Abstract
Purpose
Although the quality of life (QoL) of prostate cancer (PCa) patients is a major issue, there is no unified and useful methodology for assessing QoL. The Expanded Prostate Cancer Index Composite (EPIC) is a globally used tool to measure QoL after PCa treatment that comprises urinary, bowel, sexual, and hormonal domains. Acknowledging the need for such a tool applicable to Korean PCa patients, we translated EPIC into Korean and validated the new version.
Materials and Methods
The Korean version of EPIC was devised by translation, back-translation, and reconciliation. Subsequently, we randomly selected 153 patients with localized PCa treated with radical perineal prostatectomy (67, 43.8%), radical retropubic prostatectomy (19, 12.4%), laparoscopic radical prostatectomy (12, 7.8%), robot-assisted laparoscopic radical prostatectomy (36, 23.5%), and high-intensity focused ultrasound ablation of the prostate (19, 12.4%) and asked them to complete EPIC. Reliability was assessed by test-retest correlation and Cronbach's alpha. Validity was assessed by factor analysis, interscale correlation, and correlation with Functional Assessment of Cancer Therapy-Prostate (FACT-P).
Results
Test-retest correlation and Cronbach's alpha were high in each of the domains (0.92, 0.91, 0.76, 0.84 and 0.86, 0.84, 0.92, 0.83, p<0.0001). Interscale correlation among the domains was low (r<0.37), which indicated that EPIC is composed of proper domains. Interscale correlation between the function and bother subscales was high (0.94, 0.81, 0.84 and 0.80, p<0.0001). EPIC domains had low correlation with FACT-P, permitting complementary use.
Conclusions
The Korean version of EPIC was developed by a proper process, as evident by its high reliability and validity. Therefore, it is a reliable, comprehensive, systematic method that evaluates QoL in Korean patients after PCa treatment. Furthermore, it can be adapted as an objective methodology for research globally.
Keywords: Prostatic neoplasms, Quality of life, Reproducibility of results
INTRODUCTION
Prostate cancer (PCa) is the fifth most common cancer in the Korean male population as well as the most rapidly growing cancer in the population group [1]. Owing to early diagnosis through the introduction of prostate-specific antigen (PSA), followed by transrectal ultrasonography-guided prostate biopsy and the development of multiple advanced treatment modalities, early-diagnosed, localized PCa achieves a survival rate of close to 100% [2]. However, as life expectancy increases and the average age at the time of cancer diagnosis decreases, quality of life (QoL) after PCa treatment becomes increasingly important. Today's medical knowledge and technology offer a variety of choices of PCa treatment modalities, such as radical perineal prostatectomy (RPP), radical retropubic prostatectomy (RRP), laparoscopic radical prostatectomy (LRP), robot-assisted laparoscopic radical prostatectomy (RLRP), high-intensity focused ultrasound ablation of the prostate (HIFU), cryotherapy, external beam radiotherapy (EBRT), brachytherapy, and proton beam therapy. However, recent data show no definite evidence favoring one modality over another [3]. When patients are faced with the choice, it is very hard to choose a particular treatment. Hence, the responsibility for suggesting the most appropriate treatment option for an individual patient rests with the urologist [4]. This is why QoL after PCa treatment is becoming an important issue.
Although multiple methodologies have been used to evaluate the QoL of cancer patients, definitive and universally accepted methodologies that focus on PCa have been rare until now. As such, questionnaires for chronic illnesses or general cancer were adapted or individual symptoms and related QoL were studied. The Expanded Prostate Cancer Index Composite (EPIC) was developed by researchers at the University of Michigan and UCLA. EPIC expanded the UCLA-PCI (UCLA prostate cancer index) to evaluate health-related QoL in order to reflect PCa treatment-related symptoms and their negative impact more sensitively [5,6]. EPIC's major categories include the urinary, bowel, and sexual symptoms that patients face following treatment of localized PCa [7]. Furthermore, a section to evaluate hormonal symptoms frequently associated with hormonal deprivation and their consequence in QoL was added [6]. EPIC enables clinicians to evaluate the physical and mental aspects of QoL simultaneously. Since its advent, EPIC has been acknowledged a useful, systemic, and comprehensive tool that is widely used. However, there has not been any attempt to develop a Korean version of EPIC.
When translating a self-administered questionnaire into a different language, special attention must be paid to accommodate linguistic as well as cultural differences, and the questionnaire must also be verified through a proper validation procedure [8]. The Korean version of EPIC was developed by the aforementioned criteria to faithfully reflect the virtues of the original version.
MATERIALS AND METHODS
1. Composition of EPIC
EPIC consists of 50 questions in total divided into four domains: bowel, urinary, sexual, and hormonal aspects. Each domain is divided into two categories, functional and bother subscales, which evaluate types of symptoms and the extent of suffering. In addition, the urinary domain is subdivided into incontinence and irritative/obstructive subscales, according to the symptom characteristics. Each question is in the Likert scale format with scores from 0 to 100. The higher the score, the higher the QoL [6].
Development of the Korean version of EPIC (Appendix)
We initiated this process by obtaining the initial authors' agreements. EPIC was translated into Korean by two proficient translators; this was the forward translation. The prototype of the Korean version of EPIC was generated by combining these two manuscripts with the inputs of the authors, one other urologist, and two translators. Subsequently, this prototype was translated into English by another proficient translator; this was the back translation. The final version of the Korean EPIC was developed after a second round of compromise and discussions.
A pilot test was performed on 10 PCa patients treated with radical prostatectomy at the Samsung Medical Center to determine the extent of comprehension and difficulties. We then finalized the Korean version of EPIC.
3. Patient inclusion and validation
From March 2009 to May 2009, a total of 153 patients treated and followed up for localized PCa were randomized during their regular visit at the Samsung Medical Center. The mean interval from PCa treatment to the study was 27 months. The research protocol was approved by the Institutional Review Board and all interviews were performed after obtaining informed consent from patients. Treatment modalities included RPP (67 patients, 43.8%), RRP (19 patients, 12.4%), LRP (12 patients, 7.8%), RLRP (36 patients, 23.5%), and HIFU (19 patients, 12.4%). Of all patients, 37 patients (24.0%) underwent hormonal treatment (adjuvant treatment: 11 patients; neo-adjuvant treatment plus adjuvant treatment: 22 patients; orchiectomy: 2 patients; neo-adjuvant treatment: 2 patients). All patients were requested to answer the Korean version of EPIC and the Functional Assessment of Cancer Therapy-Prostate (FACT-P) - a previously proven questionnaire - simultaneously for comparison. To confirm test-retest reliability, a repeat EPIC questionnaire was mailed out 4 weeks later without informing patients of the intent to conduct the same questionnaire. In total, 78 patients replied to the retest mail.
4. Statistical analysis
Statistical analyses of reliability and validity were performed with SPSS for Windows (version 17.0 K, SPSS, Chicago, IL, USA). To confirm reliability, test-retest correlation was assessed by intra-class correlation coefficient, and the internal consistency of each subscale was assessed by Cronbach's alpha coefficient. To confirm validity, the propriety of composition of the domain categories, inter-scale correlation between the function and bother subscales of each domain, and the inter-scale correlation among each subscale were analyzed. The propriety of composition of the domain categories was verified by factor analysis with varimax rotation. Inter-scale correlation between the function and bother subscales of each domain and the inter-scale correlation among each of the subscales for independency and redundancy were verified by Spearman's correlation coefficient.
RESULTS
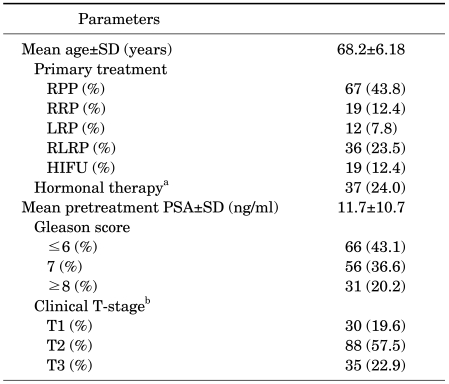
The mean age of the patients was 68±6.18 years. All patients were treated and followed up for localized PCa at the Samsung Medical Center. The patients' treatment modality and clinicopathological characteristics are summarized in Table 1. The study sample included diverse treatments groups, Gleason scores, and T stages of PCa.
TABLE 1.
Treatment modality and clinicopathological characteristics of the patients
RPP: radical perineal prostatectomy, RRP: radical retropubic prostatectomy, LRP: laparoscopic radical prostatectomy, RLRP: robot-assisted laparoscopic radical prostatectomy, HIFU: high-intensity focused ultrasound ablation of prostate, PSA: prostate-specific antigen, a: neo-adjuvant treatment: 2 patients, adjuvant treatment: 11 patients, neo-adjuvant treatment+adjuvant treatment: 22 patients, orchiectomy: 2 patients, b: 2002 TNM staging system.
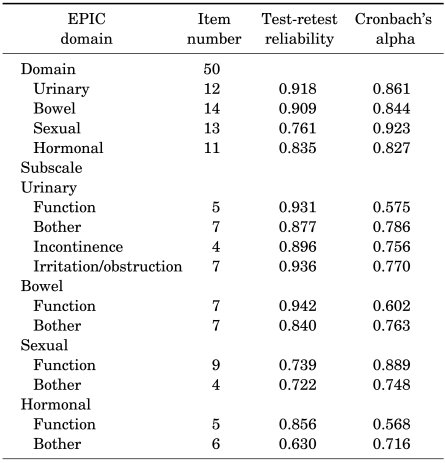
The reliability of each domain and subscale is summarized in Table 2. Test-retest correlations of the urinary, bowel, sexual, and hormonal domains were 0.92, 0.91, 0.76, and 0.84, respectively, and all exhibited very strong correlations (p<0.0001). Cronbach's alpha coefficients of each domain were 0.86, 0.84, 0.92, and 0.83, respectively (p<0.0001). The internal consistency of all domains, according to Cronbach's alpha coefficients, was very high. Therefore, the reliability of the Korean version of EPIC was validated.
TABLE 2.
Reliability assessment of the Korean version EPIC by test-retest correlation and Cronbach's alpha
Test-retest reliability greater than 0.70 means that the reliability is high. A Cronbach's alpha coefficient for the individual subscale greater than 0.70 means that the internal consistency is high. EPIC: Expanded Prostate Cancer Index Composite. p<0.0001
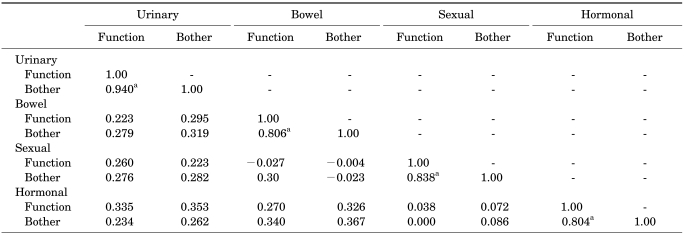
In the factor analysis, all answers were evaluated with varimax rotation. While all translated questions on the urinary, bowel, and sexual domains coincided with the original version of EPIC, the hormonal domain questions were divided into three subcategories. However, we concluded that it appeared acceptable, considering that the hormonal domain contained diverse symptom spans, such as hot flushes, breast tenderness, depression, lack of energy, and weight loss. The interscale correlations between each domain were low (r<0.37). From these results, we concluded that all domains were divided properly and the independency of each domain was confirmed. To confirm that the subscale of each domain adequately reflected the health-related QoL of PCa patients, the inter-scale correlations of each of the function and bother subscales were evaluated (Table 3). The Spearman correlation coefficients (r) of each domain were 0.94, 0.81, 0.84, and 0.80 (p<0.0001), which means that each subscale had a strong correlation. We interpreted this to mean that the bother subscales of each domain were highly correlated with the function subscales.
TABLE 3.
Validity assessment of the Korean version of EPIC by Spearman's correlation coefficients between the function and bother subscales
A value of Spearman's correlation greater than 0.40 between subscales indicates that the scales are meaningfully related, and a value greater than 0.70 between subscales indicates that they are highly related. EPIC: Expanded Prostate Cancer Index Composite, a: Spearman's correlation coefficients between function and bother subscale ≥0.7 indicates that the bother subscales of each domain were highly correlated with the function subscales. p<0.0001
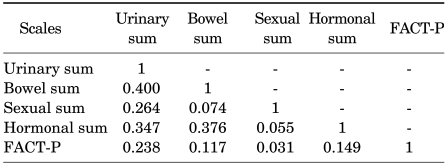
The correlation between each domain score of the Korean version of EPIC and a previously validated and currently used questionnaire, FACT-P, was analyzed. FACT-P and each domain of EPIC showed low correlation (r<0.24), which could be interpreted to mean that these two questionnaires could be used complementarily without redundancy in the evaluation of QoL in PCa patients (Table 4).
TABLE 4.
Validity assessment of the Korean version of EPIC by Spearman's correlation coefficients between EPIC domains and FACT-P
A value of Spearman's correlation greater than 0.40 between subscales indicates that the scales are meaningfully related, and a value greater than 0.70 between subscales indicates that they are highly related. EPIC: Expanded Prostate Cancer Index Composite, FACT-P: Functional Assessment of Cancer Therapy-Prostate. p<0.0001
DISCUSSION
PCa is the second most commonly diagnosed cancer and the sixth most common cause of death in males worldwide [9]. Its prevalence is also confirmed by recent data (2004-2006) that 1 in every 6 males experience PCa in their lifetime [10]. In fact, PCa has shown the fastest growth in incidence, which it may explain the explosively increasing interest in this disease in the general population in Korea [1]. Without doubt, the primary goal of PCa treatment is to improve survival. Despite the development of diverse modalities to manage PCa, only scarce comparative studies on the long-term survival rates of these modalities are available, limiting the data available for patients regarding comparative survival associated with different treatment modalities.
Moreover, QoL after the treatment of PCa is important because of three characteristics of the disease: most PCa progresses slowly, most treatments leave certain residual symptoms, and the average survival after treatment is gradually increasing [11,12]. Hence, QoL research could provide additional information for choosing a treatment. Furthermore, the report that about 16% of patients who undergo treatment for localized PCa regret their treatment choice emphasizes the importance of providing the most comprehensive information possible to patients about the treatments before they choose their treatment [13,14].
The most common complications after treatment of localized PCa are urinary incontinence, bleeding, bowel toxicity, and erectile dysfunction [7]. More specifically, 4-50% of patients suffer mild stress incontinence, 0-15% suffer severe stress incontinence, 0.5-15% suffer bladder neck stricture [14], and 60-90% suffer erectile dysfunction after surgical management [7]. In the case of EBRT, it has been reported that more than 12% of patients experience a moderate to severe degree of urinary incontinence, 20-50% experience diarrhea, more than 39% experience rectal pain, and 80% experience erectile dysfunction [14]. Reports on QoL after HIFU are inadequate, but it has been reported that approximately 12% of patients experience stress urinary incontinence and 55-70% experience erectile dysfunction [14]. Hormonal therapy for PCa also severely affects QoL. Prevalences of 50-100% for erectile dysfunction, 13-70% for gynecomastia, and 55-80% for hot flushes in treated patients have been reported [15]. This is why close attention needs to be paid to complications when we consider QoL after PCa treatment.
Although there are multiple methodologies to evaluate QoL in chronically ill or cancer patients, such as FACIT, SF-36, and FACT-G, a more specialized method for examining the QoL of PCa patients treated with multiple modalities has not been established. As such, these methodologies have been used as research tools to study QoL after PCa treatments, despite their limitations, which include a lack of reflection in symptom severity, bother, limitation of one's life, and eventually quality of life.
FACT-P, a recently developed questionnaire to overcome this limitation, has been translated into Korean and has been validated [16]. It was achieved by extending FACT-G, adding certain prostate-related symptoms, which allows it to provide objective and consistent data on cancer treatment and rough prostate-related symptoms. However, its limitation lies in that the research does not offer detailed information on symptom-related QoL after PCa treatment. In contrast, EPIC is systemically organized with common symptom-related domains and is divided into the physical and mental aspects of QoL [17]. In addition, hormone-related symptom domains were added, which were neglected in other studies on QoL [6]. These properties were supported by our results that showed a low correlation coefficient between EPIC and FACT-P (r<-0.1). This result can be interpreted to mean that EPIC and FACT-P can be used simultaneously with complementary purposes, or indeed independently.
To describe the word 'domain', one translator proposed '계' initially. However, we revised this to '영역' because '계' appeared to be awkward. There was a minor opinion enquiring about 'leaked urine' in Question 1 and 'urinary control' in Question 4 in the urinary domain, which appeared to have similar meaning in Korean, but we agreed that these expressions were acceptable, considering the context of the initial questionnaire. In the bowel domain, 'rectal pain' was a problem because it was ambiguous and difficult to distinguish from rectum; therefore, we changed 'rectal pain' to '항문 통증', meaning anal pain. In the hormonal domain, we translated 'hot flush' to '열감' initially; however, it was then changed to '일과성 열감' because '열감' was confused with fever sensation. All translators chose '유방의 민감도' for 'Breast tenderness', but we substituted this expression with '유방통증'. There were two opinions on 'small', 'moderate', and 'big' on the bother subscale of each domain. One was '조금', '보통' and '큰'; the other was '경증', '중등도' and '중증'. One raised an objection to the first opinion because the word '보통' is usually used in case of normal and it was confusing. But the majority of our group came to consensus on the first opinion, because '중증의 문제가 됨' was somewhat awkward and '보통' was not confusing in this context.
Although studies on PCa QoL with EPIC are on the increase, there are not enough efforts to publish the result of its translation into other languages and subsequent validation. As far as we know, Japanese [18], Spanish [19], and partially Dutch [20] versions of EPIC have been published. It is probably because EPIC is not yet generalized to urologists or other oncologists. However, according to our experience, large numbers of questions and advanced patient age may explain this. EPIC consists of 50 questions that could be excessive for elderly patients who have difficulties maintaining concentration and compliance. In our cases, many patients complained that it took a lot of time to complete the informed consent, EPIC, and FACT-P simultaneously, mainly due to poor vision. After realizing this, we decided to compare EPIC with FACT-P only after consulting with a statistician, and arranged for a monitor until completion of the questionnaire. This could have improved patient compliance. With these processes, we achieved reliability and validity that can be compared with the original version of EPIC. An outstanding point of this research is the diverse inclusion of target patients. In other words, we included patients who underwent RPP, RRP, LRP, RLRP, and HIFU. Therefore, our validation of the Korean version of EPIC in this group of patients can offer a more solid basis to the outcomes on QoL after PCa treatment.
CONCLUSIONS
The Korean version of EPIC was developed to reflect the original version of EPIC and was adapted to the Korean culture and language. Reliability and validity were acceptable; therefore, it can be used as a validated tool to evaluate treatment-related QoL in Korean PCa patients. Furthermore, it can be used as an objective reference to compare the mental and physical aspects of various treatment modalities. The Korean version of EPIC can be accepted as an accurate methodology with cross-cultural variety in international multicenter research.
From the patient's perspective, the Korean version of EPIC can offer important clues on choosing a treatment option by providing knowledge about differences in QoL associated with individual modalities. In addition, it may help patients to deal with the postoperative state and overcome complications with appropriate and early interventions.
Footnotes
The authors have nothing to disclose.
Appendix
The Expanded Prostate Cancer Index Composite
References
- 1.Ministry of Health and Welfare. Annual report of Korea central cancer registry. 2005. pp. 11–24. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 4.Zeliadt SB, Moinpour CM, Blough DK, Penson DF, Hall IJ, Smith JL, et al. Preliminary treatment considerations among men with newly diagnosed prostate cancer. Am J Manag Care. 2010;16:e121–e130. [PubMed] [Google Scholar]
- 5.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–1012. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 7.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Chung TG, Lee TK, Chung S, Lee MS, Kim YS, Ahn TY. The Korean version of the International Index of Erectile Function (IIEF): reliability and validation study. Korean J Urol. 1999;40:1334–1343. [Google Scholar]
- 9.Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, et al. Global cancer facts and figures 2007. Available from: http://www.cancer.org/docroot/STT/content/STT_1x_Global_cancer_Facts_and_Figures_2007.asp.
- 10.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER cancer statistics review, 1975-2006 (posted to the web site, 2009) Available from: http://seer.cancer.gov/csr/1975_2007/index.html.
- 11.Penson DF, Litwin MS, Aaronson NK. Health related quality of life in men with prostate cancer. J Urol. 2003;169:1653–1661. doi: 10.1097/01.ju.0000061964.49961.55. [DOI] [PubMed] [Google Scholar]
- 12.Efficace F, Bottomley A, van Andel G. Health related quality of life in prostate carcinoma patients: a systematic review of randomized controlled trials. Cancer. 2003;97:377–388. doi: 10.1002/cncr.11065. [DOI] [PubMed] [Google Scholar]
- 13.Moul JW, Anderson J, Penson DF, Klotz LH, Soloway MS, Schulman CC. Early prostate cancer: prevention, treatment modalities, and quality of life issues. Eur Urol. 2003;44:283–293. doi: 10.1016/s0302-2838(03)00296-3. [DOI] [PubMed] [Google Scholar]
- 14.Heidenreich A. Guidelines and counselling for treatment options in the management of prostate cancer. Recent Results Cancer Res. 2007;175:131–162. doi: 10.1007/978-3-540-40901-4_9. [DOI] [PubMed] [Google Scholar]
- 15.Kumar RJ, Barqawi A, Crawford ED. Adverse events associated with hormonal therapy for prostate cancer. Rev Urol. 2005;7(Suppl 5):S37–S43. [PMC free article] [PubMed] [Google Scholar]
- 16.Hong JH, Jeon SS, Lee HM, Choi YH, Kim S, Choi HY. The Functional Assessment of Cancer Therapy-Prostate (FACT-P) scales in men with prostate cancer: reliability and validity of the Korean version. J Korean Med Sci. 2006;21:295–299. doi: 10.3346/jkms.2006.21.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson JF, Swanson DA, Levy LB, Kuban DA, Lee AK, Kudchadker R, et al. Urinary side effects and complications after permanent prostate brachytherapy: the MD Anderson Cancer Center experience. Urol. 2009;74:601–605. doi: 10.1016/j.urology.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 18.Takegami M, Suzukamo Y, Sanda MG, Kamoto T, Namiki S, Arai Y, et al. The Japanese translation and cultural adaptation of Expanded Prostate Cancer Index Composite (EPIC) Nippon Hinyokika Gakkai Zasshi. 2005;96:657–669. doi: 10.5980/jpnjurol1989.96.657. [DOI] [PubMed] [Google Scholar]
- 19.Ferrer M, Garin O, Pera J, Prats JM, Mendivil J, Alonso J, et al. Evaluation of the quality of patients with localized prostate cancer: validation of the Spanish version of the EPIC. Med Clin (Barc) 2009;132:128–135. doi: 10.1016/j.medcli.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Korfage IJ, Roobol M, de Koning HJ, Kirkels WJ, Schröder FH, Essink-Bot ML. Does "normal" aging imply urinary, bowel, and erectile dysfunction? A general population survey. Urology. 2008;72:3–9. doi: 10.1016/j.urology.2008.01.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Expanded Prostate Cancer Index Composite