Abstract
Contact between sister chromatids from S phase to anaphase depends on cohesin, a large multi-subunit protein complex. Mutations in sister chromatid cohesion proteins underlie the human developmental condition, Cornelia de Lange Syndrome. Roles for cohesin in regulating gene expression, sometimes in combination with CCCTC-binding factor (CTCF), have emerged. We analyzed zebrafish embryos null for cohesin subunit rad21 using microarrays to determine global effects of cohesin on gene expression during embryogenesis. This identified Rad21-associated gene networks that included myca (zebrafish c-myc), p53 and mdm2. In zebrafish, cohesin binds to the transcription start sites of p53 and mdm2, and depletion of either Rad21 or CTCF increased their transcription. In contrast, myca expression was strongly downregulated upon loss of Rad21 while depletion of CTCF had little effect. Depletion of Rad21 or the cohesin-loading factor Nipped-B in Drosophila cells also reduced expression of myc and Myc target genes. Cohesin bound the transcription start site plus an upstream predicted CTCF binding site at zebrafish myca. Binding and positive regulation of the c-Myc gene by cohesin is conserved through evolution, indicating this regulation is likely to be direct. The exact mechanism of regulation is unknown, but local changes in histone modification associated with transcription repression at the myca gene were observed in rad21 mutants.
Keywords: Cohesin, Zebrafish, Cornelia de Lange Syndrome, Myc
Introduction
Sister chromatid cohesion during cell division is mediated by cohesin, a large multimeric complex that also has a DNA repair function (Nasmyth and Haering, 2009; Watrin and Peters, 2006). Cohesin forms a large ring-like complex that concatenates replicated sister chromatids (Haering et al., 2008). The cohesin ring contains four subunits: structural maintenance of chromosomes subunits Smc1 and Smc3, plus two non-SMC subunits, Mcd1/Scc1/Rad21, and Scc3/Stromalin (SA). Loading of cohesin onto chromosomes happens in telophase in most organisms, and is facilitated by a protein complex containing Scc2 (Nipped-B in Drosophila and NIPBL in human) and Scc4/MAU-2 (Ciosk et al., 2000; Rollins et al., 2004; Seitan et al., 2006). Cohesin's role in sister chromatid cohesion is relatively well characterized (Losada, 2008; Nasmyth and Haering, 2005, 2009), but it also has an enigmatic role in the regulation of gene expression (Dorsett, 2007).
In Drosophila, the cohesin-loading factor Nipped-B/Scc2 facilitates expression of the cut gene through long-range enhancer-promoter interactions (Dorsett et al., 2005; Rollins et al., 2004; Rollins et al., 1999). The effects of Nipped-B and cohesin on gene expression are direct, vary greatly in magnitude, and can be both positive and negative, suggesting that they regulate transcription via multiple mechanisms (Schaaf et al., 2009). In zebrafish, cohesin is expressed in both proliferating and non-proliferating cells (Mönnich et al., 2009) and is required for early tissue-specific transcription of runx1 and runx3 during embryogenesis (Horsfield et al., 2007). In mouse, the cohesin-associated proteins Pds5a and Pds5b have essential non-cell cycle related functions (Zhang et al., 2009; Zhang et al., 2007), and mice heterozygous for the Nipped-B ortholog Nipbl have severe developmental deficits and altered gene expression in the absence of cell cycle or sister chromatid cohesion defects (Kawauchi et al., 2009). Cohesin is required for axon pruning in post-mitotic neurons of Drosophila mushroom bodies (Pauli et al., 2008; Schuldiner et al., 2008), clearly demonstrating a developmental function separable from its cell cycle role.
Loss-of-function mutations in NIPBL or missense mutations in the SMC1A or SMC3 cohesin subunits cause Cornelia de Lange syndrome (CdLS), which displays diverse and highly variable mental deficits and structural abnormalities (Deardorff et al., 2007; Krantz et al., 2004; Musio et al., 2006; Tonkin et al., 2004). It is widely believed that the pathology of CdLS is caused by altered expression of developmental genes, rather than by cell cycle anomalies (Dorsett, 2009; Liu and Krantz, 2008; Strachan, 2005). In a mouse NIPBL model of CdLS, a large number of gene expression changes that are small in magnitude (≤2 fold) were observed (Kawauchi et al., 2009). Transcript profiling of lymphoblastoid cell lines from CdLS patients also identified consistent gene expression alterations (Liu et al., 2009). Cohesin binds a high proportion of the affected genes at their transcriptional start sites (Liu et al., 2009).
Genome-scale mapping of cohesin binding sites provides further evidence that it directly regulates transcription. In Drosophila, Nipped-B and cohesin co-localize genome-wide, and associate preferentially with active genes (Gause et al., 2008; Misulovin et al., 2008). Similar mapping experiments in mammalian cells identified extensive co-localization between cohesin and the CCCTC-binding factor (CTCF), a highly conserved zinc finger protein (Parelho et al., 2008; Stedman et al., 2008; Wendt et al., 2008). CTCF functions at transcriptional insulators that disrupt enhancer-promoter communication (Wallace and Felsenfeld, 2007). Recruitment of cohesin to CTCF binding sites may require interaction with CTCF (Rubio et al., 2008), and studies suggest that cohesin influences the activity of cis-regulatory elements that bind CTCF (Bowers et al., 2009; Hadjur et al., 2009). However, cohesin also binds several sites in the human genome independently of CTCF (Schmidt et al., 2010). Sites bound by cohesin independently of CTCF in human cell lines were highly tissue specific and corresponded with known transcription factor binding sites and active gene expression (Schmidt et al., 2010).
Both CTCF and cohesin can regulate epigenetic silencing of gene expression by PcG proteins. Trimethylation of lysine 27 in histone H3 (H3K27Me3) is associated with PcG silencing (Schuettengruber et al., 2007), and its distribution strongly anti-correlates with cohesin binding on Drosophila chromosomes (Misulovin et al., 2008). In those rare exceptions where cohesin and H3K27Me3 overlap, which include several genes that regulate development, both cohesin and PcG proteins are needed to restrict transcription (Schaaf et al., 2009). In imprinting of the vertebrate Igf2 locus, CTCF recruits Polycomb Repressive Complex 2 to mediate allele-specific H3K27Me3 (Li et al., 2008). Cohesin also regulates the H19/Igf2 locus by participating in chromosome looping (Nativio et al., 2009).
Myc proteins are key regulators of protein synthesis, growth and proliferation in diverse organisms, and Myc overexpression contributes to many cancers (Pelengaris et al., 2002; Vita and Henriksson, 2006). Cohesin binds a CTCF site upstream of the mammalian c-Myc gene (Rubio et al., 2008; Stedman et al., 2008), which in some cells resides in a chromatin domain with hyperacetylated histones (H3K9Ac) characteristic of transcriptionally active chromatin. In turn, this active locus is itself flanked by regions containing inactive chromatin enriched in lysine 9-methylated histone H3 (H3K9Me). A potential barrier element called MINE (Myc Insulator Element) containing a CTCF binding site, is positioned between the active and inactive chromatin 2.5 kb upstream of c-Myc (Gombert et al., 2003). Surprisingly, however, c-Myc expression occurs independently of CTCF binding to the MINE (Gombert et al., 2003), and mutation of the CTCF binding site in the MINE has no effect on c-Myc transcription (Gombert and Krumm, 2009).
A null mutation in the zebrafish rad21 gene (rad21nz171) was isolated in a screen for positive regulators of runx1 transcription in the early zebrafish embryo (Horsfield et al., 2007). Here we identify additional genetic pathways regulated by cohesin during early zebrafish development through microarray analysis of rad21nz171 mutants. A network of genes connected with myc (myca, NM_131412), p53 and mdm2 are dysregulated, and some are highly sensitive to rad21 gene dosage (ascl1b, sox11a, and aqp). A subset of cohesin-regulated genes, including p53 and mdm2, are also sensitive to reduced CTCF. Cohesin binds a CTCF binding site upstream of myca and to the transcription start sites of myca, p53 and mdm2. Strikingly, loss of cohesin strongly reduces myca expression, while depletion of CTCF has no detectable effect; furthermore, cohesin can still bind myca in CTCF-depleted embryos. The H3K27Me3 silencing modification increases at the myca transcription start site in the absence of cohesin, while H3K9Ac (a mark of transcriptionally active chromatin) is reduced. Reduction of cohesin or Nipped-B in Drosophila cells also downregulates myc and its target genes without cell cycle defects or activation of p53. The Drosophila myc locus lacks CTCF binding sites, but is nevertheless directly bound by cohesin. Furthermore, known myc regulators are not affected upon Nipped-B or cohesin depletion, indicating that cohesin directly facilitates myc transcription. The combined results argue that regulation of the Myc growth and cell proliferation pathway by cohesin is an evolutionarily conserved mechanism that may occur independently of c-Myc regulation by CTCF.
Materials and Methods
Zebrafish lines
Zebrafish were maintained as described previously (Westerfield, 1995). All zebrafish research was approved by the University of Otago Animal Ethics Committee.
Microarray and analysis
Total RNA was extracted from 24 hours post-fertilization (h.p.f.) and 48 h.p.f. wild type and rad21nz171 mutants using Trizol (Invitrogen) and purified using Qiagen RNeasy columns. Hybridization to Affymetrix Zebrafish Genome Arrays and data acquisition were performed at The University of Auckland School of Biological Sciences. A full description of the microarray analysis is available on request, and the data has been deposited at GEO (acc. no. GSE18795). The BG3 cell cohesin and Nipped-B ChIP-chip data are from Misulovin et al. 2008 (GEO acc. no. GSE9248) and the BG3 cell gene expression data are from Schaaf et al. (2009) (GEO acc. no.16152). The ChIP-chip and gene expression data were processed and correlated as previously described (Schaaf et al., 2009). The dm mutant larvae gene expression data that were compared to the BG3 gene expression data are from Pierce et al. (2008).
Microinjection
Morpholino oligonucleotides were obtained from GeneTools LLC and diluted in water. For microinjection, 1 nl of morpholino was injected into the yolk of wild type embryos at the 1- to 2-cell stages. Morpholino oligonucleotides used were smc3ATG-MO, 5′-TGTACATGGCGGTTTATGC-3′; smc3Spl-MO, 5′-GTGAGTCGCATCTTACCTG-3′; ctcfSplx2-MO, 5′-CCAAAACAGATCACAAACCTGAAAG-3′; ctcfATG-MO, 5′-CATGGGTAATACCTACATTGGTTAA-3′. All morpholinos were effective over the range of 0.75-1.0 pmol injected. See Supplementary Methods and Fig. S2 for further information.
Quantitative RT-PCR
Total RNA from pools of 30-50 embryos was extracted using Trizol, DNAse-treated, and used to synthesize random-primed cDNA (SuperScriptIII, Invitrogen). Individual embryos from rad21nz171 heterozygous incrosses were genotyped by sequencing amplified exon 8 of the rad21 gene. Equal amounts of total RNA from approximately 10 genotyped single embryos was then pooled into groups of homozygous wild type, heterozygous rad21nz171 and homozygous rad21nz171 RNA, from which random primed cDNA was synthesized in triplicate. SYBR Green PCR Master Mix (Applied Biosystems) was used to amplify cDNA, and relative quantities were normalized to β-actin and wnt5a expression. Samples were analyzed using an Applied Biosystems 7300 Real-Time PCR System. All PCR primers are listed in Table S3.
Whole mount in situ hybridization
In situ hybridization was performed as described previously (Kalev-Zylinska et al., 2002).
CTCF-binding site prediction
CTCF-binding sites were predicted using the CTCFBSDB tool at http://insulatordb.utmem.edu/ (Bao et al., 2008). The best hits using the four position weight matrices (PWM) that represent core motifs for CTCFBS sequences, with PWM scores >10.0, are presented.
Antibodies
Antibodies used for ChIP assays were: anti-Rad21 (raised in rabbit against a 15 amino acid peptide of the zebrafish protein, GenScript Corporation, USA), anti-acetylated histone H3 (06-599; Upstate Biotechnology), anti-trimethylated histone H3 (Lys 9) (07-442; Upstate Biotechnology), anti-trimethylated histone H3 (Lys 4) (9751; Cell Signaling Technology), anti-trimethylated histone H3 (Lys27) (9756; Cell Signaling Technology) and anti-pan histone H3 (05-928; Millipore).
Chromatin Immunoprecipitation (ChIP)
ChIP was performed essentially as described previously (Eroglu et al., 2006) on wild type and ctcf morphant 24 h.p.f embryos using anti-Rad21 and anti-pan H3; on wild type and rad21nz171 27 h.p.f embryos using anti-H3K4Me3, anti-H3K27Me3 and anti-pan H3; and on wild type and rad21nz171 30 h.p.f embryos using anti-H3K9Ac, anti-H3K9Me3 and anti-pan H3. qPCR analysis was performed as described above. All PCR primers are listed in Table S3. The full ChIP protocol is described in the Supplementary Methods.
Statistical analysis was performed using the Statistics/Data analysis programme STATA, version 9.1 (StataCorp, USA). To compare Rad21 enrichment between wild type and ctcf morphants a two-sample t-test with equal variances was used.
Results
A network of genes functionally related to myca and p53 is dysregulated in the rad21nz171 mutant
RNA from rad21nz171 mutant and wild type zebrafish embryos collected at two developmental time points 24 and 48 h.p.f. was used to prepare probes that were hybridized to Affymetrix microarrays. This revealed differential expression of many transcripts between mutant and wild type embryos at both time points as illustrated by the heat maps in Figure 1. A significance cut-off was set at ANOVA p≤0.05, and additional filtering was applied to include only transcripts that were up or downregulated 2-fold or more. These correspond to false discovery rates of 0.31 at 24 h.p.f. and 0.19 at 48 h.p.f. Selected data are presented in Table S1, and all data are available in the GEO database (acc. no. GSE18795). Over half the genes regulated by Rad21 at 24 h.p.f. were also regulated at 48 h.p.f.; Figure S1 shows that 69 transcripts were regulated by Rad21 inactivation at both times.
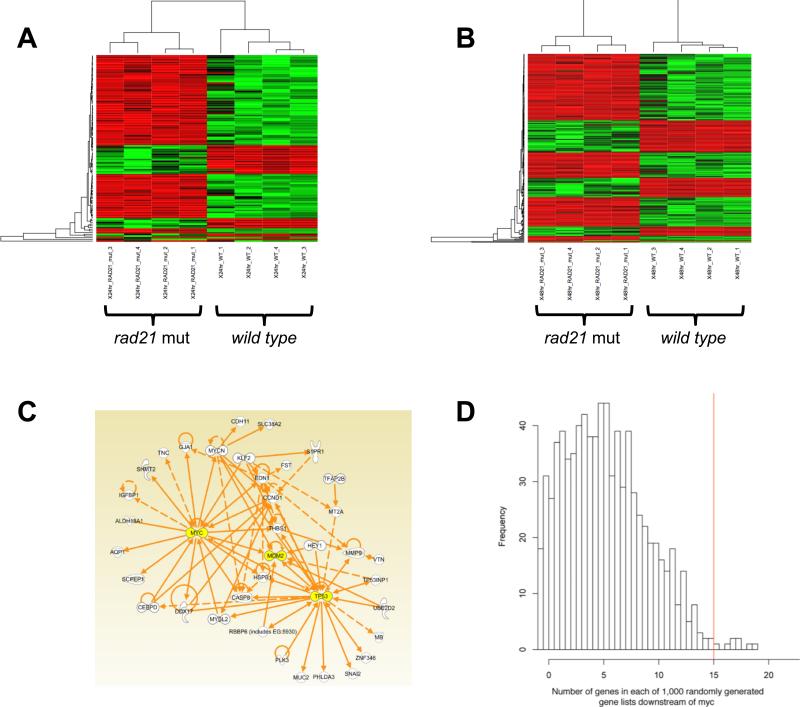
Figure 1. Affymetrix microarray analysis of the rad21nz171 mutant.
A, Heat map of mRNAs differentially abundant between wild type and rad21nz171 mutant embryos at 24 h.p.f. Colour is proportional to mRNA abundance after transformation to Z-scores across rows, with mean abundance for any gene shown as black, higher than mean abundance shown as red, lower than mean abundance shown as green. Both genes and microarrays have been clustered using Ward's method. B, Heat map of mRNAs differentially abundant between wild type and rad21nz171 mutant embryos at 48 h.p.f. C, A subset of the most significant 100 RNAs differentially abundant between wild type and rad21nz171 mutant embryos at 48 h.p.f. constitute a putative molecular network, in which 15 mRNAs have known relationships to myca. D, Significantly more of the differentially abundant genes were associated with myca than would be expected due to chance alone. 1,000 gene lists, each the same size as the list of genes regulated by rad21 disruption at 48 h.p.f. were randomly drawn from the genes available on the Affymetrix chip used in this study. The number of genes in each of the 1,000 lists that associated in IPA networks with myca are plotted in the histogram. Only 0.008 of the randomly chosen gene lists contained more genes associated with myca than the experimentally derived gene list.
Gene Ontology analysis was performed with the 24 h.p.f. and 48 h.p.f. differentially abundant transcript lists (p≤0.005 and fold change ≤-2 fold or ≥+2 fold). While the transcripts regulated only at 24 h.p.f. were not significantly enriched for any specific function, those regulated only at 48 h.p.f. or at both time points were enriched for genes involved in embryo development (GO:0007275, p≤0.0001; aldh1a2, ascl1a, ascl1b, bambi, edn1, emx2, eomes, ebp41, fzd8a, gsc, igfbp1, otp, pax9, pou50, six2.1, slc4a1, sox9b, tnc, tnnt, vox, wif) and transcription (GO:0006351, p≤0.0001; cebpd, myca, emx2, eomes, foxd5, gsc, hey1, maf, otp, pax9, pou50, six2.1, sox11a, sox11b, sox9b, tbx15, tp53, vox). Ingenuity Pathways Analysis was used to interrogate a gene product functional database, which revealed that genes with altered expression in 48 h.p.f. rad21 mutants are enriched for genes involved in tissue development (20 molecules, max p≤3.0×10-3), cellular development (26 molecules, max p≤3.2×10-3), cancer (32 genes, max p≤3.6×10-3), cell cycle (12 molecules, max p≤3.2×10-3), gene expression (20 molecules, max p≤3.4×10-3) and cell death (26 molecules, max p≤3.6×10-3).
The microarray data were also analyzed for the putative signatures of molecular pathways using the networks function of Ingenuity Pathways. This uncovered a network incorporating myca, p53, and mdm2 (Fig. 1C). By permutation analysis (Fig. 1D) it is unlikely that this network is due to chance alone (p≤0.008). The core genes in the network, myca, p53 and mdm2, were significantly dysregulated upon loss of rad21. myca was downregulated more than 5-fold at 24 and 48 h.p.f., while p53 was upregulated 1.5-fold (24 h.p.f.) to over 3-fold (48 h.p.f.) and mdm2 upregulated over 3-fold at 48 h.p.f. (Table S1). These results were confirmed independently using quantitative PCR (qPCR) on wild type, heterozygous rad21nz171 and homozygous rad21nz171 embryos (Table S1, Fig. 2). Analysis of mRNA levels from 48 h.p.f. embryos using qPCR showed a >5-fold reduction in myca, a >6-fold up-regulation of p53, and a >10-fold upregulation of mdm2 in mutants compared with wild type (Fig. 2B-D). We also used qPCR to confirm the regulation downstream of Rad21 of other genes found in the microarray analysis (Fig. 2E-H, Table S1).
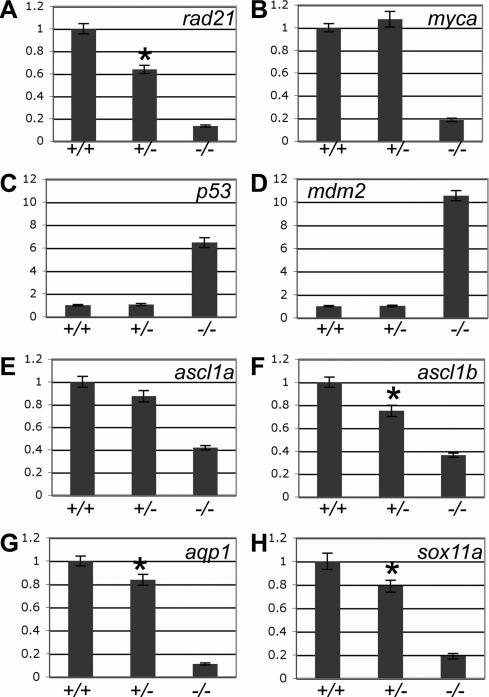
Figure 2. The effect of rad21 gene dose on the expression of genes regulated downstream of Rad21 in 48 h.p.f. embryos.
A-H, quantitative PCR was used to measure the expression of rad21 (A), myca (B), p53 (C), mdm2 (D), ascl1a (E), ascl1b (F), aqp1 (G) and sox11a (H) from cDNA generated from pools of wild type (+/+), heterozygous rad21nz171 (+/-) and homozygous rad21nz171 (-/-) embryos. An asterisk indicates where the difference in expression between wild type and heterozygous rad21nz171 is statistically significant (p-value < 0.05). Values are relative to wild type and represent the mean ±s.e.m. of three cDNA samples each run in duplicate.
Halving the gene dose of rad21 reduced the levels of rad21 mRNA to 60% of wild type in 48 h.p.f. embryos (Fig. 2A). We therefore asked whether selected genes regulated downstream of Rad21 respond to rad21 gene dose. Some of the Rad21-responsive genes, such as ascl1a, ascl1b, aqp1, sox11a, (Fig. 2E-H) and edn1 (not shown) exhibited a consistent sensitivity to rad21 gene dose. Embryos heterozygous for rad21nz171 showed a small but statistically significant (p<0.05) reduction in expression of ascl1b, aqp1 and sox11a. However, expression of myca, p53, and mdm2 was not sensitive to halving the dose of rad21 (Fig. 2B-D).
A subset of genes regulated by Rad21 are also regulated by CTCF
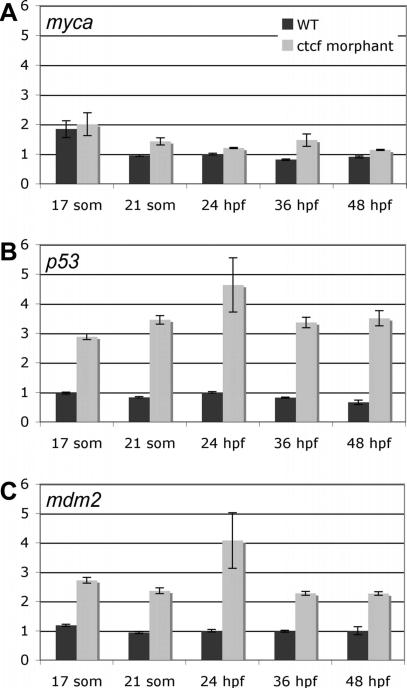
Many cohesin binding sites in the mammalian genome coincide with binding sites for CTCF, therefore we asked whether certain genes regulated by rad21 in the microarray analysis are also regulated by CTCF. A single zebrafish ctcf gene (Ensembl ENSDARG00000056621) is expressed ubiquitously in early embryogenesis, later becoming restricted to the brain (Pugacheva et al., 2006). We used antisense morpholino oligonucleotides (MOs) targeting the ATG start codon (ctcfATG-MO), or the 5′ donor of the exon/intron boundary of intron 2 in both known splice variants of ctcf (ctcfSplx2-MO), to create knockdown “morphant” embryos. Both MOs produced an identical phenotype characterized by developmental delay with head and posterior defects (Fig. S2A), and had synergistic effects when co-injected (Fig. S2C). RT-PCR was used to confirm aberrant splicing of ctcf transcripts targeted by the MO (Fig. S2B). qPCR was used to analyze the expression of selected genes that were significantly regulated by Rad21 (Table 1). Some, but not all of the genes regulated by Rad21 were also regulated by CTCF. Genes that showed statistically significant dysregulation in ctcf morphants included p53, mdm2, ascl1a, ascl1b, aqp1 and sox11b. Genes regulated by Rad21 but unaffected in ctcf morphants included rad21, myca, sox11a, cdh11, hey1, edn1, foxd5, emx2, tnc, pax9, fzd8a (Table 1 and data not shown). The p53 and mdm2 genes were both dramatically upregulated in ctcf morphants (Table 1 and Fig. 3). Unlike rad21 mutants, p53 upregulation in ctcf morphants was not associated with an increase in apoptosis (Fig. S3). Unexpectedly, transcription of the zebrafish myca locus, which is strongly regulated by Rad21, was not affected in ctcf morphants (Figs 3A, S2C). This finding is surprising since the MINE element bound by CTCF near the mammalian c-Myc locus (Gombert et al., 2003) appears to be conserved in zebrafish (see below).
Table 1.
Expression of Rad21-responsive genes in ctcf morphants relative to wild type, with locations of predicted CTCF binding sites.
| Gene | Fold-change expression in ctcf morphants | CTCF binding site(s)a | |||||
|---|---|---|---|---|---|---|---|
| | |||||||
| 17 somites | 21 somites | 24 h.p.f. | 36 h.p.f. | 48 h.p.f. | Relative to TSSb (kb) | Exonic/Intronic | |
| rad21 | 1.0 | 1.1 | 1.2 | 1.1 | 1.2 | -2.4 | - |
| -1.3 | - | ||||||
| +7.0 | intron/exon boundary | ||||||
| +8.5 | intronic | ||||||
| +13.9 | intronic | ||||||
| myca | 1.2 | -1.3 | -1.2 | -1.2 | 1.0 | -10.53 | - |
| -1.27 | - | ||||||
| -0.76 | - | ||||||
| p53 | 4.1* | 4.2* | 4.4* | 3.5* | 3.3* | -0.16 | - |
| +1.0 | exonic | ||||||
| +13.1 | - | ||||||
| mdm2 | 2.7* | 2.1* | 2.5* | 2.3* | 1.5 | -0.02 | - |
| +3.4 | intronic | ||||||
| +8.5 | exonic | ||||||
| +9.6 | - | ||||||
| ascl1a | -1.6* | -1.7 | -1.8* | -1.3 | -1.1 | - | |
| ascl1b | -1.5 | -1.6* | -1.6 | -2.0 | -1.4* | - | |
| aqp1 | -3.7* | 1.0 | -2.0* | -1.7* | -2.0* | +7.2 | intronic |
| +10.3 | intronic | ||||||
| sox11a | -1.3 | -1.3 | -1.6 | -1.4 | -1.4 | -0.4 | - |
| sox11b | -1.4* | -1.4 | -1.5 | -1.5 | -1.3 | - | |
Statistically significant change in expression relative to wild type (p-value < 0.05), data are the average of RT-qPCR results from three independent experiments
CTCF binding sites were predicted using CTCFBSDB (Bao et al., 2008) over regions of genomic DNA 3 kb up- and downstream of the gene, sites with a PWM score >10.0 are presented
TSS, transcriptional start site.
Figure 3. Expression of selected Rad21-responsive genes in ctcf morphants.
A-C, quantitative PCR was used to measure the expression of myca (A), p53 (B) and mdm2 (C) in ctcf morphants relative to that in wild type embryos during early stages of embryonic development: 17 somites, 21 somites, 24 h.p.f., 36 h.p.f., 48 h.p.f.. Values are shown relative to wild type expression at 24 h.p.f., and are the mean ±s.e.m. of cDNA generated from pooled embryos run in duplicate. Data from three independent experiments are combined in Table 1, and graphs of one representative experiment for each gene are shown here.
Cohesin binds the zebrafish myca locus and regulates its transcription
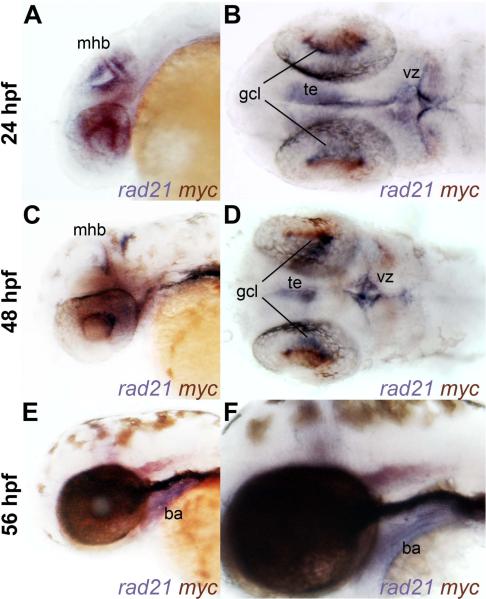
Binding of cohesin to the mammalian c-Myc gene (Rubio et al., 2008; Stedman et al., 2008) and downregulation of c-Myc in lymphoblastoid cell lines derived from CdLS patients (Liu et al., 2009) and brain of heterozygous Nipbl mutant mice (Kawauchi et al., 2009) suggest that cohesin might directly regulate c-Myc gene expression. If cohesin directly regulates c-Myc expression, the gene products should be present in the same cells. To define regions of overlap between rad21 and myca expression, we performed double in situ hybridization with riboprobes detecting myca and rad21. At 24 h.p.f., overlap was found in the tegmentum (te), the midbrain-hindbrain boundary (mhb), the retinal ganglion cell layer (gcl) and cells of the ventricular zone (vz) (Fig. 4A,B). At 48 h.p.f., myca and rad21 overlap persisted in the retinal ganglion cell layer, the tegmentum and midbrain-hindbrain boundary (Fig. 4C,D). Many of these cells are likely to be proliferating, since a high proportion of cells in these regions are in S phase (Mönnich et al., 2009). However, by 56 h.p.f., overlap between myca and rad21 expression was less obvious (Fig. 4E,F). rad21 expression in the branchial arches (ba) is robust at this stage, whereas myca expression in this tissue is negligible.
Figure 4. Overlapping expression of rad21 and myca in wild-type embryos.
A-F, whole-mount wild type embryos stained for rad21 (blue) and myca (red-purple) expression at 24 h.p.f. (A-B), 48 h.p.f. (C-D) and 56 h.p.f. (E-F). Lateral views (Panels A, C, E and F) and dorsal views (Panels B and D) are shown of anterior regions. There is overlapping expression of rad21 and myca in cells of the ventricular zone (vz) at 24 h.p.f., and in tegmentum (te), midbrain-hindbrain boundary (mhb) and retinal ganglion cell layer (gcl) at 24 and 48 h.p.f. Only rad21 is expressed in the branchial arches (ba) at 56 h.p.f.
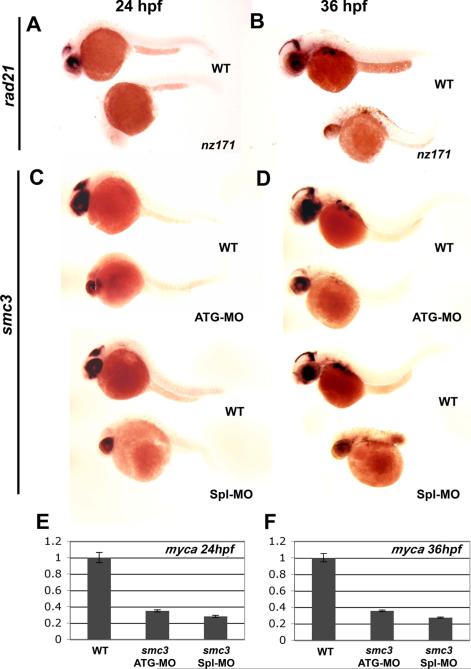
Whole mount in situ hybridization with a myca riboprobe confirmed down-regulation of myca expression in rad21nz171 embryos. myca transcripts were markedly reduced in the brain and eye of rad21nz171 mutants at 24 and 36 h.p.f. (Fig. 5A,B), consistent with the reduced expression detected by qPCR. Expression of myca in rad21nz171 mutants was rescued by microinjection of wild type rad21 mRNA into 1-cell embryos, but not by microinjection of the rad21nz171 mutant mRNA (data not shown). To determine whether the whole cohesin complex is necessary for myca regulation we knocked down smc3 (Fig. 5C-F) with MOs targeting the smc3 start codon (smc3ATG-MO) or the splice site of exon 1, 3′ donor (smc3Spl-MO) to create smc3 morphants. MO efficacy was previously verified (Horsfield et al., 2007). smc3 morphants displayed a dramatic reduction in myca expression at 24 and 36 h.p.f. as detected by in situ hybridization with a myca riboprobe (Fig. 5C,D), and by qPCR (Fig. 5E,F). These results indicate that the whole cohesin complex contributes to myca regulation.
Figure 5. Reduced myca expression in rad21nz171 mutants and smc3 morphants.
A, B, expression pattern of myca in whole-mount wild type and rad21nz171 embryos at 24 h.p.f. and 36 h.p.f. respectively (anterior to the left). myca expression (purple) in the brain and eye of wild type is absent in rad21nz171 embryos. C, D, Expression of myca is also greatly reduced in smc3 morphants at 24 h.p.f. and 36 h.p.f. respectively (anterior to the left). Embryos were injected with antisense morpholino oligonucleotides targeting the start codon (smc3ATG-MO) or the 3′ donor site of exon 1 (smc3Spl1-MO) of the smc3 gene to create two smc3 morphants. E, F, The expression of myca in smc3 morphants (smc3ATG-MO and smc3Spl-MO) is significantly reduced compared to wild type embryos as measured by quantitative PCR at 24 h.p.f. and 36 h.p.f. respectively.
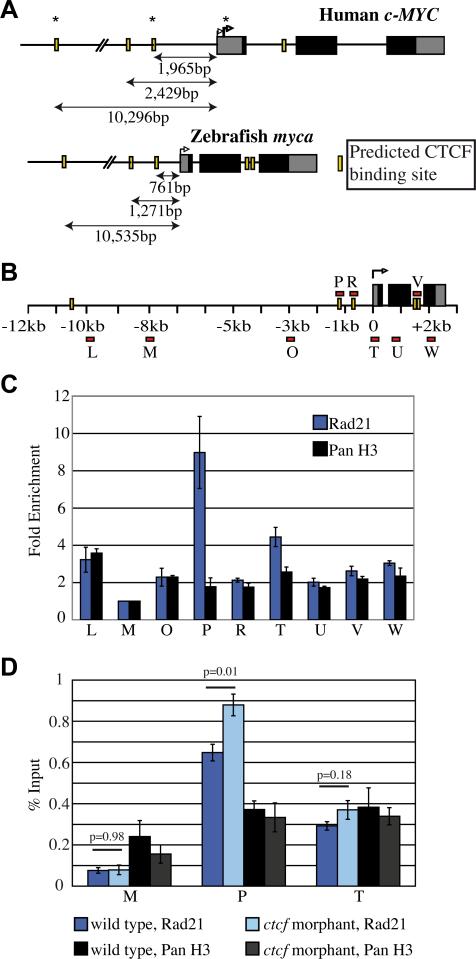
To determine if regulation of zebrafish myca by cohesin could be direct, we first asked whether potential CTCF and cohesin binding sites exist in zebrafish myca. The CTCF-binding site database (CTCFBSDB) (Bao et al., 2008) was used to predict CTCF-binding sites around the myca locus. We found two sites that strongly match the CTCF consensus 0.76 kb and 1.27 kb upstream of the TSS of zebrafish myca (Fig. 6A). At the human c-MYC locus CTCFBSDB predicted two similarly spaced CTCF sites 1.97 kb and 2.43 kb upstream of the TSS. The spacing between these upstream CTCF sites is similar between human (464 bp) and zebrafish (510 bp) but the zebrafish sites are closer in proximity to the TSS (Fig. 6A). Although CTCF binds to the human c-MYC P2 promoter (Gombert et al., 2003; Gombert and Krumm, 2009), CTCFBSDB does not predict a CTCF binding site within this region for either human or zebrafish. However, two CTCF sites are predicted to reside within the second intron of zebrafish myca under slightly less stringent criteria.
Figure 6. Rad21 binding at zebrafish myca.
A, Schematic of human c-MYC and zebrafish myca genes comparing relative positions of predicted CTCF binding sites from the transcriptional start site. Black solid boxes indicate translated regions, yellow bars indicate predicted CTCF binding sites and right-angled arrows indicate the TSS and P2 (bold arrow). In vivo binding of CTCF is denoted by an asterisk. B, Schematic of the zebrafish myca gene indicating the location of primer sets (red bars) used for amplification of immunoprecipitated DNA following ChIP. C, anti-Rad21 ChIP in wild type zebrafish embryos at 24 h.p.f. Binding at each site was determined relative to primer M (where no Rad21 binding was predicted) to give fold enrichment. Anti-pan histone H3 (panH3) ChIP was used as a control. Results shown are the averages of four independent ChIP experiments for Rad21 and two independent ChIP experiments for panH3 ±s.e.m. D, Rad21 enrichment in ctcf morphants compared to wild type embryos at 24 h.p.f. % Input corresponds to a fold enrichment (relative to M) of 8.5 for wild type and 11.1 for ctcf morphants at (P), and 3.8 for wild type and 4.7 for ctcf morphants at (T). There is a statistically significant increase in Rad21 enrichment in ctcf morphants at the – 1.27kb binding site (primer P) compared to wild type embryos (p=0.01), but no statistically significant difference in Rad21 enrichment at the transcriptional start site (primer T) between ctcf morphants and wild type embryos (p=0.18). Results shown are the average of 5 independent ChIP experiments for Rad21 and 3 independent ChIP experiments for panH3, ±s.e.m. Aberrant splicing of ctcf in the morphants was confirmed for each ChIP experiment (data not shown).
We next asked if the predicted zebrafish CTCF sites recruit cohesin. Using chromatin immunoprecipitation (ChIP) with antibodies detecting zebrafish Rad21 (Fig. S4), we scanned for cohesin binding from -10 kb upstream of the myca gene to +2 kb downstream of the TSS (Fig. 6B). We found significant Rad21 binding at the predicted CTCF site (P) 1.27 kb upstream of the TSS of myca, and at the TSS itself (T, Fig. 6C). We did not detect cohesin binding to the predicted upstream 0.76 kb CTCF site (R), or the predicted intronic sites (V, Fig. 6C). Therefore in zebrafish, as in human cells, cohesin locates to two specific binding sites in myca that are also predicted to recruit CTCF. Surprisingly, cohesin robustly bound both sites in CTCF-depleted embryos (Figs S2, 6D). Although it is not known if CTCF binds to the same sites as cohesin in zebrafish (as it does in human), its depletion did not affect cohesin binding or myca expression.
Cohesin has the potential to directly regulate mdm2 and p53
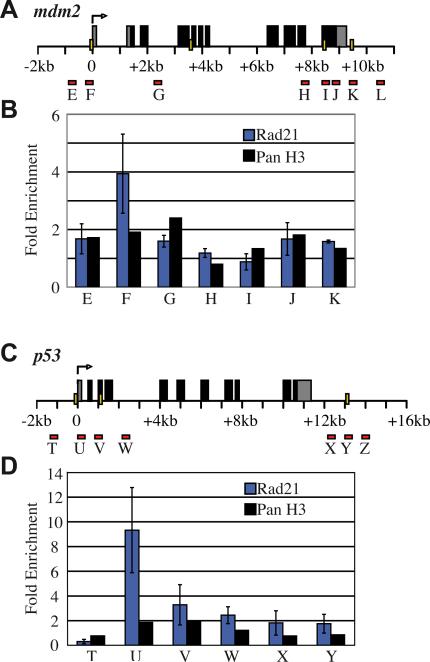
Transcription of zebrafish mdm2 and p53 increased markedly upon depletion of either Rad21 or CTCF (Figs 1, 2), and the CTCFBSDB (Bao et al., 2008) predicts CTCF binding sites at various locations throughout both p53 and mdm2 (Table 1, Fig. 7A,C). This raises the possibility that CTCF and cohesin could bind directly to regulatory regions of these genes and control their transcription. To determine if cohesin binds mdm2 and p53, we performed anti-Rad21 ChIP on chromatin from wild type 24 h.p.f. embryos to scan the predicted CTCF binding sites at both loci. We found that Rad21 binds at a single predicted CTCF binding site immediately adjacent to the TSS of both genes (Fig. 7B,D). Although several other CTCF binding sites were predicted for both genes (Table 1 and Fig. 7A,C), these sites were not bound by cohesin in vivo (Fig. 7B,D).
Figure 7. Rad21 binding at zebrafish mdm2 and p53 genes.
A, C, Schematics of the zebrafish mdm2 (A) and p53 (C) genes showing the locations of predicted CTCF binding sites (yellow bars) and primers (red bars) used to amplify immunoprecipitated DNA following ChIP. B, D, Rad21 ChIP at mdm2 (B) and p53 (D) in wild type zebrafish embryos at 24 h.p.f. Rad21 binds to a single predicted CTCF binding site immediately adjacent to the TSS of both mdm2 and p53. Results shown are the averages of two independent ChIP experiments for Rad21 ±s.e.m whilst one ChIP experiment is shown for panH3.
Loss of cohesin leads to altered histone marks conferring transcription repression at the myca transcription start site
The conserved arrangement of predicted CTCF binding sites and in vivo binding of cohesin at myca suggests that the -1.27 site may be a zebrafish MINE that separates actively transcribed chromatin from repressed chromatin, similar to human c-MYC. Furthermore, myca downregulation could be explained if loss of MINE integrity in the absence of cohesin leads to spread of repressive chromatin marks into myca, decreasing transcription. To explore this idea, we first determined the relative proportion of active (acetylated) to repressive (methylated) histone marks in the myca region.
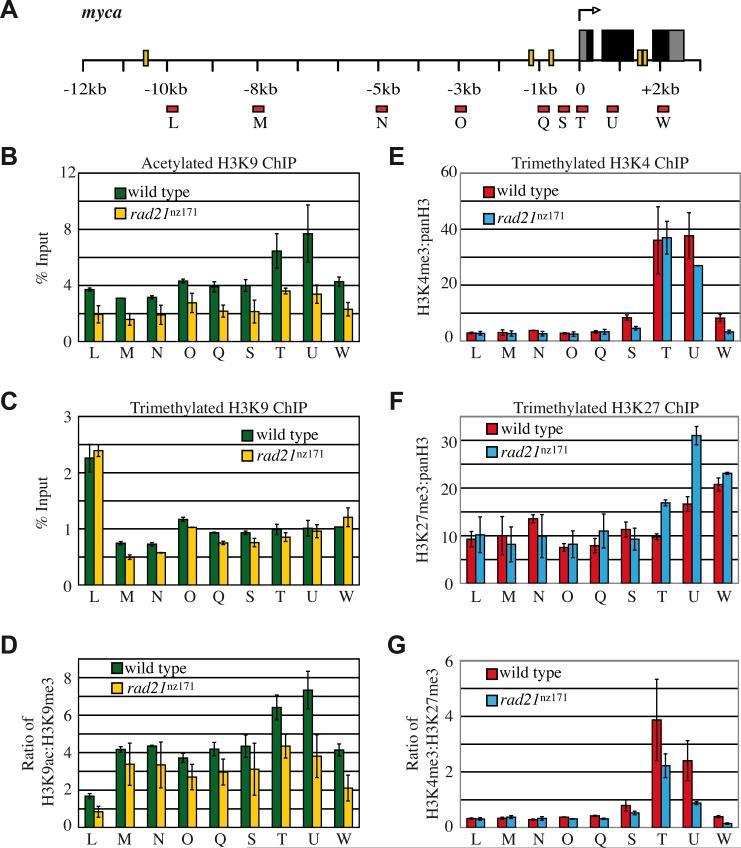
We used ChIP to determine the enrichment of histone H3 either methylated on lysine 9 (H3K9Me3) or acetylated on lysine 9 (H3K9Ac) from 10 kb upstream to 2 kb downstream of myca (Fig. 8A-C). There was a sharp, greater than 2-fold enrichment of H3K9Me3 at -10 kb compared with -8 kb (Fig. 8C, L compared with M). Conversely, enrichment of H3K9Ac increased from around -3 kb through the myca gene (Fig. 8B). In human c-MYC, a chromatin boundary exists at the -2.5 kb MINE (Gombert et al., 2003). However, in zebrafish increased H3K9Ac enrichment starting from 3 kb upstream of myca does not seem to coincide with a predicted CTCF or an in vivo cohesin binding site. Interestingly, there is a predicted CTCF binding site (conserved in human) and a slight enrichment of cohesin at -10.53 kb upstream of myca (Fig. 6C, L). Enrichment of H3K9Me3 near this site raises the possibility that a chromatin boundary may be present there. It is unclear whether a conserved chromatin boundary exists for zebrafish myca, however, a comparison of human and zebrafish chromatin structure across the Myc region is summarized in Figure S5.
Figure 8. Enrichment of histone modifications at the zebrafish myca locus in wild type and rad21nz171 mutants.
A, myca gene schematic showing the location of predicted CTCF binding sites (yellow bars) and position of primer sets for qPCR of immunoprecipitated DNA following ChIP (red bars). ChIP was performed on 30 h.p.f. wild type and rad21nz171 mutant embryos using anti-H3K9Ac, H3K9Me3, and panH3. B, C, Enrichment of H3K9Ac (B) and H3K9Me3 across the myca locus expressed as % Input. Results shown are the averages of two separate ChIP experiments ±s.e.m. D, Loss of H3K9Ac contributes to the lower ratio of active to repressive histone marks through the myca locus in rad21nz171 mutants. E, F, ChIP was performed on 27 h.p.f. wild type and rad21nz171 mutant embryos using anti-H3K4Me3, H3K27Me3, and panH3. To account for the difference in panH3 enrichment between wild type and rad21nz171 mutant embryos in this particular experiment, graphs show the ratio of either H3K4Me3 (E) or H3K27Me3 (F) enrichment relative to panH3 enrichment. H3K27Me3 is markedly increased at the TSS of myca in rad21nz171 mutants compared to wild type. G, dividing H3K4Me3 enrichment by H3K27Me3 enrichment shows that rad21nz171 mutants have a lower ratio of active to repressive histone marks at the myca TSS.
While there was essentially no difference in chromatin enrichment of H3K9Me3 between rad21nz171 mutants and wild type (Fig. 8C), there was a marked decrease in H3K9Ac in the rad21nz171 mutants (Fig. 8B). Loss of acetylation was most pronounced at the myca gene itself. Overall, the wild type myca locus contains a greater proportion of acetylated to methylated histones than rad21nz171 mutants (Fig. 8D), due to loss of H3K9Ac in the mutants.
In Drosophila, Rad21 was shown to have TrxG activity in some tissues (Hallson et al., 2008), which promotes H3K4Me3, a mark of gene activation. Moreover, cohesin binding to Drosophila chromosomes is predominantly excluded from regions enriched in the transcription repression mark H3K27Me3 (Misulovin et al., 2008). Therefore, we asked if these histone marks are altered across the myca locus in rad21nz171 mutants. We used ChIP to scan the myca locus for relative enrichment of H3K27Me3 and H3K4Me3 from 10 kb upstream to 2 kb downstream of the myca TSS (Fig. 8A,E-F). We found that H3K4Me3 was enriched at the TSS of myca (Fig. 8E) in both wild type and rad21nz171 mutant embryos. In contrast, H3K27Me3 enrichment increased about 2-fold at the myca TSS and a downstream site in rad21nz171 mutants (Fig. 8F). The H3K4Me3 to H3K27Me3 ratio was substantially decreased in rad21nz171 mutants (Fig. 8G), indicative of transcription repression. H3K27Me3 enrichment at the myca TSS in wild type is gene-specific, as it was not found at the TSS of cohesin-responsive genes mdm2 and p53 (Fig. S6). Significantly, H3K9Ac depletion and H3K27Me3 enrichment in rad21nz171 mutants relative to wild type was predominantly localized to the TSS.
Together the results indicate that loss of cohesin function in zebrafish does not lead to the spread of silencing from an upstream region of condensed chromatin, but rather, confers a specific set of histone modifications at the myca gene itself that are consistent with transcription repression.
Cohesin regulation of c-Myc is a cross-species phenomenon that accounts for concomitant indirect regulation of a subset of cohesin-responsive genes
Downregulation of c-Myc upon partial NIPBL reduction in human cells (Liu et al., 2009) and mouse brain (Kawauchi et al., 2009) suggests that regulation of c-Myc by cohesin is evolutionarily conserved. To explore this idea, we reanalyzed genome-wide ChIP and gene expression data from Drosophila ML-DmBG3 (BG3) cells derived from 3rd instar larvae central nervous system (Misulovin et al., 2008; Schaaf et al., 2009).
Drosophila contains a single myc ortholog called diminutive (dm). In BG3 cells, dm/myc is located in an 84 kb region bound by cohesin and Nipped-B (Misulovin et al., 2008). RNAi knockdown of Rad21 or Nipped-B by 80% reduced dm/myc expression by 65-70% (Schaaf et al., 2009). Some genes downregulated in response to cohesin RNAi in BG3 cells are not bound by cohesin, and therefore cannot be directly regulated by it (Misulovin et al., 2008). Examples shown in Figure 9 (pit, Surf6, Nop60B, ppan, Fib) are also Dm/Myc target genes that show decreased expression of similar magnitude to the decrease in dm/myc transcripts (Fig. 9C-G). Knockdown of the SA cohesin subunit also reduced expression of dm/myc and Myc target genes, indicating that the cohesin complex is responsible (Fig. S7). Moreover, decreased expression of the known Dm/Myc target genes is likely due to dm/myc downregulation, because dm/myc RNAi reduces their expression in BG3 cells (Fig. S7), and genes encoding Myc's partner Max (Gallant et al., 1996), the Mnt repressor protein that competes with Myc for interaction with Max (Loo et al., 2005; Pierce et al., 2008) and the Ago protein that destabilizes Myc (Moberg et al., 2004) are all unaffected by cohesin RNAi (Fig. S8). In addition, the reduction in dm/myc transcripts caused by cohesin RNAi is not due to effects on other upstream genes that regulate dm/myc function (Fig. S8). Therefore, we conclude that reduced dm/myc transcription accounts for the downregulation of a subset of cohesin-responsive genes.
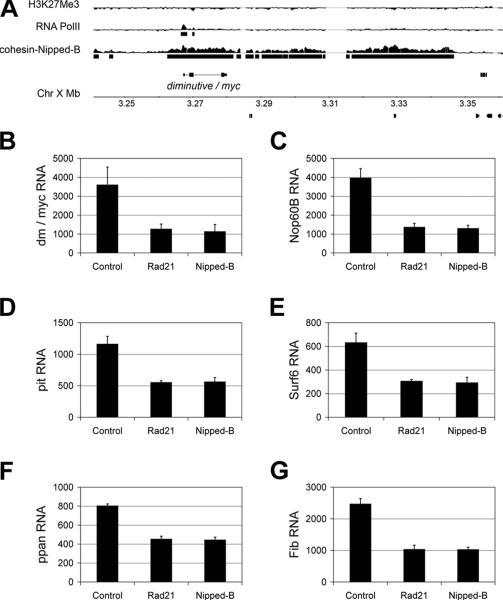
Figure 9. Drosophila dm/myc is located in a cohesin binding region, and is downregulated upon cohesin depletion, along with selected downstream targets.
A, a region on chromosome 1 (X) containing dm/myc is coated with cohesin and Nipped-B in ML-DmBG3 (BG3) cells. RNA PolII binding at the dm/myc gene shows that it is actively transcribed, and a paucity of H3K27Me3 indicates a lack of transcriptional repression across the region. Similar cohesin, PolII and H3K27Me3 patterns are also observed in Kc and Sg4 cells (Misulovin et al., 2008). B, dm/myc transcripts are reduced approximately 4-fold in response to depletion of either Rad21 or Nipped-B. C-G, expression of selected cohesin-responsive genes that do not bind cohesin and are also Dm/Myc targets is reduced to the same degree as dm/myc transcripts in response to depletion of Rad21 or Nipped-B (see A). For each graph, the Y axis indicates the transcript levels in the control, Rad21 RNAi and Nipped-B RNAi samples as measured using the Affymetrix Drosophila GeneChip 2.0 microarrays. The values shown are the average of the control, Rad21 RNAi and Nipped-B RNAi samples after 3, 4 and 6 days post RNAi treatment; error bars are standard errors. The individual values for each sample are in Table S2.
Strikingly, the effects of Rad21 or Nipped-B knockdown on expression of genes downstream of dm/myc in BG3 cells are extremely close to those that occur in dm/myc mutant larvae (Pierce et al., 2008). Of the 110 genes that showed the most decreased expression in dm/myc (dm4) mutant 1st instar larvae (Pierce et al., 2008), 98% also showed decreased expression with cohesin knockdown in BG3 cells (Table S2). Genes that increase in expression in dm/myc mutant larvae are also largely affected by cohesin knockdown in BG3 cells, although less consistently than seen with the genes that decrease (Table S2). Altogether, 90% of examined in vivo dm/myc-sensitive genes are also sensitive to cohesin knockdown in BG3 cells, which is greater than the fraction of cohesin-binding genes that respond to cohesin knockdown in BG3 cells (Schaaf et al., 2009). The results of our analysis argue strongly that effects of cohesin knockdown on expression of Dm/Myc-regulated genes in BG3 cells is caused by the decrease in dm/myc expression, and that cohesin plays a conserved role in regulating the Myc growth and proliferation pathway.
Discussion
In this study we conducted a microarray gene expression analysis of zebrafish embryos null for the cohesin subunit rad21, in which we previously reported dysregulated runx1 and runx3 expression (Horsfield et al., 2007). The dysregulated genes are significantly enriched for those involved in tissue and cellular development, cancer, cell cycle, gene expression and cell death. A statistically significant dependency on rad21 gene dose was observed for the expression of some genes, consistent with a cell cycle-independent role for cohesin, and supporting the idea that quantities of cohesin that are sufficient for cell proliferation may be insufficient for normal gene expression.
The most statistically significant finding from our microarray analysis was the regulation by cohesin of a network of genes that included the well-known oncogene c-Myc.
Cohesin regulation of the c-Myc proliferation pathway is cell cycle independent
The genes regulated by Rad21 in zebrafish included a network of cancer-associated genes – the hubs of this network included myca, p53 and mdm2. While myca was dramatically downregulated in rad21nz171 mutants, p53 and mdm2 were upregulated. mdm2 and p53 also respond (in the same direction as Rad21 loss) to reduction in CTCF. Cohesin binds to predicted CTCF binding sites at the TSS of myca, p53 and mdm2 (Figs 6,7), indicating that both proteins have the potential to directly regulate transcription of these genes. Although binding of cohesin to the TSS of mdm2 and p53 raises the possibility of their direct regulation in zebrafish, it is also possible that their increased expression in rad21 mutants results from activation of a repair response due to near-complete loss of cohesin function late in development.
A previous study in zebrafish describes p53-dependent apoptosis as the primary consequence of cohesin subunit Smc3 knock down (Ghiselli, 2006). However, evidence suggests that neither p53-dependent apoptosis, nor a cell cycle blockade, is responsible for myca downregulation in rad21nz171 mutant embryos. p53 is a known repressor of c-Myc expression (Ho et al., 2005; Moberg et al., 1992; Ragimov et al., 1993). In ctcf morphants and rad21 mutants, p53 and mdm2 are upregulated to comparable levels (Figs 2,3). Because myca is not downregulated in ctcf morphants, excess p53 is unlikely to be responsible for its downregulation in rad21 mutants. Furthermore, there was no increased apoptosis in ctcf morphants despite the raised p53 (Fig. S3), suggesting that p53 is not solely responsible for apoptosis in rad21 mutants.
In accordance with our results, microarray databases from human (Liu et al., 2009), mouse (Kawauchi et al., 2009) and Drosophila (Schaaf et al., 2009) all show downregulation of c-Myc in response to cohesin or Nipbl deficiency. In Nipped-B- or Rad21-depleted Drosophila cells, virtually all ribosomal protein and aminoacyl-tRNA synthetase transcripts are reduced 10 to 30%, and the most statistically significant Gene Ontology category for transcripts that decrease in response to Rad21 or Nipped-B knockdown is protein translation (GO:0006412, p=1.86E-77) (Schaaf et al., 2009), consistent with a decrease in Dm/Myc function. In Drosophila, Rad21 or Nipped-B knockdown by 80% has no substantial effect on cell division or sister chromatid cohesion other than a mild G2/M delay, and essentially no effect on expression of DNA repair or cell cycle genes, with the exception of a slight increase in cyclin B transcripts (Schaaf et al., 2009). In lymphocytes from CdLS patients (Liu et al., 2009), c-MYC is downregulated to the same extent as NIPBL (20 to 30%), and in Nipbl/+ mouse brain (Kawauchi et al., 2009), c-Myc is again downregulated by 20-30%. The absence of proliferation defects in either case indicates c-Myc regulation by cohesin is independent of cell cycle effects. Thus, cohesin regulation of the c-Myc gene and the downstream cell growth and proliferation pathway is independent of DNA repair and cell cycle regulation. Moreover, our analysis of the Drosophila data indicates that a sizeable fraction of cohesin-responsive genes may be regulated consequential to c-Myc downregulation rather than regulated directly by cohesin per se. The combined human, mouse, zebrafish and Drosophila data argue that positive regulation of c-Myc expression by cohesin is direct, and conserved between invertebrates and vertebrates.
CTCF depletion does not affect myca expression or cohesin binding
Recent genome-wide studies have shown extensive overlap of cohesin and CTCF binding in the mammalian genomes (Wendt and Peters, 2009), including the MINE and P2 promoter regions of the c-MYC locus (Rubio et al., 2008; Stedman et al., 2008). In fish and human, two equally spaced CTCF binding sites are predicted upstream of myca and c-MYC with the sites located closer to the TSS in zebrafish than in human. In human cells, the promoter-proximal of the two sites interacts with CTCF and RAD21 (Liu and Krantz, pers. comm.), while in zebrafish the more distal of the two sites is bound by Rad21 (Figs 6C, S5). The evolutionary conservation of the CTCF binding sequences and their similar spacing between fish and human implies that these sites are functional, but the nature of this function remains to be determined. Additional in vivo binding sites for CTCF (Gombert and Krumm, 2009) and Rad21 (Liu and Krantz, pers. comm.) are present at the P2 promoter (human) and the TSS (zebrafish) although these sites were not predicted in silico. Therefore, the c-Myc locus Rad21 and CTCF sites are highly conserved between zebrafish and human.
Even though CTCF binds to the MINE and P2 in mammals, the roles of these sites in c-Myc regulation have been difficult to establish. CTCF binds to the MINE regardless of whether or not c-Myc is expressed (Gombert et al., 2003) and deletion of the CTCF binding site in the MINE has no effect on c-Myc expression (Gombert and Krumm, 2009). Altered c-Myc expression occurs only when mammalian CTCF sites at both the MINE and P2 promoter regions are deleted, and this modestly reduces expression (Gombert and Krumm, 2009). In lymphoblastoid cell lines derived from CdLS patients where c-MYC is downregulated, cohesin binding at the MINE CTCF site is unaffected, but binding is reduced at the P2 site (Liu and Krantz, pers. comm.). In Drosophila BG3 cells, the entire dm/myc gene binds cohesin, with the highest peak at the TSS (Misulovin et al., 2008) while the closest CTCF binding sites are ~40 kb upstream and downstream of the transcription unit (modENCODE). In zebrafish, depletion of CTCF had no effect on myca expression, while loss of Rad21 dramatically reduced expression. However, CTCF depletion did influence the transcription of a subset of other genes regulated by cohesin (e.g. mdm2, p53, ascl1a/1b, aqp), in the same direction as cohesin. Furthermore, the aqp1 and mdm2 genes responded incrementally to ctcf morpholino dose (Fig. S2C) while myca levels remained unchanged. Unexpectedly, Rad21 binding to the -1.27 CTCF binding site and the TSS persisted in CTCF-depleted embryos (Fig. 6). While it is possible that depletion of CTCF in these embryos was not complete enough to influence cohesin binding, these data raise the possibility that cohesin can bind the zebrafish myca gene independently of CTCF. Indeed, there was a statistically significant increase in cohesin binding (p=0.01) at the -1.27 site upon CTCF depletion (Fig. 6D), arguing against the idea that CTCF is essential for cohesin binding to this site. Furthermore, depletion of CTCF in HCT116 cells did not eliminate cohesin binding at the MINE and P2 of c-MYC (JMR and JAH, unpublished data).
The combined data from human, mouse, Drosophila and zebrafish indicate that the functions of cohesin and CTCF in transcriptional regulation of c-Myc may be separable. Since the MINE appears to be dispensable for regulation of c-Myc transcription (Gombert and Krumm, 2009), we propose that cohesin regulates myca expression independently of CTCF through the TSS/P2 promoter binding site. A recent study showed that cohesin binds specific sites in the human genome independently of CTCF (Schmidt et al., 2010), in combination with tissue-specific transcription factors. Since multiple transcriptional regulators bind c-Myc in a context-dependent manner, it is possible that cohesin binding depends on the spatiotemporal availability of other DNA binding factors in addition to CTCF.
Refining the mechanism of Myc regulation by cohesin
Chromatin structure of the zebrafish myca region compared with that of human c-MYC is suggestive of c-Myc regulatory elements that are conserved through evolution, and are thus likely to be important for c-Myc regulation. A boundary dividing condensed from hyperacetylated chromatin coincides with the MINE ~2 kb upstream of human c-MYC (Gombert et al., 2003). In contrast, profiling of H3K9Ac across the myca locus revealed that this boundary does not appear to be conserved in zebrafish. However, enrichment of H3K9Me ~10 kb upstream of myca coincident with a cohesin binding site suggests that a boundary may exist at this location (Fig. S5). The absence of a chromatin boundary at the zebrafish MINE-equivalent cohesin binding site raises the possibility that MINE function can be separated from chromatin barrier positioning.
In some cases, CTCF and cohesin may function as a ‘barrier insulator’ by blocking the spread of silencing chromatin structures (Wendt and Peters, 2009). However, although we observed specific changes in histone modification in rad21 mutants compared with wild type, these were strongly localized to the TSS and the start of the myca gene itself. Therefore, downregulation of myca in rad21 mutants is unlikely to be due to spreading of silenced chromatin from an upstream region.
In rad21 mutants, we found an enrichment of histone marks localized to the myca TSS that reflect a state of transcription repression. The H3K27Me3 histone modification is a mark of PcG repression, and is highly enriched at the myca locus in wild type embryos (Fig. S6), indicating that H3K27Me3 normally plays a role in switching off myca. Significantly, the enrichment of this histone mark is doubled at the myca TSS in rad21 mutants. However, there was no difference in the repressive histone modification H3K9Me3 between rad21 mutants and wild type. The H3K4Me3 histone mark denotes gene activation and was unchanged in rad21 mutants compared with wild type, however, there was a 2-fold depletion of H3K9Ac (also a signature of active transcription). Therefore, loss of cohesin results in very specific and localized changes in histone modification at the myca TSS that are consistent with repression of its transcription. How cohesin depletion causes changes in histone modification remains to be determined.
Perhaps cohesin regulates c-Myc transcription by mediating long-range enhancer-promoter interactions (Hadjur et al., 2009; Wendt and Peters, 2009). Investigation of cancer-associated SNPs in the 8q24 gene desert (near c-MYC) found that several of these are located in transcriptional enhancers (Jia et al., 2009). One such SNP, strongly linked with prostate and colorectal cancers, is within an enhancer that physically interacts with the c-MYC promoter ~335 kb downstream (Pomerantz et al., 2009; Tuupanen et al., 2009). Moreover, mutations in cohesin subunits have been linked to colorectal cancer (Barber et al., 2008). It is possible cohesin brings the c-MYC promoter into proximity with distant enhancers, such as those recently found in the 8q24 gene desert. Further studies will be needed to define the exact mechanism by which cohesin regulates c-MYC and other cancer-related genes.
Conclusion
Whatever the mechanism of cohesin regulation of c-Myc transcription, it is remarkable that this regulation is highly evolutionarily conserved, from flies to human. It is tempting to speculate that conserved cohesin-dependent regulation of c-Myc expression may provide a mechanism for the coordination of cell division, where cohesin has a key function, with cell growth controlled by Myc. This in turn could influence cell fate decisions that underlie development.
Supplementary Material
Acknowledgements
We thank Judy Rodda for expert management of the zebrafish facility, Aaron Jeffs for advice on qPCR, Liam Williams for performing the micorarray hybridizations, Fiona Wardle for invaluable advice on ChIP and Mike Eccles for helpful discussions. The zebrafish work was funded by grants to JAH from the Breast Cancer Research Trust, Genesis Oncology Trust and Maurice & Phyllis Paykel Trusts of NZ, Lottery Health Research NZ, the Marsden Fund and the Health Research Council of NZ. Support for the Drosophila analysis was provided by grants to DD from the USA National Institutes of Health (R01 GM055683, P01 HD052860).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bao L, Zhou M, Cui Y. CTCFBSDB: a CTCF-binding site database for characterization of vertebrate genomic insulators. Nucleic Acids Res. 2008;36:D83–87. doi: 10.1093/nar/gkm875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D, Barrett I, Nouhi Y, Spencer F, Markowitz S, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C, Hieter P. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci U S A. 2008;105:3443–3448. doi: 10.1073/pnas.0712384105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers SR, Mirabella F, Calero-Nieto FJ, Valeaux S, Hadjur S, Baxter EW, Merkenschlager M, Cockerill PN. A conserved insulator that recruits CTCF and cohesin exists between the closely related but divergently regulated interleukin-3 and granulocyte-macrophage colony-stimulating factor genes. Mol Cell Biol. 2009;29:1682–1693. doi: 10.1128/MCB.01411-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciosk R, Shirayama M, Shevchenko A, Tanaka T, Toth A, Nasmyth K. Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol Cell. 2000;5:243–254. doi: 10.1016/s1097-2765(00)80420-7. [DOI] [PubMed] [Google Scholar]
- Deardorff MA, Kaur M, Yaeger D, Rampuria A, Korolev S, Pie J, Gil-Rodriguez C, Arnedo M, Loeys B, Kline AD, Wilson M, Lillquist K, Siu V, Ramos FJ, Musio A, Jackson LS, Dorsett D, Krantz ID. Mutations in cohesin complex members SMC3 and SMC1A cause a mild variant of cornelia de Lange syndrome with predominant mental retardation. Am J Hum Genet. 2007;80:485–494. doi: 10.1086/511888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D. Roles of the sister chromatid cohesion apparatus in gene expression, development, and human syndromes. Chromosoma. 2007;116:1–13. doi: 10.1007/s00412-006-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D. Cohesin, gene expression and development: lessons from Drosophila. Chromosome Res. 2009;17:185–200. doi: 10.1007/s10577-009-9022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D, Eissenberg JC, Misulovin Z, Martens A, Redding B, McKim K. Effects of sister chromatid cohesion proteins on cut gene expression during wing development in Drosophila. Development. 2005;132:4743–4753. doi: 10.1242/dev.02064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu B, Wang G, Tu N, Sun X, Mivechi NF. Critical role of Brg1 member of the SWI/SNF chromatin remodeling complex during neurogenesis and neural crest induction in zebrafish. Dev Dyn. 2006;235:2722–2735. doi: 10.1002/dvdy.20911. [DOI] [PubMed] [Google Scholar]
- Gallant P, Shiio Y, Cheng PF, Parkhurst SM, Eisenman RN. Myc and Max homologs in Drosophila. Science. 1996;274:1523–1527. doi: 10.1126/science.274.5292.1523. [DOI] [PubMed] [Google Scholar]
- Gause M, Webber HA, Misulovin Z, Haller G, Rollins RA, Eissenberg JC, Bickel SE, Dorsett D. Functional links between Drosophila Nipped-B and cohesin in somatic and meiotic cells. Chromosoma. 2008;117:51–66. doi: 10.1007/s00412-007-0125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli G. SMC3 knockdown triggers genomic instability and p53-dependent apoptosis in human and zebrafish cells. Mol Cancer. 2006;5:52. doi: 10.1186/1476-4598-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombert WM, Farris SD, Rubio ED, Morey-Rosler KM, Schubach WH, Krumm A. The c-myc insulator element and matrix attachment regions define the c-myc chromosomal domain. Mol Cell Biol. 2003;23:9338–9348. doi: 10.1128/MCB.23.24.9338-9348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombert WM, Krumm A. Targeted deletion of multiple CTCF-binding elements in the human C-MYC gene reveals a requirement for CTCF in C-MYC expression. PLoS ONE. 2009;4:e6109. doi: 10.1371/journal.pone.0006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjur S, Williams LM, Ryan NK, Cobb BS, Sexton T, Fraser P, Fisher AG, Merkenschlager M. Cohesins form chromosomal cis-interactions at the developmentally regulated IFNG locus. Nature. 2009;460:410–413. doi: 10.1038/nature08079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering CH, Farcas AM, Arumugam P, Metson J, Nasmyth K. The cohesin ring concatenates sister DNA molecules. Nature. 2008;454:297–301. doi: 10.1038/nature07098. [DOI] [PubMed] [Google Scholar]
- Hallson G, Syrzycka M, Beck SA, Kennison JA, Dorsett D, Page SL, Hunter SM, Keall R, Warren WD, Brock HW, Sinclair DA, Honda BM. The Drosophila cohesin subunit Rad21 is a trithorax group (trxG) protein. Proc Natl Acad Sci U S A. 2008;105:12405–12410. doi: 10.1073/pnas.0801698105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho JS, Ma W, Mao DY, Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol. 2005;25:7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsfield J, Anagnostou S, Hu JKH, Cho KH-Y, Geisler R, Lieschke G, Crosier K, Crosier P. Cohesin-dependent regulation of runx genes. Development. 2007;134:2639–2649. doi: 10.1242/dev.002485. [DOI] [PubMed] [Google Scholar]
- Jia L, Landan G, Pomerantz M, Jaschek R, Herman P, Reich D, Yan C, Khalid O, Kantoff P, Oh W, Manak JR, Berman BP, Henderson BE, Frenkel B, Haiman CA, Freedman M, Tanay A, Coetzee GA. Functional enhancers at the gene-poor 8q24 cancer-linked locus. PLoS Genet. 2009;5:e1000597. doi: 10.1371/journal.pgen.1000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, Horsfield JA, Flores MV, Postlethwait JH, Vitas MR, Baas AM, Crosier PS, Crosier KE. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- Kawauchi S, Calof AL, Santos R, Lopez-Burks ME, Young CM, Hoang MP, Chua A, Lao T, Lechner MS, Daniel JA, Nussenzweig A, Kitzes L, Yokomori K, Hallgrimsson B, Lander AD. Multiple organ system defects and transcriptional dysregulation in the Nipbl(+/-) mouse, a model of Cornelia de Lange Syndrome. PLoS Genet. 2009;5:e1000650. doi: 10.1371/journal.pgen.1000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krantz ID, McCallum J, DeScipio C, Kaur M, Gillis LA, Yaeger D, Jukofsky L, Wasserman N, Bottani A, Morris CA, Nowaczyk MJ, Toriello H, Bamshad MJ, Carey JC, Rappaport E, Kawauchi S, Lander AD, Calof AL, Li HH, Devoto M, Jackson LG. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat Genet. 2004;36:631–635. doi: 10.1038/ng1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Hu JF, Qiu X, Ling J, Chen H, Wang S, Hou A, Vu TH, Hoffman AR. CTCF regulates allelic expression of Igf2 by orchestrating a promoter-polycomb repressive complex 2 intrachromosomal loop. Mol Cell Biol. 2008;28:6473–6482. doi: 10.1128/MCB.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Krantz ID. Cohesin and Human Disease. Annu Rev Genomics Hum Genet. 2008;9:303–320. doi: 10.1146/annurev.genom.9.081307.164211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang Z, Bando M, Itoh T, Deardorff MA, Clark D, Kaur M, Tandy S, Kondoh T, Rappaport E, Spinner NB, Vega H, Jackson LG, Shirahige K, Krantz ID. Transcriptional dysregulation in NIPBL and cohesin mutant human cells. PLoS Biol. 2009;7:e1000119. doi: 10.1371/journal.pbio.1000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo LW, Secombe J, Little JT, Carlos LS, Yost C, Cheng PF, Flynn EM, Edgar BA, Eisenman RN. The transcriptional repressor dMnt is a regulator of growth in Drosophila melanogaster. Mol Cell Biol. 2005;25:7078–7091. doi: 10.1128/MCB.25.16.7078-7091.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A. The regulation of sister chromatid cohesion. Biochim Biophys Acta. 2008;1786:41–48. doi: 10.1016/j.bbcan.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Misulovin Z, Schwartz YB, Li XY, Kahn TG, Gause M, MacArthur S, Fay JC, Eisen MB, Pirrotta V, Biggin MD, Dorsett D. Association of cohesin and Nipped-B with transcriptionally active regions of the Drosophila melanogaster genome. Chromosoma. 2008;117:89–102. doi: 10.1007/s00412-007-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg KH, Mukherjee A, Veraksa A, Artavanis-Tsakonas S, Hariharan IK. The Drosophila F box protein archipelago regulates dMyc protein levels in vivo. Curr Biol. 2004;14:965–974. doi: 10.1016/j.cub.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Moberg KH, Tyndall WA, Hall DJ. Wild-type murine p53 represses transcription from the murine c-myc promoter in a human glial cell line. J Cell Biochem. 1992;49:208–215. doi: 10.1002/jcb.240490213. [DOI] [PubMed] [Google Scholar]
- Mönnich M, Banks S, Eccles M, Dickinson E, Horsfield J. Expression of cohesin and condensin genes during zebrafish development supports a non-proliferative role for cohesin. Gene Expr Patterns. 2009 doi: 10.1016/j.gep.2009.08.004. In press. [DOI] [PubMed] [Google Scholar]
- Musio A, Selicorni A, Focarelli ML, Gervasini C, Milani D, Russo S, Vezzoni P, Larizza L. X-linked Cornelia de Lange syndrome owing to SMC1L1 mutations. Nat Genet. 2006;38:528–530. doi: 10.1038/ng1779. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH. Cohesin: Its Roles and Mechanisms. Annu Rev Genet. 2009 doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- Nativio R, Wendt KS, Ito Y, Huddleston JE, Uribe-Lewis S, Woodfine K, Krueger C, Reik W, Peters JM, Murrell A. Cohesin Is Required for Higher-Order Chromatin Conformation at the Imprinted IGF2-H19 Locus. PLoS Genet. 2009;5:e1000739. doi: 10.1371/journal.pgen.1000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parelho V, Hadjur S, Spivakov M, Leleu M, Sauer S, Gregson HC, Jarmuz A, Canzonetta C, Webster Z, Nesterova T, Cobb BS, Yokomori K, Dillon N, Aragon L, Fisher AG, Merkenschlager M. Cohesins Functionally Associate with CTCF on Mammalian Chromosome Arms. Cell. 2008;132:422–433. doi: 10.1016/j.cell.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Pauli A, Althoff F, Oliveira RA, Heidmann S, Schuldiner O, Lehner CF, Dickson BJ, Nasmyth K. Cell-type-specific TEV protease cleavage reveals cohesin functions in Drosophila neurons. Dev Cell. 2008;14:239–251. doi: 10.1016/j.devcel.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Yost C, Anderson SA, Flynn EM, Delrow J, Eisenman RN. Drosophila growth and development in the absence of dMyc and dMnt. Dev Biol. 2008;315:303–316. doi: 10.1016/j.ydbio.2007.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz MM, Ahmadiyeh N, Jia L, Herman P, Verzi MP, Doddapaneni H, Beckwith CA, Chan JA, Hills A, Davis M, Yao K, Kehoe SM, Lenz HJ, Haiman CA, Yan C, Henderson BE, Frenkel B, Barretina J, Bass A, Tabernero J, Baselga J, Regan MM, Manak JR, Shivdasani R, Coetzee GA, Freedman ML. The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet. 2009;41:882–884. doi: 10.1038/ng.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugacheva EM, Kwon YW, Hukriede NA, Pack S, Flanagan PT, Ahn JC, Park JA, Choi KS, Kim KW, Loukinov D, Dawid IB, Lobanenkov VV. Cloning and characterization of zebrafish CTCF: Developmental expression patterns, regulation of the promoter region, and evolutionary aspects of gene organization. Gene. 2006;375:26–36. doi: 10.1016/j.gene.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Ragimov N, Krauskopf A, Navot N, Rotter V, Oren M, Aloni Y. Wild-type but not mutant p53 can repress transcription initiation in vitro by interfering with the binding of basal transcription factors to the TATA motif. Oncogene. 1993;8:1183–1193. [PubMed] [Google Scholar]
- Rollins RA, Korom M, Aulner N, Martens A, Dorsett D. Drosophila nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol Cell Biol. 2004;24:3100–3111. doi: 10.1128/MCB.24.8.3100-3111.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins RA, Morcillo P, Dorsett D. Nipped-B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics. 1999;152:577–593. doi: 10.1093/genetics/152.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ED, Reiss DJ, Welcsh PL, Disteche CM, Filippova GN, Baliga NS, Aebersold R, Ranish JA, Krumm A. CTCF physically links cohesin to chromatin. Proc Natl Acad Sci U S A. 2008;105:8309–8314. doi: 10.1073/pnas.0801273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf CA, Misulovin Z, Sahota G, Siddiqui AM, Schwartz YB, Kahn TG, Pirrotta V, Gause M, Dorsett D. Regulation of the Drosophila Enhancer of split and invected-engrailed gene complexes by sister chromatid cohesion proteins. PLoS ONE. 2009;4:e6202. doi: 10.1371/journal.pone.0006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Schwalie P, Ross-Innes CS, Hurtado A, Brown G, Carroll J, Flicek P, Odom D. A CTCF-independent role for cohesin in tissue-specific transcription. Genome Res. 2010 doi: 10.1101/gr.100479.109. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Schuldiner O, Berdnik D, Levy JM, Wu JS, Luginbuhl D, Gontang AC, Luo L. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev Cell. 2008;14:227–238. doi: 10.1016/j.devcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitan VC, Banks P, Laval S, Majid NA, Dorsett D, Rana A, Smith J, Bateman A, Krpic S, Hostert A, Rollins RA, Erdjument-Bromage H, Tempst P, Benard CY, Hekimi S, Newbury SF, Strachan T. Metazoan Scc4 Homologs Link Sister Chromatid Cohesion to Cell and Axon Migration Guidance. PLoS Biol. 2006;4:e242. doi: 10.1371/journal.pbio.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman W, Kang H, Lin S, Kissil JL, Bartolomei MS, Lieberman PM. Cohesins localize with CTCF at the KSHV latency control region and at cellular c-myc and H19/Igf2 insulators. Embo J. 2008;27:654–666. doi: 10.1038/emboj.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strachan T. Cornelia de Lange Syndrome and the link between chromosomal function, DNA repair and developmental gene regulation. Curr Opin Genet Dev. 2005;15:258–264. doi: 10.1016/j.gde.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Tonkin ET, Wang TJ, Lisgo S, Bamshad MJ, Strachan T. NIPBL, encoding a homolog of fungal Scc2-type sister chromatid cohesion proteins and fly Nipped-B, is mutated in Cornelia de Lange syndrome. Nat Genet. 2004;36:636–641. doi: 10.1038/ng1363. [DOI] [PubMed] [Google Scholar]
- Tuupanen S, Turunen M, Lehtonen R, Hallikas O, Vanharanta S, Kivioja T, Bjorklund M, Wei G, Yan J, Niittymaki I, Mecklin JP, Jarvinen H, Ristimaki A, Di-Bernardo M, East P, Carvajal-Carmona L, Houlston RS, Tomlinson I, Palin K, Ukkonen E, Karhu A, Taipale J, Aaltonen LA. The common colorectal cancer predisposition SNP rs6983267 at chromosome 8q24 confers potential to enhanced Wnt signaling. Nat Genet. 2009;41:885–890. doi: 10.1038/ng.406. [DOI] [PubMed] [Google Scholar]
- Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol. 2006;16:318–330. doi: 10.1016/j.semcancer.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr Opin Genet Dev. 2007;17:400–407. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrin E, Peters JM. Cohesin and DNA damage repair. Exp Cell Res. 2006;312:2687–2693. doi: 10.1016/j.yexcr.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Wendt KS, Peters JM. How cohesin and CTCF cooperate in regulating gene expression. Chromosome Res. 2009;17:201–214. doi: 10.1007/s10577-008-9017-7. [DOI] [PubMed] [Google Scholar]
- Wendt KS, Yoshida K, Itoh T, Bando M, Koch B, Schirghuber E, Tsutsumi S, Nagae G, Ishihara K, Mishiro T, Yahata K, Imamoto F, Aburatani H, Nakao M, Imamoto N, Maeshima K, Shirahige K, Peters JM. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- Westerfield M. A Guide for the Laboratory Use of Zebrafish (Danio rerio) 3rd edition University of Oregon Press; Eugene, OR: 1995. The Zebrafish Book. [Google Scholar]
- Zhang B, Chang J, Fu M, Huang J, Kashyap R, Salavaggione E, Jain S, Shashikant K, Deardorff MA, Uzielli ML, Dorsett D, Beebe DC, Jay PY, Heuckeroth RO, Krantz I, Milbrandt J. Dosage effects of cohesin regulatory factor PDS5 on mammalian development: implications for cohesinopathies. PLoS ONE. 2009;4:e5232. doi: 10.1371/journal.pone.0005232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Jain S, Song H, Fu M, Heuckeroth RO, Erlich JM, Jay PY, Milbrandt J. Mice lacking sister chromatid cohesion protein PDS5B exhibit developmental abnormalities reminiscent of Cornelia de Lange syndrome. Development. 2007;134:3191–3201. doi: 10.1242/dev.005884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.