage of argatroban is 2 μg/kg/min administered as a continuous infusion. The aPTT should be checked 2 hours after the initiation of therapy and after every dosage adjustment (not to exceed 10 μg/kg/min), until the steady-state aPTT is 1.5–3.0 times the initial baseline value (not to exceed 100 seconds).5
In adult patients with HIT or HITTS undergoing PCI, after determining the baseline ACT, argatroban infusion should be started at 25 μg/kg/min and an intravenous (IV) bolus of 350 μg/kg administered over 3–5 minutes. The ACT should be checked 5–10 minutes after the bolus is completed. If the ACT is less than 300 seconds, an additional IV bolus dose of 150 μg/kg should be administered, the infusion dose increased to 30 μg/kg/min, and the ACT checked 5–10 minutes later. If the ACT is greater than 450 seconds, the infusion rate should be decreased to 15 μg/kg/min, and the ACT checked 5–10 minutes later. Once a therapeutic ACT (between 300 and 450 seconds) has been achieved, this infusion dose should be continued for the duration of the procedure. In case of dissection, impending abrupt closure, thrombus formation during the procedure, or inability to achieve or maintain an ACT over 300 seconds, additional bolus doses of 150 μg/kg may be administered and the infusion dose increased to 40 μg/kg/min. The ACT should be checked after each additional bolus or change in the rate of infusion. Additional ACTs should be drawn about every 20–30 minutes during a prolonged procedure and at the end of PCI procedure. If a patient requires anticoagulation after the procedure, argatroban may be continued, but at lower infusion dosages (those recommended for patients with HIT or HITTS).5
No initial dosage adjustment is required in patients with renal impairment.5 In adult patients with hepatic impairment and HIT or HITTS, the initial infusion dosage should be reduced to 0.5 μg/kg/min and aPTT should be monitored closely; similarly, in adult patients with HIT or HITTS and hepatic impairment who are undergoing PCI, argatroban dosages should be titrated carefully to achieve therapeutic ACTs.5 Use of high doses of argatroban in PCI patients with clinically significant hepatic disease or alanine aminotransferase/aspartate aminotransferase levels ≥3 times the upper limit of normal should be avoided.5
Although the approved initial dosage of argatroban (as recommended by the manufacturer) for adults with HIT or HITTS is 2 μg/kg/min in patients with normal hepatic function and 0.5 μg/kg/min in patients with hepatic dysfunction, there is evidence that a reduced initial dose may also be prudent for patients with heart failure, multiple organ-system failure, severe anasarca, or after cardiac surgery (ie, conditions associated with hepatic congestion/dysfunction, which may potentially decrease argatroban clearance).6–12 Based on these observations of mean doses used in recent reports of argatroban-treated patients13–16 and in a multicenter registry,17,18 the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (Eighth Edition) suggests lower initial dosages of argatroban (0.5–1.2 μg/kg/min) in the aforementioned clinical settings, as well as for patients with hepatic impairment. Moreover, no initial dosage adjustment is suggested in patients with renal impairment, however, in the absence of the aforementioned conditions4.
Argatroban is contraindicated in patients with overt major bleeding. Extreme caution is advised during the use of argatroban in conditions or circumstances that increase the risk of hemorrhage, such as severe hypertension; immediately following lumbar puncture; spinal anesthesia; major surgery, especially involving the brain, spinal cord, or eye; and hematologic conditions associated with increased bleeding tendencies such as congenital or acquired bleeding disorders and gastrointestinal lesions such as ulcerations.5
There is no specific antidote to argatroban overdose; thus, if life-threatening bleeding occurs or excessive plasma concentrations of argatroban are suspected, therapy should be discontinued immediately, aPTT should be performed, and symptomatic therapy should be provided to the patient.5
When switching to oral anticoagulant therapy after argatroban treatment, the potential for combined effects of argatroban and warfarin on INR measurements should be taken into consideration. It is suggested that argatroban and warfarin therapy should be overlapped to avoid prothrombotic effects and to ensure continuous anticoagulation when initiating warfarin. Warfarin therapy should be initiated at the expected daily dose; a loading dose of warfarin should not be used. The INR values should be measured daily while coadministering these agents. Generally, argatroban can be discontinued when the INR is >4 following treatment with argatroban (up to 2 μg/kg/min) and warfarin. The INR should be measured again 4–6 hours after discontinuation of argatroban, and if the repeat INR is below the therapeutic range, argatroban infusion may be resumed. This procedure should be repeated daily until the desired therapeutic INR is achieved with warfarin alone.5
Argatroban in adults with HIT
Two prospective multicenter, nonrandomized, open-label studies (ARG-911 and ARG-915 studies) evaluated the efficacy and safety of argatroban as an anticoagulant in patients with HIT or HITTS. When these studies were initiated, no approved alternative agent was available for use as an active comparator; therefore, historical control subjects were studied for comparison. Patients were assigned at enrollment to either the HIT study arm (for those with isolated HIT) or the HITTS study arm (for those with HIT complicated by thrombosis that occurred after heparin initiation).19,20
Eligible patients had thrombocytopenia, defined as a platelet count <100 ×109/L, or a 50% reduction in count after heparin therapy with no explanation besides HIT. Patients who had a documented history of positive HIT antibody (ie, latent disease) and who required anticoagulation were eligible for the HIT arm, in the absence of thrombocytopenia or heparin challenge.19,20
All patients with an unexplained aPTT value >2 times the control at baseline; a documented coagulation disorder or bleeding diathesis unrelated to HITTS; a lumbar puncture within the past 7 days; or a history of previous aneurysm, hemorrhagic stroke, or recent (within 6 months) thrombotic stroke unrelated to HITTS were excluded.19,20
Historical control cases were identified by means of prospectively agreed on, documented approaches, which included a review of the laboratory logs of patients who were tested for HIT or were thrombocytopenic. Control subjects were treated according to the local standard of practice at the time of HIT diagnosis, with typical treatments being heparin discontinuation and/or oral anticoagulation.19,20 The historical controls used in the ARG-915 study were drawn from the same historical controls used in the ARG-911 study. Study ARG-915 included an initial phase in which 264 patients were enrolled and an extension phase in which the remaining patients were enrolled.
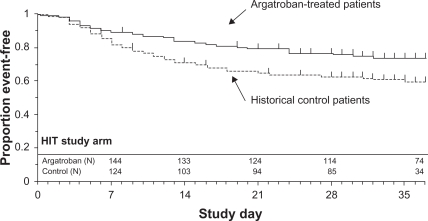
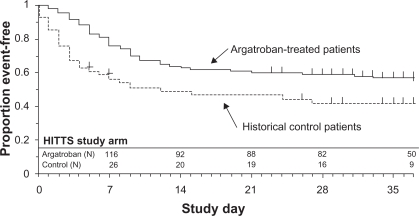
The ARG-911 study showed that the incidence of the primary efficacy end point, a composite of all-cause death, all-cause amputation, or new thrombosis, was reduced significantly in argatroban-treated patients vs control subjects with HIT (25.6% vs 38.8%, P = 0.014). In HITTS, the composite incidence in argatroban-treated patients was 43.8% vs 56.5% in control subjects (P = 0.13). Significant differences between groups were noted by time-to-event analysis of the composite end point–favored argatroban treatment in HIT (P = 0.01, Figure 2) and HITTS (P = 0.014, Figure 3). Argatroban therapy, relative to control subjects, also significantly reduced new thrombosis and death caused by thrombosis (P <0.05).19
Figure 2.
Time to first event for composite end point: HIT study arm.
Reprinted with permission from Lewis BE, Wallis DE, Berkowitz SD, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103:1838–1843.19 Copyright © 2001 Wolters Kluwer Health.
Abbreviation: HIT, heparin-induced thrombocytopenia.
Figure 3.
Time to first event for composite end point: HITTS study arm.
Reprinted with permission from Lewis BE, Wallis DE, Berkowitz SD, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103:1838–1843.19 Copyright © 2001 Wolters Kluwer Health.
Abbreviation: HITTS, heparin-induced thrombocytopenia thrombosis syndrome.
The ARG-915 study showed that in the HIT arm, the composite end point was significantly reduced in argatroban-treated patients vs control subjects with HIT (28.0% vs 38.8%, P = 0.04). In the HITTS arm, the composite end point occurred in 41.5% of argatroban-treated patients vs 56.5% of control subjects (P = 0.07). According to the time-to-event analysis of the composite end point, argatroban therapy was significantly better than historical control therapy in HIT (P = 0.02, Figure 2) and HITTS (P = 0.008, Figure 3) cases. Argatroban therapy also significantly reduced new thrombosis in HIT and HITTS cases and death due to thrombosis in HITTS.20 In both studies, bleeding rates were similar among groups.19,20
Argatroban in adults with HIT undergoing PCI
In 3 prospective, multicenter, open-label studies (ARG-216, ARG-310, and ARG-311 studies) in which HIT patients underwent PCI, argatroban was evaluated as the anticoagulant therapy. The studies – a dose confirmation study and 2 efficacy and safety studies – were similar in design with respect to patient inclusion and exclusion criteria, the argatroban dosing regimen, primary efficacy outcomes, and recorded outcomes. The investigators performed a pooled analysis of these studies and reported the outcomes of 91 HIT patients who underwent 112 separate coronary interventions on a total of 177 treated lesions (149 in the initial group and 28 in the repeat group).21
Eligible patients were males or nonpregnant females >18 years old with a documented history of HIT as evidenced by a previous or current positive HIT antibody test, or a previous or current clinical diagnosis of HIT. The HIT condition was defined as a platelet count <100 × 109/L or a 50% reduction in platelet count after the initiation of heparin with no other apparent cause than HIT. Eligible patients also had coronary artery disease requiring elective, urgent, or emergent PCI. Use of any approved device including balloon angioplasty, atherectomy, or stent implantation was allowed. Patients were excluded if they had clinically relevant hepatic dysfunction; uncontrolled hypertension; documented bleeding diathesis; uncontrolled peptic ulcer disease, or gastrointestinal bleeding within 6 weeks; hypersensitivity to aspirin or argatroban; previous history of hemorrhagic stroke; suffered thrombotic stroke within the past 7 days; were pregnant or lactating; or had received heparin within 6 hours of dosing, a thrombolytic agent within 24 hours, or warfarin within 72 hours. Concomitant use of a glycoprotein IIb/IIIa receptor antagonist was disallowed.21
Patients received oral aspirin (325 mg) 2–24 hours before PCI. After sheath placement, a 350 μg/kg bolus of argatroban was administered over 3–5 minutes, and a continuous IV infusion was initiated at 25 μg/kg/min (30 μg/kg/min in ARG-216). The ACT was measured 5–10 minutes after completion of the bolus. The infusion dose was adjusted between 15–40 μg/kg/min (20–40 μg/kg/min in ARG-216), and up to 3 additional bolus doses of 150 μg/kg were allowed to achieve and maintain ACTs of 300–450 seconds during PCI. The ACT was measured after each bolus dose, at the end of PCI, and as clinically indicated during and after PCI. At the investigator’s discretion, argatroban anticoagulation was continued postprocedurally at a reduced dose (<10 μg/kg/min), adjusted to achieve an aPTT 1.5–3.0 times the preprocedural value. The mean dosages of argatroban during the infusion were 23 and 22 μg/kg/min in the initial and the repeat PCI groups, which were administered for a mean of 1.2 hours; after PCI, 44 patients received reduced argatroban dosages (<10 μg/kg/min) for 2.1 days.21
Patients were followed during argatroban infusion and for 24 hours after its cessation. The primary efficacy outcomes were subjective assessments by the investigator of the attainment of a satisfactory outcome of the procedure and of adequate anticoagulation during the procedure. Secondary objective assessments of efficacy included the lack of acute major complications (ie, the absence of death, emergent coronary artery bypass graft surgery, or Q-wave myocardial infarction), attainment of angiographic success (ie, a final stenosis of <50% in ≥1 lesion attempted), and attainment of clinical success (ie, angiographic success plus the lack of acute major complications). The incidence of death, any myocardial infarction, or revascularization (emergency coronary artery bypass graft surgery or repeat PCI) at 24 hours was determined for each group.21
The primary end point of a satisfactory outcome of the procedure was attained in 94.5% of the initial group and 100% of the repeat group. An unsatisfactory outcome of the procedure was reported in 5 patients in the initial group.21
The other coprimary efficacy end point was the achievement of adequate anticoagulation during the procedure. In the initial and repeat groups, respectively, 97.8% and 100% of patients achieved adequate anticoagulation. Inadequate anticoagulation was noted in 2 patients in the initial group.
Of these 2 patients, 1 never attained an ACT in the target range of 300–450 seconds (however, PCI was performed and clinical success was achieved in 1 patient, as the investigators considered the anticoagulation to be sufficient), whereas the other patient required emergent revascularization despite achieving clotting times within the target range.21
No major acute complications (ie, death, emergent coronary artery bypass graft surgery, or Q-wave myocardial infarction) occurred in 97.8% and 100%, respectively, of patients in the initial and repeat groups. In the initial group, no patient died, repeat PCI occurred in 3 (3%) patients, emergent coronary artery bypass graft surgery occurred in 2 (2%) patients (one of whom first underwent repeat PCI), and myocardial infarction (each non-Q-wave) occurred in 4 (4%) patients. In the repeat group, there was no death, periprocedural revascularization, or myocardial infarction. Overall, the composite of these events occurred in 7 (7.7%) patients in the initial group and in none of the patients (0%) in the repeat group.21
Conclusion
Argatroban improved clinical outcomes and was generally well tolerated in adults with HIT. In 2 pivotal, open-label, historically controlled studies conducted on adults with HIT, the incidence of the primary composite end point was significantly lower in argatroban recipients than in historical controls, and more argatroban recipients than historical controls stayed event-free during the study. Based on this evidence, for patients with strongly suspected (or confirmed) HIT, whether or not complicated by thrombosis, the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (Eighth Edition) recommend the use of argatroban (grade of recommendation 1C), as well as other nonheparin anticoagulants (danaparoid [grade of recommendation 1B], lepirudin [grade of recommendation 1C], fondaparinux [grade of recommendation 2C], or bivalirudin [grade of recommendation 2C]), over the further use of UFH or low-molecular-weight heparin therapy or initiation/continuation of vitamin K antagonists (grade of recommendation 1B).4
Moreover, argatroban was found to be an effective anticoagulant in patients with HIT undergoing PCI from the pooled data from 3 small, uncontrolled trials, which showed that most patients achieved a satisfactory outcome of the PCI procedure and adequate anticoagulation.21 Accordingly, these data support argatroban as an alternative anticoagulant for HIT patients during PCI.
Finally, although the approved initial dosage of argatroban (as recommended by the manufacturer) for adults with HIT or HITTS is 2 μg/kg/min in patients with normal hepatic function and 0.5 μg/kg/min in patients with hepatic dysfunction, there is evidence that a reduced initial dose may also be prudent for patients with heart failure, multiple organ-system failure, severe anasarca, or after cardiac surgery (ie, conditions associated with hepatic congestion/dysfunction, which may potentially decrease argatroban clearance). Coagulation parameters should be analyzed carefully.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Warkentin TE. Heparin-induced thrombocytopenia: diagnosis and management. Circulation. 2004;110:e454–e458. doi: 10.1161/01.CIR.0000147537.72829.1B. [DOI] [PubMed] [Google Scholar]
- 2.Baroletti SA, Goldhaber SZ. Heparin-induced thrombocytopenia. Circulation. 2006;114:e355–e356. doi: 10.1161/CIRCULATIONAHA.106.632653. [DOI] [PubMed] [Google Scholar]
- 3.Jang IK, Hursting MJ. When heparins promote thrombosis: review of heparin-induced thrombocytopenia. Circulation. 2005;111:2671–2683. doi: 10.1161/CIRCULATIONAHA.104.518563. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th editor) Chest. 2008;133:340S–380S. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 5.GlaxoSmithKline (Argatroban injection) [US prescribing information] 2009. Available from: http://www.us.gsk.com/products/assets/us_argatroban.pdf.
- 6.Koster A, Buz S, Hetzer R, Kuppe H, Breddin K, Harder S. Anticoagulation with argatroban in patients with heparin-induced thrombocytopenia antibodies after cardiovascular surgery with cardiopulmonary bypass: first results from the ARG-E03 trial. J Thorac Cardiovasc Surg. 2006;132:699–700. doi: 10.1016/j.jtcvs.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Koster A, Hentschel T, Groman T, et al. Argatroban anticoagulation for renal replacement therapy in patients with heparin-induced thrombocytopenia after cardiovascular surgery. J Thorac Cardiovasc Surg. 2007;133:1376–1377. doi: 10.1016/j.jtcvs.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Williamson DR, Boulanger I, Tardif M, Albert M, Gregoire G. Argatroban dosing in intensive care patients with acute renal failure and liver dysfunction. Pharmacotherapy. 2004;24:409–414. doi: 10.1592/phco.24.4.409.33168. [DOI] [PubMed] [Google Scholar]
- 9.Reddy BV, Grossman EJ, Trevino SA, Hursting MJ, Murray PT. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia requiring renal replacement therapy. Ann Pharmacother. 2005;39:1601–1605. doi: 10.1345/aph.1G033. [DOI] [PubMed] [Google Scholar]
- 10.Beiderlinden M, Treschan TA, Gorlinger K, Peters J. Argatroban anticoagulation in critically ill patients. Ann Pharmacother. 2007;41:749–754. doi: 10.1345/aph.1H569. [DOI] [PubMed] [Google Scholar]
- 11.Reichert MG, MacGregor DA, Kincaid EH, Dolinski SY. Excessive argatroban anticoagulation for heparin-induced thrombocytopenia. Ann Pharmacother. 2003;37:652–654. doi: 10.1345/aph.1C187. [DOI] [PubMed] [Google Scholar]
- 12.de Denus S, Spinler SA. Decreased argatroban clearance unaffected by hemodialysis in anasarca. Ann Pharmacother. 2003;37:1237–1240. doi: 10.1345/aph.1C493. [DOI] [PubMed] [Google Scholar]
- 13.Arpino PA, Hallisey RK. Effect of renal function on the pharmacodynamics of argatroban. Ann Pharmacother. 2004;38:25–29. doi: 10.1345/aph.1D163. [DOI] [PubMed] [Google Scholar]
- 14.Kodityal S, Nguyen PH, Kodityal A, Sherer J, Hursting MJ, Rice L. Argatroban for suspected heparin-induced thrombocytopenia: contemporary experience at a large teaching hospital. J Intensive Care Med. 2006;21:86–92. doi: 10.1177/0885066605284590. [DOI] [PubMed] [Google Scholar]
- 15.Smythe MA, Stephens JL, Koerber JM, Mattson JC. A comparison of lepirudin and argatroban outcomes. Clin Appl Thromb Hemost. 2005;11:371–374. doi: 10.1177/107602960501100403. [DOI] [PubMed] [Google Scholar]
- 16.Kiser TH, Jung R, MacLaren R, Fish DN. Evaluation of diagnostic tests and argatroban or lepirudin therapy in patients with suspected heparin-induced thrombocytopenia. Pharmacotherapy. 2005;25:1736–1745. doi: 10.1592/phco.2005.25.12.1736. [DOI] [PubMed] [Google Scholar]
- 17.Bartholomew JR, Pietrangeli CE, Hursting MJ. Argatroban anticoagulation for heparin-induced thrombocytopenia in elderly patients. Drugs Aging. 2007;24:489–499. doi: 10.2165/00002512-200724060-00005. [DOI] [PubMed] [Google Scholar]
- 18.Rice L, Hursting MJ, Baillie GM, McCollum DA. Argatroban anticoagulation in obese versus nonobese patients: implications for treating heparin-induced thrombocytopenia. J Clin Pharmacol. 2007;47:1028–1034. doi: 10.1177/0091270007302951. [DOI] [PubMed] [Google Scholar]
- 19.Lewis BE, Wallis DE, Berkowitz SD, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103:1838–1843. doi: 10.1161/01.cir.103.14.1838. [DOI] [PubMed] [Google Scholar]
- 20.Lewis BE, Wallis DE, Leya F, Hursting MJ, Kelton JG. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch Intern Med. 2003;163:1849–1856. doi: 10.1001/archinte.163.15.1849. [DOI] [PubMed] [Google Scholar]
- 21.Lewis BE, Matthai WH, Jr, Cohen M, Moses JW, Hursting MJ, Leya F. Argatroban anticoagulation during percutaneous coronary intervention in patients with heparin-induced thrombocytopenia. Catheter Cardiovasc Interv. 2002;57:177–184. doi: 10.1002/ccd.10276. [DOI] [PubMed] [Google Scholar]