Abstract
At present, the treatment of T-cell-dependent autoimmune diseases relies exclusively on strategies leading to nonspecific suppression of the immune systems causing a substantial reduced ability to control concomitant infections or malignancies. Furthermore, long-term treatment with most drugs is accompanied by several serious adverse effects and does not consequently result in cure of the primary immunological malfunction. By contrast, antigen-specific immunotherapy offers the potential to achieve the highest therapeutic efficiency in accordance with minimal adverse effects. Therefore, several studies have been performed utilizing antigen-presenting cells specifically engineered to deplete allo- or antigen-specific T cells (‘guided missiles’). Many of these strategies take advantage of the Fas/Fas ligand signaling pathway to efficiently induce antigen-presenting cell-mediated apoptosis in targeted T cells. In this article, we discuss the advantages and shortcomings of a novel non-cell-based ‘killer artificial antigen-presenting cell’ strategy, developed to overcome obstacles related to current cell-based approaches for the treatment of T-cell-mediated autoimmunity.
Keywords: antigen-presenting cell, apoptosis, artificial, killer artificial antigen-presenting cell, T cell
Approximately 5% of the Western population are suffering from autoimmune diseases. Those individuals are in need of a long-term treatment to prevent organ damage and to reduce disease-related mortality. Still, the most common treatments of autoimmune diseases rely on corticosteroids as well as immunosuppressive drugs such as purine analogs, alkylating agents and calcineurin inhibitors [1]. Even though the use of such immuno suppressive drugs has greatly improved the prognosis of patients suffering from autoimmune diseases, these treatments do not provide a cure for the underlying immunological malfunction, and the ability to control concomitant infections or malignancies are frequently impaired. Furthermore, treatment does not result in prolonged periods of drug-free remissions.
Recently, new biological agents including monoclonal antibodies (mAbs) have been introduced, targeting inflammatory effector molecules or defined proteins and receptors expressed on the surface of pathogenic immune cells. Promising results have been observed in several autoimmune diseases including systemic lupus erythematosus, rheumatoid arthritis (RA) and multiple sclerosis by utilizing mAbs against CD20, leading to an efficient B-cell depletion with an excellent safety record at the same time (reviewed in [2]). Anti-TNF mAb and soluble recombinant TNF receptor–immunoglobulin (Ig) fusion proteins have been demonstrated to suppress inflammation and improve function in patients with RA efficiently [3]. In the meantime, numerous mAbs or constructs targeted towards cytokines, receptors, integrins and chemokines have been recently approved or are currently under investigation in clinical trials. But all these strategies are not exclusively restricted to pathogenetically relevant cells or mechanisms, leading to potentially severe side effects [4–6]. Therefore, there is still a necessity for the development of novel selective antigen-specific immunotherapies to further improve the treatment outcomes with reduced adverse effects at the same time.
The idea of an antigen-specific therapy dates back to the 1970s and 1980s, when several groups discovered the existence of naturally occurring antigen-presenting cells (APCs) that suppressed peripheral T cells in an antigen-specific fashion. Such ‘veto cells’ presented autoantigens on their cell surface, facilitating elimination of T cells recognizing such antigens (reviewed in [7]). These findings were essentially amplified in the late 1980s and early 1990s through the discovery that natural APCs can be artificially modified to become ‘artificial veto cells’. These cells were decorated with specific protein constructs, such as HLA-A2, to modulate the antigenic display; costimulators such as CD80, CD86 and cytokines such as IL-2 via decay-accelerating factor-mediated binding of a glycosylinositol phospholipid moiety. The artificial veto cell concept was based on the enforced expression of ‘co-inhibitors’ such as CD8, which induces inhibition of T cells upon antigenic recognition (reviewed in [8]).
Based on these initial findings, it has been assumed that trans-signaling molecules known to induce apoptosis or programmed cell death, such as Fas ligand (FasL) and TNF, or the overexpression of immunosuppressive molecules including IL-10, TGF-β and cytotoxic T-lymphocyte antigen 4 would facilitate the therapeutic use of artificial veto cells, leading to the concept of a ‘killer APC’ or ‘guided missile’ (reviewed in [9]). Over the years, killer APCs and guided missiles were reinvented several times and in multiple facets, always aiming for an antigen-specific suppression of auto- or allo-reactive T-cell responses. Thus, dendritic cells (DCs) emerged as an attractive target owing to their pivotal potency in uptaking, processing and presenting foreign and self-antigens to T cells (reviewed in [10]).
The major advantage of the killer APC or guided missile concept compared with conventional treatment strategies originates in an antigen-specific elimination or suppression of disease-causing T cells without restraining the immune response towards viral, bacterial or tumor antigens. Furthermore, there is a reasonable possibility that treatment with such guided missiles in vivo will have limited side effects.
This article will focus on the development of an artificial guided missile-derived novel strategy utilizing the Fas/FasL signaling pathway to deplete autoreactive T cells in an antigen-specific manner. We will provide an overview of cell-based and artificial concepts that finally led to the development of a killer artificial APC (KaAPC).
Cell-based depletion strategies
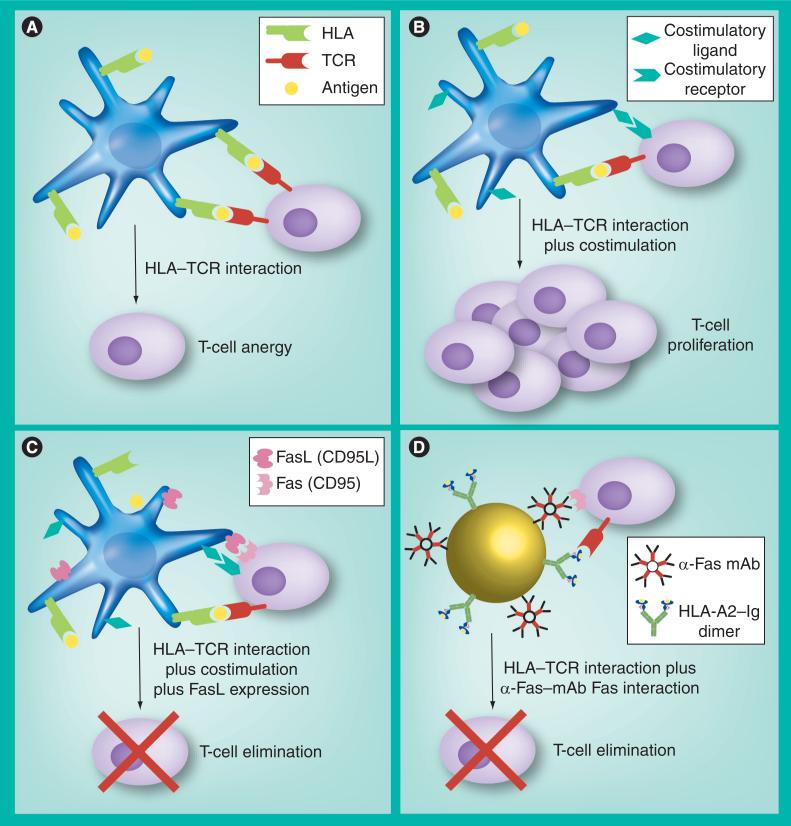
Prerequisite to all these concepts is the tight interaction of T cells with professional APC. If only a T-cell receptor–HLA interaction occurs without further stimulation, functional anergy is induced in responder T cells (Figure 1A). However, the concomitant presentation of a prominent costimulatory signal leads to efficient activation and induction of clonal expansion in T cells (Figure 1B). Besides these two signals, killer APCs provide an additional apoptosis-inducing ‘third signal’ mediated through death-inducing trans-signaling molecules such as FasL. Thus killer APCs induces antigen-specific apoptosis in activated Fas+ T cells, whereas FasL expression can be achieved in APCs utilizing gene transduction or exogenous loading with Fas-activating molecules (Figure 1C).
Figure 1. Possible T-cell–antigen-presenting cell interactions.
(A) If solely a TCR–HLA interaction occurs without further stimulation, functional anergy is induced in interacting T cells. (B) The additional presentation of a prominent costimulatory signal leads to efficient activation and induction of clonal expansion in T cells. (C) Together with these two signals, killer APCs provide an additional ‘third signal’ as these cells express FasL that induces apotosis in Fas+ T cells. Killer artificial APCs are the synthetic embodiment of a cell-based killer APC. (D) These constructs simultaneously provide an antigen-specific signal (HLA-A2–Ig dimer) and an apoptosis signal (anti-Fas mAb) resulting in efficient antigen-specific depletion of Fas+ T cells.
APC: Antigen-presenting cell; FasL: Fas ligand; Ig: Immunoglobulin; mAb: Monoclonal antibody; TCR: T-cell receptor.
To date, FasL-expressing APCs derived from B-cell lines [11], macrophages [12] and DCs [13] have been successfully utilized for the generation of killer APCs in different in vitro and in vivo animal models resembling features of RA [14], allogeneic transplantation [15–18], allergy [19] and animal models used for the analysis of antigen-specific T-cell responses [11,12,20–22]. Thus, substantial suppression of allo- and auto-antigen-specific T-cell responses has been demonstrated. However, Fas is also expressed within and on the surface of these engineered APCs, rendering these cells susceptible for Fas/FasL-mediated autocrine or bystander apoptosis. To prevent this limitation, killer APCs have been derived from precursor cells with established defects in the Fas/FasL signaling pathway [13,16,23], Fas- cell lines [16,17,20,21] or cell lines with a defective Fas/FasL signaling pathway [11,24].
By contrast, studies performed with primary human cells are still limited. Hoves et al. could generate murine FasL-expressing human primary mature DCs that induced apoptosis in primary allogeneic Fas+ T cells, and at the same time failed to deplete primary naive T cells [25,26]. In addition, FasL-transduced human cell lines have been used to achieve alloantigen-specific T-cell tolerance and preservation of protective antiviral T-cell responses at the same time [27,28]. However, studies demonstrating antigen-specific suppression of T-cell responses utilizing human FasL-expressing killer APCs are still missing.
Besides these promising results that envisage FasL-expressing killer APCs as a potent therapeutic strategy for the treatment of allograft rejection [17,18,29], autoimmune disease [27,30–34] and chronic infections [24,35], there are several restrictions that clearly limit their application: isolation, enrichment and differentiation of APCs are time, cost and labor intensive. Multiple enrichment cycles from peripheral blood mononuclear cells are required; however, many patients exhibit cytopenia due to chemotherapy or immuno suppressive drugs. Therefore, the amount of cells is strongly limited and the quality highly variable. Hence, it is almost impossible to assure a defined reproducible immunosuppressive APC phenotype that can be potentially applied for the modulation of the immune response in vivo. Furthermore, abnormal regulation of the immune system is the major cause for the development of autoimmune diseases. Therefore, the utilization of FasL-expressing killer APCs with varying phenotypes might lead to a potential unknown hazard and increased risk of further immunological malfunction. Moreover, generation of several FasL-expressing killer APCs requires viral transduction. Consequently, an inefficient transduction of APCs would increase the risk of a secondary immune response towards viral vector antigens initiated by nonfunctional killer APCs. On the other hand, a too exalted FasL expression could result in loss of a protective immune response via unspecific bystander depletion of vector-specific T cells. In addition, even if a stable FasL expression could be achieved, FasL can be cleaved from the cell surface through a metalloproteinase-dependent mechanism, resulting in a possible inhibition or unspecific activation of the Fas/FasL signaling pathway by soluble FasL, respectively (reviewed in [36]). Therefore, studies have been performed utilizing a genetically modified FasL missing the metalloproteinase cleavage side [37]. Furthermore, killer APCs are sensitive to cytotoxic effector functions of T cells, resulting in their elimination and consequently diminished killing efficiency. Hence, the therapeutic outcome and the consequences for the immune system seem unpredictable, if utilizing killer APCs [38].
Therefore, the crucial factor for a successful antigen-specific treatment is a defined and robust phenotype of FasL-expressing killer APCs, which appears unfeasible to achieve given the currently available viral and nonviral transduction methods for cellular approaches.
Novel artificial concepts paving the way to KaAPC
The development of the MHC tetramer technology has established many new possibilities of immune modulatory strategies [39]. Tetramers not only allow for visualization of antigen-specific T cells but also permit investigation of TCR and MHC interactions [40]. Previously, it has been demonstrated that H-2Kb dimer molecules bind to H-2Kb alloreactive T-cell clones and primary T cells in nanomolecular concentrations, thereby inhibiting the cytotoxic effector functions of those T cells [41]. Further evidence for an inhibitory influence of MHC class I-tetramers was derived from experiments with female B6 mice. Multiple injections of HY-Db-tetramer prevented a graft rejection of male B6 skin transplants. These results emphasize the great potential of MHC tetramers for selective immunotherapy in allograft rejections [42].
Peptide-loaded MHC molecules have a high affinity to bind the corresponding TCR on antigen-specific T cells. Some novel approaches took advantage of this ability to deplete T cells in an antigen-specific manner. Actinum 225-conjugated MHC tetramers have been demonstrated to bind to corresponding CD8+ T-cell lines and thereby inducing apoptosis in T cells via α-particle emission. By contrast, T cells specific for irrelevant antigens were not depleted [43]. Co-culture of T cells with ribosome inhibitor saporin-conjugated MHC tetramers resulted in the elimination of 75% of antigen-specific T cells after 72 h, whereas background apoptosis was observed in control co-cultures. Apoptosis induction was dependent on the saporin–MHC tetramer to T-cell ratio and the antigen-specific T-cell affinity [44]. With regards to these observations, there is evidence to suggest that MHC tetramers could be deployed for the generation of immune modulatory ‘guided missiles’.
To overcome problems related to autologous cell-based treatment strategies, many investigators promoted the development of artificial APC (aAPCs). Currently, aAPCs are mainly used for the expansion of antigen-specific CD4+ and CD8+ T cells for adoptive immunotherapy of chronic infections and cancer. Such studies aim to infuse ex vivo activated and expanded autologous antigen-specific or tumor-reactive T cells into donors receiving standard medical treatment [45–48].
Studies focusing on the development of an optimal aAPC phenotype have utilized artificially engineered cells [49,50] or other scaffolds such as liposomes [40,51] and latex or magnetic beads [52–54]. First, nonspecific activation and expansion of T cells using anti-CD3 and anti-CD28 mAbs or 4-1BBL-coated aAPCs [49,55] have been rapidly replaced by aAPCs utilizing soluble HLA molecules to improve T-cell–APC interaction [40,52–54,56]. In addition, costimulatory signals were also directly provided by such aAPCs through immobilization of antibodies including anti-LFA-1, anti-CD80 or anti-CD86. Moreover, recombinant production of different HLA class I and II molecules rapidly extended the capacity for the creation of a customized antigen-specific aAPC. Immobilization of HLA molecules onto beads or liposomes enhanced the expansion of antigen-specific T cells in mouse and human in vitro systems. Thus, different combinations of costimulatory signals and different amounts of HLA molecules were tested (reviewed in [57]).
One such aAPC was paving the way for the development of the KaAPC. Using an aAPC generated by immobilization of HLA-A2–Ig dimer molecules and anti-CD28 mAb on to paramagnetic epoxy beads, human antigen-specific CD8+ T cells have been successfully expanded in vitro. Antigen-specificity was ensured through loading of HLA-A2–Ig dimer molecules with peptides of interest. Expansion and specificity of antigen-specific CD8+ T cells was comparable or even improved compared with expansion protocols relying on classical peptide-pulsed DCs. Furthermore, peptide-pulsed target cells were efficiently lysed by aAPC-expanded CD8+ T cells [54]. These aAPCs generated with an B7.1-Ig instead of an anti-CD28 mAb augmented the activity of adoptively transferred cytotoxic T lymphocytes and significantly delayed tumor growth in an in vivo treatment model of subcutaneous melanoma [58]. Therefore, this aAPC technology has been proved functional in vitro and in vivo, demonstrating that the combination of the MHC tetramer technology with a bead-based aAPC system could provide the advantage of specific T-cell recognition without the requirement of cell–cell interference.
The KaAPC platform technology
Based on the previous work on aAPCs [54], the KaAPC concept is founded on three pre-requisites:
■ The backbone of the KaAPC consists of a paramagnetic epoxy bead, which is inexpensive, labor efficient and easy to handle;
■ Antigen specificity of the KaAPC is warranted by a covalently linked HLA-A2–Ig dimer molecule, which can be loaded with different HLA-A2 restricted peptides;
■ Apoptosis induction is mediated via an anti-Fas mAb utilizing the Fas/FasL signaling pathway in activated antigen-specific T cells.
Based on these requirements, a KaAPC phenotype displaying maximal antigen-specific depletion of CD8+ T cells and minimal T-cell activation at the same time has been considered as the ideal KaAPC phenotype (Figure 1D).
The phenotypical and functional characterization of this optimal KaAPC revealed an antigen-specific depletion of T cells by corresponding peptide-loaded KaAPCs. KaAPCs unloaded or loaded with an irrelevant peptide did not deplete antigen-specific or polyclonal activated Fas+ CD8+ T cells. Furthermore, stimulation of antigen-specific T cells with unloaded or peptide-loaded KaAPCs did not result in T-cell activation. Neither the expression of CD107a in co-cultured T cells nor a significant production of proinflammatory cytokines could be detected. Further experiments revealed that the amount of T-cell apoptosis was directly related to the amount of KaAPCs used in T-cell co-cultures. In addition, time course experiments indicated that 30 min of co-culture was sufficient to induce almost maximal apoptosis in antigen-specific Fas+ CD8+ T cells. Using a novel four-color flow cytometric assay [59], it could be demonstrated that KaAPCs selectively deplete corresponding antigen-specific T cells, even within a mixture of T cells with heterogeneous antigen specificities. At the same time, no relevant induction of apoptosis could be detected in T cells with nonrelevant antigen specificities.
Taken together, the collective data on KaAPCs demonstrated that apoptosis induction in antigen-specific T cells was dependent on the delivery of a combined signal via the TCR and Fas. Both signals could only be sufficient provided that KaAPCs also presented the corresponding peptide, whereas KaAPCs loaded with an irrelevant peptide failed to mediate these two signals (Figure 2) [60].
Figure 2. Analysis of antigen-specific elimination of human cytotoxic T lymphocytes with different antigen specificities.
Autologous PKH26-stained FluM1-specific CTL and PKH67-stained CMVpp65-specific CTLs were mixed at a 1:1 ratio and then co-cultured with unloaded (unloadedKaAPC), Mart-1-loaded (Mart-1KaAPC) or CMVpp65-loaded (CMVpp65KaAPC) KaAPCs at a 1:1 ratio. After 48 h, co-cultures were harvested and additionally stained with annexin V and 7-actinomycin D. Anti-Fas mAb-stimulated mixed T-cell cultures served as positive control. Apoptosis was determined by differential gating on PKH26-stained and PKH67-stained T-cell populations, respectively (view protocol [60]). Numbers indicate the percentages of gated cells.
CMV: Cytomegalovirus; CTL: Cytotoxic T lymphocyte; KaAPC: Killer artificial antigen-presenting cell; mAb: Monoclonal antibody.
Therefore, KaAPCs in their present form prove efficient artificial in vitro guided missiles for the modulation of human antigen-specific T-cell responses.
KaAPC: a possible future?
Killer artificial antigen-presenting cells, conceived and developed as antigen-specific bead-based killer APCs, combine the advantages of the bead-based aAPC technology with the Fas/FasL signaling pathway to eliminate T cells in an antigen-specific fashion without hampering the T-cell repertoire.
Compared with cell-based approaches utilizing autologous APCs, generation of KaAPCs is much easier and less time consuming, preserving labor and resources. KaAPC phenotypes were stable over a long period, and the ‘off the shelf’ nature could make them an instant treatment possibility. Furthermore, signal strength and composition of the KaAPCs can be better controlled than on APCs, allowing a higher reproducibility. In addition, KaAPCs do not underlie gene-regulation issues. Furthermore, the quality of KaAPCs is not dependent on patient conditions as is required for the cell-based expansion protocols. Furthermore, KaAPCs cannot be eliminated through para- or auto-crine activation of the Fas/FasL signaling pathway. In addition, the functional capacity and the number of KaAPCs are not compromised by effector functions of cytotoxic T lymphocyte.
Killer artificial antigen-presenting cells can be customized through loading of defined peptides, avoiding the danger of tolerance induction or secondary immune responses to antigens other than the loaded peptides. However, this also represents one of the major limitations of this technology. Since KaAPCs do not phagocytose, process and present antigen, it will be necessary that at least the immune-dominant disease-relevant antigens are identified. Autologous cell-based strategies will not have such limitations as they present the complete repertoire of naturally occurring antigens. To date, many HLA-A2-restricted antigens have already been identified that account for several autoimmune diseases (Table 1). The fact that 40–50% of Caucasians feature HLA-A2 molecules in their individual HLA phenotype promises the treatment of up to 50% of all Caucasians, and the combination of 5–7 different HLA class I molecules would already cover more than 90% of the whole population. Therefore, therapeutic efficiency could be improved by the loading of different disease-related HLA-A2-restricted antigens onto the KaAPC. The almost exclusive usage of HLA-A2 molecules so far is not a limitation of this strategy alone, but cannot hide the fact that epitope mapping and peptide identification would have to be performed for other HLA class I and II subclasses to ensure an effective antigen repertoire for the generation of functional KaAPCs. It might be beneficial that at the onset of an autoimmune disease, only a few T-cell clones are activated. Therefore, a potential KaAPC treatment should be initiated in the early phase of an autoimmune disease if possible, preventing the antigen spreading observed at later disease stages [61–63].
Table 1.
Exemplarily overview of already known human HLA-A*0201 restricted disease-related antigens.
| Disease | Antigen | Position | Sequence | Ref. |
|---|---|---|---|---|
| Type 1 diabetes | GAD65 | 114–123 | VMNILLQYVV | [77] |
| IA-2 | 172–180 | SLSPLQAEL | [78] | |
| 482–490 | SLAAGVKLL | [78] | ||
| 797–805 | MVWESGCTV | [79] | ||
| GFAP | 143–151 | NLAQDLATV | [80] | |
| 192–200 | SLEEEIRFL | [80] | ||
| 214–222 | QLARQQVHV | [80] | ||
| IAPP | 5–13 | KLQVFLIVL | [78,80,81] | |
| 9–17 | FLIVLSVAL | [78,80] | ||
| IGRP | 152–160 | FLWSVFWLI | [78,80] | |
| 211–219 | NLFLFLFAV | [82] | ||
| 215–223 | FLFAVGFYL | [78,80] | ||
| 222–230 | YLLLRVLNI | [82] | ||
| 228–236 | LNIDLLWSV | [83] | ||
| 265–273 | VLFGLGFAI | [83] | ||
| 293–301 | RLLCALTSL | [80] | ||
| Insulin | B 9–18 | SHLVEALYLV | [84] | |
| B 10–18 | HLVEALYLV | [78,84–87] | ||
| B 18–27 | VCGERGFFYT | [84,85] | ||
| C 20–28 | SLQPLALEG | [84,87] | ||
| C 25–33 | ALEGSLQKR | [84] | ||
| C 29 – A 5 | SLQKRGIVEQ | [84,87] | ||
| A 1–10 | GIVEQCCTSI | [84,87] | ||
| A 12–20 | SLYQLENYC | [84,87] | ||
| L 2–10 | ALWMRLLPL | [78] | ||
| | ||||
| Multiple sclerosis | MBP | 87–95 | VVHFFKNIV | [88,89] |
| 110–118 | SLSRFSWGA | [88–90] | ||
| MAG | 287–295 | SLLLELLEEV | [90] | |
| 509–517 | LMWAKIGPV | [90] | ||
| 556–564 | VLFSSDFRI | [90] | ||
| MOG | 43–51 | ALVGDEVEL | [89] | |
| 97–105 | RTELLKDAI | [89] | ||
| 104–112 | AIGEGKVTL | [89] | ||
| 143–151 | KVEDPFYWV | [89] | ||
| 157–166 | LLAVLPVLLL | [89] | ||
| 164–171 | VLLLQITV | [89] | ||
| 185–193 | KLRAEIENL | [89] | ||
| 203–211 | RVPCWKITL | [89] | ||
| 248–255 | SLCYKQRI | [89] | ||
| 263–271 | EATRGRGGL | [89] | ||
| PLP | 3–11 | LLECCARCL | [89] | |
| 63–72 | NVIHAFQYVI | [89] | ||
| 80–88 | FLYGALLLA | [90–92] | ||
| 223–231 | NLLSICKTA | [89] | ||
| TAL | 168–176 | LLFSFAQAV | [93] | |
| | ||||
| Primary biliary cirrhosis | PDC-E2 | 159–167 | KLSEGDLLA | [94] |
| 165–174 | LLAEIETDKA | [95] | ||
However, before considering a clinical application of KaAPCs, several obstacles have to be acknowledged. Does the latex-coated ferrous paramagnetic core of KaAPCs have any side effects when applied to in vivo systems? These utilized beads are indeed available in a ‘good manufacturing practice’ grade but potential adverse effects due to size, coating and distribution have still to be evaluated. Initial aAPC data that proved in vivo functionality and biodistribution [58] encourage a potential in vivo application of KaAPCs. However, it is not yet clear if aAPC were actively directed towards tumor sites and tumor antigen-specific T cells or if the site of injection mainly determined the pattern of aAPC distribution. Hence, to date, it is nearly impossible to define the exact amount or the application strategy of KaAPCs for a potential future in vivo treatment. In addition, one might envisage the application of KaAPCs for purging of antigen-specific T cells ex vivo. In light of these limitations, future progress should focus on the development of biodegradable KaAPCs more suitable for potential in vivo studies. To this end, particles should be considered that have already been applied in pharmacological in vivo studies for targeted drug delivery [64] including liposomes. Promising results have been achieved utilizing liposomes for aAPC generation [40,51,65,66], supporting a possible transfer to the KaAPC technology.
Furthermore, the current KaAPC phenotype depleted highly activated antigen-specific T cells only. However, it remains to be elucidated if the present KaAPC phenotype might deplete T cells of different activation statuses. Since KaAPCs do not display costimulatory molecules and we did not observe any T-cell activation in co-cultures, it appears unlikely that this phenotype will be able to deplete naive antigen-specific T cells. By contrast, killer APCs display the whole range of costimulatory molecules leading eventually to T-cell activation.
In addition, the anti-Fas mAb immobilized on to KaAPCs could interfere with other Fas+ cells, causing unspecific depletion of cells other than T cells, including hepatocytes. Therefore, modification of the apoptosis-inducing signal loaded onto KaAPCs should be considered. For this purpose, ligands or mAbs targeting other receptors of the TNF-receptor superfamily appear to be suitable apoptosis [67,68] or tolerance inducers [69]. Supporting this concept, Hirata et al. demonstrated that genetically modified DCs presenting myelin oligodendrocyte glycoprotein (MOG) peptide in the context of MHC class II molecules in the presence of TNF-related apoptosis-inducing ligand or programmed death-1 ligand expression significantly reduced T-cell responses towards MOG, resulting in reduced cell infiltration into the spinal cord and reduced severity of MOG peptide-induced experimental autoimmune encephalomyelitis [70].
Furthermore, the addition of costimulatory molecules or the combined use of HLA class I and II signals is conceivable and potentially of benefit for a possible future therapeutic application [71]. Moreover, by introducing other molecules such as CD137, the KaAPC technology could be deployed for the depletion of alloreactive T cells, since these cells could already be characterized ex vivo, generated and depleted [72–75]. A combination of both HLA class I and II molecules eventually promote the complete depletion of alloreactive T cells, which would facilitate the treatment of graft rejection and might reduce its incidence. However, these concepts are still ‘dreams of the future’.
Future perspective
Killer APCs, guided missiles and inhibitory tetramers together with our KaAPCs hold promise for an effective antigen-specific treatment of T-cell-related autoimmune diseases. But clinical application in the future will depend on several factors. First, disease-related antigens and T-cell populations have to be identified and characterized to allow a targeting as specific as possible. Second, the issue of which strategy will be most efficient in vivo with minimal potential side effects is still yet to be evaluated. To date, the KaAPC technology is one of the first strategies displaying antigen-specific deletion of human T cells in vitro, but other strategies, such as inhibitory tetramers [43] or nonvirally transduced cell-based settings [76], could be better alternatives in terms of biocompatibility.
Therefore, after successful proof-of-concept, the future of antigen-specific treatment strategies fundamentally relies on an improved biocompatibility and specificity at the same time. However, given the rapid development of biomaterials, there is a fair chance that antigen-specific depletion strategies will enter the clinic within the next few decades.
Executive summary.
Current autoimmune & chronic infection treatments
■ Most current treatment strategies of autoimmune diseases rely either on corticosteroids or immunosuppressive drugs, such as purine analogs, alkylating agents and calcineurin inhibitors, which do not result in cure of the primary immunological malfunction.
■ New biological agents have been introduced, such as monoclonal antibodies (mAbs), targeting inflammatory effector molecules, defined proteins or receptors expressed on the cell surface.
■ Other mAbs targeting cytokines, receptors, integrins and chemokines have been approved or are currently under investigation in clinical trials.
■ However, these strategies are not restricted to pathogenetically relevant cells or mechanisms, leading to potentially severe side effects.
Cell-based depletion strategies utilizing the Fas/Fas ligand signaling pathway
■ Many in vitro and in vivo animal model utilized B-cell lines, macrophages and dendritic cells (DCs) as killer antigen-presenting cells (APCs) to demonstrate antigen-specific depletion of T cells.
■ Killer APCs were successfully tested in in vivo animal models resembling features of rheumatoid arthritis, allogenic transplantation and allergy, as well as in models for studying antigen-specific T-cell responses.
■ It has been demonstrated that human killer APCs do not deplete naive human T cells but eliminate alloantigen-specific T cells, thereby preserving protective antiviral T-cell responses.
Artificial concepts for antigen-specific immunotherapy
- ■ Development of cell- and bead-based artificial APCs:
- Unspecific (anti-CD3 and anti-CD28) and specific approaches utilizing HLA class I and II molecules in combination with different costimulatory molecules (anti-CD80, anti-CD86 and anti-LFA-1) have been tested.
- Artificial APCs (HLA-A2-Ig; B7.1-Ig) display a prominent in vivo functionality by augmentation of adoptively transferred cytotoxic T lymphocyte and consequently delayed tumor growth.
- ■ Depletion strategies utilizing MHC-tetramers linked to cell-toxic agents:
- Actinum 225-conjugated MHC tetramers specific for human viral antigens have been demonstrated to deplete antigen-specific T cells via α particle emission.
- Ribosome inhibitor saporin-conjugated MHC tetramers resulted in the depletion of antigen-specific murine T cells.
Killer artificial antigen-presenting cell technology
■ Killer artificial antigen-presenting cells (KaAPC)s are generated by covalently bound HLA-A2–Ig and anti-CD28 mAbs onto the surface of paramagnetic epoxy beads.
■ KaAPCs deplete antigen-specific T cells in an antigen-specific manner, even from T-cell mixtures of heterogeneous antigen specificities.
■ Depletion efficiency is dependent on the amount of KaAPC used and the co-culture interval, and is not related to activation-induced cell death.
Advantages over cell-based approaches
■ No induction of tolerance to unwanted antigens.
■ No negative impact on the protective T-cell immune response.
■ Not susceptible to cytotoxic effector functions of cytotoxic T lymphocyte.
■ ‘Off the shelf’ nature.
■ Time-, cost- and labor-saving technology.
Shortcomings of this new concept
■ Paramagnetic backbone is approved for in vivo studies but data related to biodistribution, clearance and toxicity are sparsely published.
■ Efficient use of KaAPCs requires the identification of immune-dominant disease-related antigens.
■ The current KaAPC phenotype efficiently depletes only highly activated T cells.
Conclusion
■ Proof-of-concept of KaAPC-mediated human antigen-specific T-cell elimination utilizing a HLA-A2–Ig dimer and anti-Fas mAb has been accomplished.
■ KaAPC technology could provide a robust and highly flexible platform for individual treatment strategies if the utilization of other costimulatory or apoptosis-inducing molecules is applicable.
■ For a putative clinical application, the KaAPC technology has to first be adapted to a biodegradable or biocompatible backbone and also requires better in vivo characterization in terms of distribution, toxicity and clearance.
Acknowledgments
Financial & competing interests disclosure
Primary work on killer artificial antigen-presenting cells, was supported in parts by Deutsche Forschungsgemeinschaft KFO146 (to Andreas Mackensen and Martin Fleck), Wilhelm Sander Stiftung (to Martin Fleck), Department of Defense grant PC 040972 (to Mathias Oelke) and NIH grants RO1 CA108835, RO1 AI44129, ROI AI29575 and ROI AI072677 (to Jonathan P Schneck). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
- 1.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003;348(17):1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y. B cell targeting therapy using the anti-CD20 antibody in autoimmune diseases. Yakugaku Zasshi. 2009;129(6):675–679. doi: 10.1248/yakushi.129.675. [DOI] [PubMed] [Google Scholar]
- 3.Lawton JA, Ghosh P. Novel therapeutic strategies based on Toll-like receptor signaling. Curr. Opin. Chem. Biol. 2003;7(4):446–451. doi: 10.1016/s1367-5931(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 4.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006;354(9):899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 5.Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs interferon β-1a in early multiple sclerosis. N. Engl. J. Med. 2008;359(17):1786–1801. doi: 10.1056/NEJMoa0802670. [DOI] [PubMed] [Google Scholar]
- 6.Kremer JM, Dougados M, Emery P, et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a Phase IIb, double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52(8):2263–2271. doi: 10.1002/art.21201. [DOI] [PubMed] [Google Scholar]
- 7■.Fink PJ, Shimonkevitz RP, Bevan MJ. Veto cells. Annu. Rev. Immunol. 1988;6:115–137. doi: 10.1146/annurev.iy.06.040188.000555. [Reviews the discovery of veto cells.] [DOI] [PubMed] [Google Scholar]
- 8■.Tykocinski ML, Kaplan DR, Medof ME. Antigen-presenting cell engineering. The molecular toolbox. Am. J. Pathol. 1996;148(1):1–16. [Reviews the generation of artificial veto cells.] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L, Lee WC, Takayama T, et al. Genetic engineering of dendritic cells to express immunosuppressive molecules (viral IL-10, TGF-β, and CTLA4IG). J. Leukoc. Biol. 1999;66(2):293–296. doi: 10.1002/jlb.66.2.293. [DOI] [PubMed] [Google Scholar]
- 10.Hackstein H, Morelli AE, Thomson AW. Designer dendritic cells for tolerance induction: guided not misguided missiles. Trends Immunol. 2001;22(8):437–442. doi: 10.1016/s1471-4906(01)01959-7. [DOI] [PubMed] [Google Scholar]
- 11.Kosiewicz MM, Krishnan A, Worthington MT, Matriano JA, Ross WG. B cells engineered to express Fas ligand suppress pre-sensitized antigen-specific T cell responses in vivo. Eur. J. Immunol. 2002;32(6):1679–1687. doi: 10.1002/1521-4141(200206)32:6<1679::AID-IMMU1679>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Zhang HG, Su X, Liu D, et al. Induction of specific T cell tolerance by Fas ligand-expressing antigen-presenting cells. J. Immunol. 1999;162(3):1423–1430. [PubMed] [Google Scholar]
- 13.Buonocore S, Paulart F, Le Moine A, et al. Dendritic cells overexpressing CD95 (Fas) ligand elicit vigorous allospecific T-cell responses in vivo. Blood. 2003;101(4):1469–1476. doi: 10.1182/blood-2002-07-2042. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Kim S, Oligino TJ, Robbins PD. Effective treatment of established mouse collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express FasL. Mol. Ther. 2002;6(5):584–590. [PubMed] [Google Scholar]
- 15.Walczak H, Krammer PH. The CD95 (Apo-1/Fas) and the TRAIL (Apo-2L) apoptosis systems. Exp. Cell. Res. 2000;256(1):58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 16.Min WP, Gorczynski R, Huang XY, et al. Dendritic cells genetically engineered to express Fas ligand induce donor-specific hyporesponsiveness and prolong allograft survival. J. Immunol. 2000;164(1):161–167. doi: 10.4049/jimmunol.164.1.161. [DOI] [PubMed] [Google Scholar]
- 17.Kusuhara M, Matsue K, Edelbaum D, Loftus J, Takashima A, Matsue H. Killing of naive T cells by CD95L-transfected dendritic cells (DC): in vivo study using killer DC–DC hybrids and CD4+ T cells from DO11.10 mice. Eur. J. Immunol. 2002;32(4):1035–1043. doi: 10.1002/1521-4141(200204)32:4<1035::AID-IMMU1035>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Matsue H, Matsue K, Kusuhara M, et al. Immunosuppressive properties of CD95L-transduced “killer” hybrids created by fusing donor- and recipient-derived dendritic cells. Blood. 2001;98(12):3465–3472. doi: 10.1182/blood.v98.12.3465. [DOI] [PubMed] [Google Scholar]
- 19.Chuang YH, Suen JL, Chiang BL. Fas-ligand-expressing adenovirus-transfected dendritic cells decrease allergen-specific T cells and airway inflammation in a murine model of asthma. J. Mol. Med. 2006;84(7):595–603. doi: 10.1007/s00109-006-0047-3. [DOI] [PubMed] [Google Scholar]
- 20.Matsue H, Matsue K, Walters M, Okumura K, Yagita H, Takashima A. Induction of antigen-specific immunosuppression by CD95L cDNA-transfected ‘killer’ dendritic cells. Nat. Med. 1999;5(8):930–937. doi: 10.1038/11375. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe T, Asseman C, Hughes A, Matsue H, Takashima A, Von Herrath MG. Reduction of antiviral CD8 lymphocytes in vivo with dendritic cells expressing Fas ligand-increased survival of viral (lymphocytic choriomeningitis virus) central nervous system infection. J. Immunol. 2002;169(9):4867–4872. doi: 10.4049/jimmunol.169.9.4867. [DOI] [PubMed] [Google Scholar]
- 22.Wu B, Wu JM, Miagkov A, Adams RN, Levitsky HI, Drachman DB. Specific immunotherapy by genetically engineered APCs: the ‘guided missile’ strategy. J. Immunol. 2001;166(7):4773–4779. doi: 10.4049/jimmunol.166.7.4773. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HG, Fleck M, Kern ER, et al. Antigen presenting cells expressing Fas ligand down-modulate chronic inflammatory disease in Fas ligand-deficient mice. J. Clin. Invest. 2000;105(6):813–821. doi: 10.1172/JCI8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu JM, Wu B, Miagkov A, Adams RN, Drachman DB. Specific immunotherapy of experimental myasthenia gravis in vitro: the “guided missile” strategy. Cell. Immunol. 2001;208(2):137–147. doi: 10.1006/cimm.2001.1778. [DOI] [PubMed] [Google Scholar]
- 25.Hoves S, Krause SW, Halbritter D, et al. Mature but not immature Fas ligand (CD95L)-transduced human monocyte-derived dendritic cells are protected from Fas-mediated apoptosis and can be used as killer APC. J. Immunol. 2003;170(11):5406–5413. doi: 10.4049/jimmunol.170.11.5406. [DOI] [PubMed] [Google Scholar]
- 26.Hoves S, Krause SW, Herfarth H, et al. Elimination of activated but not resting primary human CD4+ and CD8+ T cells by Fas ligand (FasL/CD95L)-expressing killer-dendritic cells. Immunobiology. 2004;208(5):463–475. doi: 10.1078/0171-2985-00293. [DOI] [PubMed] [Google Scholar]
- 27.Dulat HJ, Von Grumbkow C, Baars W, Schröder N, Wonigeit K, Schwinzer R. Down-regulation of human alloimmune responses by genetically engineered expression of CD95 ligand on stimulatory and target cells. Eur. J. Immunol. 2001;31(7):2217–2226. doi: 10.1002/1521-4141(200107)31:7<2217::aid-immu2217>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28■.Strauss G, Osen W, Knape I, Jacobsen EM, Muller SM, Debatin KM. Membrane-bound CD95 ligand expressed on human antigen-presenting cells prevents alloantigen-specific T cell response without impairment of viral and third-party T cell immunity. Cell Death. Differ. 2006;14(3):480–488. doi: 10.1038/sj.cdd.4402019. [Depletion of human alloreactive T cells utilizing human killer antigen-presenting cells.] [DOI] [PubMed] [Google Scholar]
- 29.Georgantas RW III, Leong KW, August JT. Antigen-specific induction of peripheral T cell tolerance in vivo by codelivery of DNA vectors encoding antigen and Fas ligand. Hum. Gene Ther. 2000;11(6):851–858. doi: 10.1089/10430340050015464. [DOI] [PubMed] [Google Scholar]
- 30.Whartenby KA, Straley EE, Kim H, et al. Transduction of donor hematopoietic stem-progenitor cells with Fas ligand enhanced short-term engraftment in a murine model of allogeneic bone marrow transplantation. Blood. 2002;100(9):3147–3154. doi: 10.1182/blood-2002-01-0118. [DOI] [PubMed] [Google Scholar]
- 31.Elhalel MD, Huang JH, Schmidt W, Rachmilewitz J, Tykocinski ML. CTLA-4. FasL induces alloantigen-specific hyporesponsiveness. J. Immunol. 2003;170(12):5842–5850. doi: 10.4049/jimmunol.170.12.5842. [DOI] [PubMed] [Google Scholar]
- 32.Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17(6):795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 33.Yagita H, Seino K, Kayagaki N, Okumura K. CD95 ligand in graft rejection. Nature. 1996;379(6567):682. doi: 10.1038/379682a0. [DOI] [PubMed] [Google Scholar]
- 34.Lau HT, Yu M, Fontana A, Stoeckert CJ., Jr Prevention of islet allograft rejection with engineered myoblasts expressing FasL in mice. Science. 1996;273(5271):109–112. doi: 10.1126/science.273.5271.109. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B-lymphocyte stimulator in systemic lupus erythematosus. J. Immunol. 2001;166(1):6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 36■.Askenasy N, Yolcu ES, Yaniv I, Shirwan H. Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood. 2005;105(4):1396–1404. doi: 10.1182/blood-2004-06-2364. [Reviews the role of Fas/Fas ligand signaling in immunotherapeutical strategies.] [DOI] [PubMed] [Google Scholar]
- 37.Symes JC, Siatskas C, Fowler DH, Medin JA. Retrovirally transduced murine T lymphocytes expressing FasL mediate effective killing of prostate cancer cells. Cancer Gene Ther. 2009;16(5):439–452. doi: 10.1038/cgt.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J. Immunol. 2000;164(6):3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 39.Constantin CM, Bonney EE, Altman JD, Strickland OL. Major histocompatibility complex (MHC) tetramer technology: an evaluation. Biol. Res. Nurs. 2002;4(2):115–127. doi: 10.1177/1099800402238332. [DOI] [PubMed] [Google Scholar]
- 40.Prakken B, Wauben M, Genini D, et al. Artificial antigen-presenting cells as a tool to exploit the immune ‘synapse’. Nat. Med. 2000;6(12):1406–1410. doi: 10.1038/82231. [DOI] [PubMed] [Google Scholar]
- 41.Dal Porto J, Johansen TE, Catipovic B, et al. A soluble divalent class I major histocompatibility complex molecule inhibits alloreactive T cells at nanomolar concentrations. Proc. Natl Acad. Sci. USA. 1993;90(14):6671–6675. doi: 10.1073/pnas.90.14.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maile R, Wang B, Schooler W, Meyer A, Collins EJ, Frelinger JA. Antigen-specific modulation of an immune response by in vivo administration of soluble MHC class I tetramers. J. Immunol. 2001;167(7):3708–3714. doi: 10.4049/jimmunol.167.7.3708. [DOI] [PubMed] [Google Scholar]
- 43■.Yuan RR, Wong P, Mcdevitt MR, et al. Targeted deletion of T-cell clones using α-emitting suicide MHC tetramers. Blood. 2004;104(8):2397–2402. doi: 10.1182/blood-2004-01-0324. [MHC tetramer-based antigen-specific T-cell depletion strategy utilizing Actinum225.] [DOI] [PubMed] [Google Scholar]
- 44■.Hess PR, Barnes C, Woolard MD, et al. Selective deletion of antigen-specific CD8+ T cells by MHC class I tetramers coupled to the type I ribosome-inactivating protein saporin. Blood. 2007;109(8):3300–3307. doi: 10.1182/blood-2006-06-028001. [Saporin-conjugated MHC-tetramer technology demonstrating a 75% depletion efficiency of corresponding antigen-specific T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl Acad. Sci. USA. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J. Clin. Oncol. 2006;24(31):5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 47.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein–Barr virus + Hodgkin's disease. J. Exp. Med. 2004;200(12):1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maus MV, Thomas AK, Leonard DG, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat. Biotechnol. 2002;20(2):143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 50.Sasawatari S, Tadaki T, Isogai M, Takahara M, Nieda M, Kakimi K. Efficient priming and expansion of antigen-specific CD8+ T cells by a novel cell-based artificial APC. Immunol. Cell. Biol. 2006;84(6):512–521. doi: 10.1111/j.1440-1711.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- 51.Zappasodi R, Di Nicola M, Carlo-Stella C, et al. The effect of artificial antigen-presenting cells with preclustered anti-CD28/-CD3/-LFA-1 monoclonal antibodies on the induction of ex vivo expansion of functional human antitumor T cells. Haematologica. 2008;93(10):1523–1534. doi: 10.3324/haematol.12521. [DOI] [PubMed] [Google Scholar]
- 52.Tham EL, Jensen PL, Mescher MF. Activation of antigen-specific T cells by artificial cell constructs having immobilized multimeric peptide-class I complexes and recombinant B7-FC proteins. J. Immunol. Methods. 2001;249(1–2):111–119. doi: 10.1016/s0022-1759(00)00335-5. [DOI] [PubMed] [Google Scholar]
- 53.Maus MV, Riley JL, Kwok WW, Nepom GT, June CH. HLA tetramer-based artificial antigen-presenting cells for stimulation of CD4+ T cells. Clin. Immunol. 2003;106(1):16–22. doi: 10.1016/s1521-6616(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 54.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat. Med. 2003;9(5):619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 55.Levine BL, Cotte J, Small Cc, et al. Large-scale production of CD4+ T cells from HIV-1-infected donors after CD3/CD28 costimulation. J. Hematother. 1998;7(5):437–448. doi: 10.1089/scd.1.1998.7.437. [DOI] [PubMed] [Google Scholar]
- 56.Latouche JB, Sadelain M. Induction of human cytotoxic T lymphocytes by artificial antigen-presenting cells. Nat. Biotechnol. 2000;18(4):405–409. doi: 10.1038/74455. [DOI] [PubMed] [Google Scholar]
- 57■.Oelke M, Krueger C, Schneck JP. Technological advances in adoptive immunotherapy. Drugs Today (Barc.) 2005;41(1):13–21. doi: 10.1358/dot.2005.41.1.875775. [Reviews the artificial antigen-presenting cell (APC) technology.] [DOI] [PubMed] [Google Scholar]
- 58■.Ugel S, Zoso A, De Santo C, et al. In vivo administration of artificial antigen-presenting cells activates low-avidity T cells for treatment of cancer. Cancer Res. 2009;69(24):9376–9384. doi: 10.1158/0008-5472.CAN-09-0400. [First in vivo data of artificial APCs generated with HLA-A2–Ig dimer and anti-CD28 monoclonal antibodies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schütz C, Fischer K, Volkl S, et al. A new flow cytometric assay for the simultaneous analysis of antigen-specific elimination of T cells in heterogeneous T cell populations. J. Immunol. Methods. 2009;344(2):98–108. doi: 10.1016/j.jim.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 60■.Schütz C, Fleck M, Mackensen A, et al. Killer artificial antigen-presenting cells: a novel strategy to delete specific T cells. Blood. 2008;111(7):3546–3552. doi: 10.1182/blood-2007-09-113522. [Presents the original data on the killer artificial APC technology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat. Rev. Immunol. 2002;2(2):85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 62.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of Type 1 diabetes in Nod mice. Nature. 2005;435(7039):220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krishnamurthy B, Dudek NL, Mckenzie MD, et al. Responses against islet antigens in nod mice are prevented by tolerance to proinsulin but not IGRP. J. Clin. Invest. 2006;116(12):3258–3265. doi: 10.1172/JCI29602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hild WA, Breunig M, Goepferich A. Quantum dots – nano-sized probes for the exploration of cellular and intracellular targeting. Eur. J. Pharm. Biopharm. 2008;68(2):153–168. doi: 10.1016/j.ejpb.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Van Rensen AJ, Wauben MH, Grosfeld-Stulemeyer MC, Van Eden W, Crommelin DJ. Liposomes with incorporated MHC class II/peptide complexes as antigen presenting vesicles for specific T cell activation. Pharm. Res. 1999;16(2):198–204. doi: 10.1023/a:1018864005620. [DOI] [PubMed] [Google Scholar]
- 66.Giannoni F, Barnett J, Bi K, et al. Clustering of T cell ligands on artificial APC membranes influences T cell activation and protein kinase C theta translocation to the T cell plasma membrane. J. Immunol. 2005;174(6):3204–3211. doi: 10.4049/jimmunol.174.6.3204. [DOI] [PubMed] [Google Scholar]
- 67.Nagata S. Apoptosis by death factor. Cell. 1997;88(3):355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 68.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20(10):1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 69.Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 2009;10(11):1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with trail or programmed death-1 ligand. J. Immunol. 2005;174(4):1888–1897. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 71.Peakman M, Dayan CM. Antigen-specific immunotherapy for autoimmune disease: fighting fire with fire? Immunology. 2001;104(4):361–366. doi: 10.1046/j.1365-2567.2001.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pittet MJ, Gati A, Le Gal FA, et al. Ex vivo characterization of allo-MHC-restricted T cells specific for a single MHC-peptide complex. J. Immunol. 2006;176(4):2330–2336. doi: 10.4049/jimmunol.176.4.2330. [DOI] [PubMed] [Google Scholar]
- 73.Weng X, Lu S, Zhong M, et al. Allo-restricted CTLs generated by coculturing of PBLs and autologous monocytes loaded with allogeneic peptide/HLA/IgG1–FC fusion protein. J. Leukoc. Biol. 2009;85(3):574–581. doi: 10.1189/jlb.0408242. [DOI] [PubMed] [Google Scholar]
- 74.Godfrey WR, Krampf MR, Taylor PA, Blazar BR. Ex vivo depletion of alloreactive cells based on cfse dye dilution, activation antigen selection, and dendritic cell stimulation. Blood. 2004;103(3):1158–1165. doi: 10.1182/blood-2003-04-1098. [DOI] [PubMed] [Google Scholar]
- 75.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107(9):3575–3583. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 76.Strauss G, Osen W, Knape I, Jacobsen EM, Muller SM, Debatin KM. Membrane-bound CD95 ligand expressed on human antigen-presenting cells prevents alloantigen-specific T cell response without impairment of viral and third-party T cell immunity. Cell Death Differ. 2007;14(3):480–488. doi: 10.1038/sj.cdd.4402019. [DOI] [PubMed] [Google Scholar]
- 77.Panina-Bordignon P, Lang R, Van Endert PM, et al. Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J. Exp. Med. 1995;181(5):1923–1927. doi: 10.1084/jem.181.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ouyang Q, Standifer NE, Qin H, et al. Recognition of HLA class I-restricted β-cell epitopes in Type 1 diabetes. Diabetes. 2006;55(11):3068–3074. doi: 10.2337/db06-0065. [DOI] [PubMed] [Google Scholar]
- 79.Takahashi K, Honeyman MC, Harrison LC. Cytotoxic T cells to an epitope in the islet autoantigen IA-2 are not disease-specific. Clin. Immunol. 2001;99(3):360–364. doi: 10.1006/clim.2001.5031. [DOI] [PubMed] [Google Scholar]
- 80.Standifer NE, Ouyang Q, Panagiotopoulos C, et al. Identification of novel HLA-A*0201-restricted epitopes in recent-onset Type 1 diabetic subjects and antibody-positive relatives. Diabetes. 2006;55(11):3061–3067. doi: 10.2337/db06-0066. [DOI] [PubMed] [Google Scholar]
- 81.Panagiotopoulos C, Qin H, Tan R, Verchere CB. Identification of a β-cell-specific HLA class I restricted epitope in Type 1 diabetes. Diabetes. 2003;52(11):2647–2651. doi: 10.2337/diabetes.52.11.2647. [DOI] [PubMed] [Google Scholar]
- 82.Jarchum I, Nichol L, Trucco M, Santamaria P, Dilorenzo TP. Identification of novel IGRP epitopes targeted in Type 1 diabetes patients. Clin. Immunol. 2008;127(3):359–365. doi: 10.1016/j.clim.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takaki T, Marron MP, Mathews CE, et al. HLA-A*0201-restricted T cells from humanized nod mice recognize autoantigens of potential clinical relevance to Type 1 diabetes. J. Immunol. 2006;176(5):3257–3265. doi: 10.4049/jimmunol.176.5.3257. [DOI] [PubMed] [Google Scholar]
- 84.Hassainya Y, Garcia-Pons F, Kratzer R, et al. Identification of naturally processed HLA-A2-restricted proinsulin epitopes by reverse immunology. Diabetes. 2005;54(7):2053–2059. doi: 10.2337/diabetes.54.7.2053. [DOI] [PubMed] [Google Scholar]
- 85.Toma A, Haddouk S, Briand JP, et al. Recognition of a subregion of human proinsulin by class I-restricted T cells in Type 1 diabetic patients. Proc. Natl Acad. Sci. USA. 2005;102(30):10581–10586. doi: 10.1073/pnas.0504230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pinkse GG, Tysma OH, Bergen CA, et al. Autoreactive CD8 T cells associated with β cell destruction in Type 1 diabetes. Proc. Natl Acad. Sci. USA. 2005;102(51):18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Endert P, Hassainya Y, Lindo V, et al. HLA class I epitope discovery in Type 1 diabetes. Ann. NY Acad. Sci. 2006;1079:190–197. doi: 10.1196/annals.1375.030. [DOI] [PubMed] [Google Scholar]
- 88.Zang YC, Li S, Rivera VM, et al. Increased CD8+ cytotoxic T cell responses to myelin basic protein in multiple sclerosis. J. Immunol. 2004;172(8):5120–5127. doi: 10.4049/jimmunol.172.8.5120. [DOI] [PubMed] [Google Scholar]
- 89.Berthelot L, Laplaud DA, Pettre S, et al. Blood CD8+ T cell responses against myelin determinants in multiple sclerosis and healthy individuals. Eur. J. Immunol. 2008;38(7):1889–1899. doi: 10.1002/eji.200838023. [DOI] [PubMed] [Google Scholar]
- 90.Tsuchida T, Parker KC, Turner RV, Mcfarland HF, Coligan JE, Biddison WE. Autoreactive CD8+ T-cell responses to human myelin protein-derived peptides. Proc. Natl Acad. Sci. USA. 1994;91(23):10859–10863. doi: 10.1073/pnas.91.23.10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biddison WE, Taub DD, Cruikshank WW, Center DM, Connor EW, Honma K. Chemokine and matrix metalloproteinase secretion by myelin proteolipid protein-specific CD8+ T cells: potential roles in inflammation. J. Immunol. 1997;158(7):3046–3053. [PubMed] [Google Scholar]
- 92.Dressel A, Chin JL, Sette A, Gausling R, Hollsberg P, Hafler DA. Autoantigen recognition by human CD8 T cell clones: enhanced agonist response induced by altered peptide ligands. J. Immunol. 1997;159(10):4943–4951. [PubMed] [Google Scholar]
- 93.Niland B, Banki K, Biddison WE, Perl A. CD8+ T cell-mediated HLA-A*0201-restricted cytotoxicity to transaldolase peptide 168–176 in patients with multiple sclerosis. J. Immunol. 2005;175(12):8365–8378. doi: 10.4049/jimmunol.175.12.8365. [DOI] [PubMed] [Google Scholar]
- 94.Kita H, Lian ZX, Van De Water J, et al. Identification of HLA-A2-restricted CD8+ cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J. Exp. Med. 2002;195(1):113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Matsumura S, Kita H, He XS, et al. Comprehensive mapping of HLA-A0201-restricted CD8 T-cell epitopes on PDC-E2 in primary biliary cirrhosis. Hepatology. 2002;36(5):1125–1134. doi: 10.1053/jhep.2002.36161. [DOI] [PubMed] [Google Scholar]